Summary
Accumulation of proteins in the endoplasmic reticulum (ER) typically induces stress and initiates the unfolded protein response (UPR) to facilitate recovery. If homeostasis is not restored, apoptosis is induced. However, adaptation to chronic UPR activation can increase resistance to subsequent acute ER stress. We therefore investigated adaptive mechanisms in Oculocutaneous albinism type 2 (Oca2)-null melanocytes where UPR signaling is arrested despite continued tyrosinase accumulation leading to resistance to the chemical ER stressor thapsigargin. Although thapsigargin triggers UPR activation, instead of Perk-mediated phosphorylation of eIF2α, in Oca2-null melanocytes, eIF2α was rapidly dephosphorylated upon treatment. Dephosphorylation was mediated by the Gadd34-PP1α phosphatase complex. Gadd34-complex inhibition blocked eIF2α dephosphorylation and significantly increased Oca2-null melanocyte sensitivity to thapsigargin. Thus, Oca2-null melanocytes adapt to acute ER stress by disruption of proapoptotic Perk signaling, which promotes cell survival. This is the first study to demonstrate rapid eIF2α dephosphorylation as an adaptive mechanism to ER stress.
Keywords: Unfolded protein response, eIF2α, melanocyte, Oculocutaneous albinism type 2, ER stress
Introduction
Normal pigmentation requires that tyrosinase, the first and rate-limiting enzyme in melanin synthesis, is efficiently folded and processed in the endoplasmic reticulum (ER), matured in the Golgi, and transported to melanosomes where melanin synthesis and deposition occur. Oculocutaneous albinism (OCA) connotes a group of hereditary disorders characterized by hypopigmentation of the skin, hair, and eye due to abnormal processing or trafficking of tyrosinase. In OCA types 1, 2 and 3 (caused by mutations in the tyrosinase, OCA2, and tyrosinase-related protein 1 genes, respectively), tyrosinase accumulates in the ER (Lee et al., 1994, Toyofuku et al., 2001, Chen et al., 2002). Stress induced by buildup of misfolded proteins in the ER typically triggers the unfolded protein response (UPR), an intricate mechanism that enables cells to restore homeostasis through increased degradation of misfolded proteins, improved ER protein folding capacity, and reduced synthesis of new proteins. If ER homeostasis cannot be restored despite these cytoprotective measures, UPR-initiated apoptosis is triggered to remove dysfunctional cells.
The UPR consists of three distinct signaling pathways controlled by inositol-requiring protein 1 (Ire1), activating transcription factor 6 (Atf6), and PKR-like endoplasmic reticulum kinase (Perk), respectively. Under normal physiological conditions, these ER transmembrane signaling proteins bind the protein chaperone BiP (also known as glucose-regulated protein 78 (Grp78)). They are activated when BiP is released and sequestered to unfolded proteins as they accumulate in the ER. Upon activation, Ire1 is phosphorylated and its endoribonuclease domain activated, leading to mRNA splicing of transcription factor X-box binding protein 1 (Xbp1). Spliced Xbp1 induces expression of genes containing the ER stress or unfolded protein response element (ERSE/UPRE) (Calfon et al., 2002, Yoshida et al., 2001), including ER chaperones (e.g. BiP), heat shock proteins, and Xbp1 itself. Atf6 traffics to the Golgi following release, where it is cleaved and then translocated to the nucleus where it activates transcription of ER stress related genes (e.g. BiP and Xbp1). Perk autophosphorylates upon release, and subsequently phosphorylates eukaryotic translation initiation factor 2α (eIF2α), which blocks cap-dependent mRNA translation, leading to decreased global protein synthesis. However, certain mRNAs (e.g. activating transcription factor 4 (Atf4)) escape repression (Vattem and Wek, 2004). Atf4 activates transcription of a number of genes including C/EBP-homologous protein (Chop, also known as growth arrest and DNA damage-inducible protein 153 (Gadd153)), which plays an important role in UPR-induced apoptosis (Marciniak et al., 2004, McCullough et al., 2001). Chop also induces expression of Gadd34, which forms a complex with the protein phosphatase 1 catalytic subunit α (PP1α), and dephosphorylates eIF2α, thus restoring protein synthesis that is necessary for cell survival (Connor et al., 2001, Marciniak et al., 2004). Although primarily induced via Perk signaling, Chop can also be upregulated via the Ire1 and Atf6 pathways (Yoshida et al., 2000).
The timing and duration of UPR signaling greatly affects cell fate. For example, Ire1 and Perk signaling have opposite effects on viability. Prolonged Ire1 signaling during persistent ER stress leads to increased cell survival, suggesting a cytoprotective role, while Perk signaling is responsible for triggering apoptosis (Lin et al., 2007, Lin et al., 2009). During acute ER stress, cytoprotective responses outweigh proapoptotic factors. When ER stress persists, Ire1 signaling is attenuated while Perk signaling remains unaffected, thereby tipping the balance in favor of apoptosis.
Despite sustained ER stress due to tyrosinase retention in the ER, melanocytes adapt and escape UPR-induced apoptosis (Manga et al., 2010). Melanocytes remain viable in individuals with OCA and melanocyte cultures have been established from the skin of humans and mice with each of the three forms of OCA. We have shown that UPR signaling is attenuated in melanocytes from mice with Oca1 and Oca3 (Manga et al., 2010). In this study we investigated the UPR in Oca2-null melanocytes. Oca2 (also known as pink-eyed dilution (P)) mutations lead to retention of tyrosinase in the ER similar to that seen in OCA1 and OCA3 (Chen et al., 2002, Toyofuku et al., 2002), and while Oca2 expression is clearly critical for melanin synthesis, the specific function of the Oca2 protein in melanocytes is not known. We hypothesized that UPR attenuation contributes to the extreme chemoresistance phenotype we observed previously in Oca2-null cells (Staleva et al., 2002).
The UPR is also thought to be activated during melanomagenesis (Hersey and Zhang, 2008, Zhuang et al., 2009), and the inherent ability of melanocytes to adapt to ER stress has been hypothesized to facilitate their transformation into melanoma. The UPR has also been proposed as a target for therapeutic intervention in melanoma (Chen et al., 2007). Identifying mechanisms by which melanocytes adapt to sustained ER stress and UPR activation may shed light on determinants of viability and provide targets for melanoma therapy. In order to elucidate the process of adaptation to ER stress in melanocytes, we have now investigated the UPR in Oca2-null mouse melanocytes, in which misfolded tyrosinase is known to accumulate in the ER, and find that loss of Oca2 expression leads to dysregulation of UPR signaling that facilitates adaptation to ER stress in melanocytes.
Results
UPR-related proteins are differentially expressed in Oca2-null melanocytes compared to wildtype cells, but UPR signaling is not sustained
Although Oca2 mutations result in ER retention and accumulation of tyrosinase, Oca2-null melanocytes are still viable despite the chronic ER stress. We hypothesized that Oca2-null melanocytes have adapted to ER stress and escape UPR-induced cell death. To identify adaptive mechanisms, we compared expression of several key UPR signaling proteins between wildtype and Oca2-null mouse melanocytes. Both cell lines were cultured in low tyrosine media (0.1 mM), resulting in dark pigmentation of wildtype cells and light grey pigmentation of Oca2-null cells, reflecting normal and reduced tyrosinase maturation, respectively. Under these cell culture conditions Oca2-null melanocytes express increased levels of Ire1 compared to wildtype (Average densitometry of Western blot analysis of four experiments: wildtype= 1.04, SD= 0.42, Oca2-null= 1.65, SD= 0.14). However, no increase in Xbp1 splicing was observed, indicating that Ire1 is not phosphorylated and the Ire1 arm of the UPR was not activated in Oca2-null melanocytes despite continued accumulation of tyrosinase in the ER (figure 1a). Interestingly, expression of Perk and its downstream target, Atf4, was decreased in Oca2-null melanocytes (Average densitometry of Western blot analysis of four experiments: Perk: wildtype= 0.94, SD 0.03, Oca2-null= 0.53, SD 0.06; Atf4: wildtype= 1.35, SD 0.65, Oca2-null= 0.03, SD 0.01). In addition, total eIF2α was slightly decreased in Oca2-null melanocytes, while levels of phosphorylated eIF2α were maintained when compared to wildtype cells (figure 1b). These data demonstrate that Ire1 and Perk, proteins that monitor ER stress and signal UPR activation, are differentially expressed in Oca2-null melanocytes compared to wildtype cells. However, associated signal transduction pathways are not activated.
Figure 1. Attenuation of UPR signaling in Oca2-null melanocytes under routine culture conditions.
Protein and RNA were extracted from melanocytes and subjected to immunoblot analysis and reverse transcriptase PCR, respectively, to determine (a) Ire1 protein expression and Xbp-1 mRNA splicing, and (b) expression of key proteins involved in Perk signaling. Xbp1-u= unspliced Xbp-1; Xbp1-s=spliced Xpb-1. A faint higher molecular band reported as representing a hybrid amplicon is also observed (Back et al., 2005).
Oca2-null melanocytes show increased resistance to ER stress-induced apoptosis
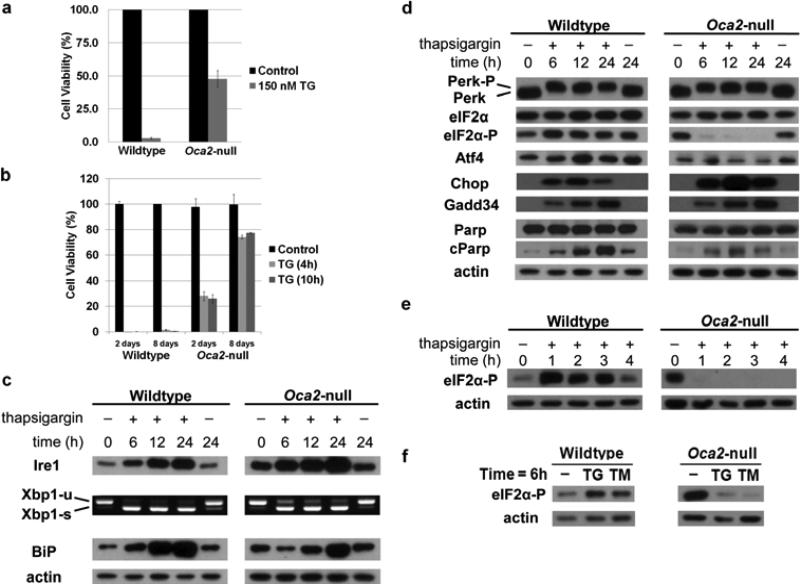
Thapsigargin disrupts ER homeostasis by inhibition of sarco/endoplasmic reticulum calcium ATPases that regulate calcium levels, resulting in UPR activation. Wildtype and Oca2-null melanocytes were treated with thapsigargin to investigate the cellular response to acute ER stress. Whereas a single dose of 150 nM thapsigargin for 48 hours reduced wildtype melanocyte viability to 3.0%, viability of Oca2-null melanocytes after 48 hours of treatment was 47.9% (figure 2a), suggesting that Oca2-null melanocytes not only adapted to chronic ER stress to escape UPR induced apoptosis, but are also more resistant to additional stress caused by UPR activating chemotoxins such as thapsigargin. When cells were dosed for either 4 hours or 10 hours and then grown over an extended period, wildtype cells were all dead within 72 hours, while Oca2-null melanocytes were still viable and had recovered significantly (8 day recovery after: 4 hour treatment = 74.2%; after 10 hour treatment = 77.6%) after 8 days (figure 2b).
Figure 2. Oca2-null melanocytes are more resistant to thapsigargin-induced ER stress, and show disruption of Perk signaling at the eIF2α level upon treatment.
(a) Wildtype and Oca2-null melanocytes were treated with 150 nM thapsigargin (TG) for 48 h, following which a crystal violet assay was performed in order to assess viability. Average viability ± SEM is shown as percentage compared to vehicle-treated controls (n = 3). *P<0.05. (b) Melanocytes were treated with 150nM TG for either 4 h or 10 h and allowed to recover in fresh medium. A crystal violet assay was performed at either day 2 or day 8. (c-e) Cells were treated with 150 nM thapsigargin for the indicated times, followed by protein extraction and immunoblot analysis of molecules involved in Ire1 and Perk signaling. Phosphorylation of Perk increases protein mass resulting in slower migration during SDS-PAGE; cParp= cleaved Parp. RNA was extracted for reverse transcriptase PCR analysis of Xbp-1 mRNA splicing. Xbp1-u= unspliced Xbp-1; Xbp1-s=spliced Xpb-1. (f) Cells were treated with 150 nM thapsigargin or 10 μg/ml tunicamycin for 6 hours, followed by protein extraction and immunoblot analysis of phosphorylated eIF2α.
Acute ER stress disrupts Perk, but not Ire1 signaling in Oca2-null melanocytes
Immunoblot analysis was performed to assess UPR activation in melanocytes treated with 150 nM thapsigargin for up to 24 hours. Ire1 expression increased in wildtype melanocytes with increasing exposure times. Furthermore, treatment resulted in Xbp1 splicing and increased BiP expression at 24 hours, indicating that Ire1 signaling was activated in response to thapsigargin-induced ER stress (figure 2c). Ire1 signaling was also activated in Oca2-null melanocytes during thapsigargin treatment as shown by increased expression of Ire1 and BiP as well as Xbp1 splicing. Ire1 signaling remained active during the 24 hour period of treatment in both cell lines (figure 2c).
Thapsigargin induced phosphorylation of Perk in both wildtype and Oca2-null melanocytes. In wildtype cells, activation of Perk led to an increase in phospho-eIF2α, Atf4, Chop, and later, Gadd34 expression indicating Perk pathway activation (figure 2d). In Oca2-null melanocytes, however, dephosphorylation of eIF2α was observed within 1 hour of treatment and persisted for the entire 24 hour period (figure 2d & e). Rapid dephosphorylation of eIF2α in response to acute ER stress disrupted Perk signaling since Atf4 expression did not increase in Oca2-null melanocytes following thapsigargin treatment. Interestingly, expression of Chop as well as Gadd34 was still induced in thapsigargin-treated Oca2-null melanocytes. Cleavage of PARP, indicating caspase-mediated apoptosis, was noticeable in both cell lines, but whereas wildtype melanocytes showed increasing levels of cleaved PARP during the 24 hour period of treatment, maximal PARP cleavage that was not robust in comparison was seen at 12 hours in Oca2-null melanocytes suggesting that Oca2-null melanocytes are more resistant to ER stress-induced apoptosis (figure 2d). Similar results were observed when cells were treated with an alternative chemical stressor, tunicamycin, which inhibits N-glycosylation of newly synthesized glycoproteins causing the proteins to be retained in the ER (data not shown), with dephosphorylation of eIF2α following treatment (figure 2f). Since phosphorylated eIF2α blocks mRNA translation and thus global protein synthesis, overall protein synthesis decreased to 20% (SD=7.5) in wildtype cells that were stressed with thapsigargin for 2 hours compared to non-treated controls, while in Oca2-null melanocytes, synthesis was reduced to 51% (SD=1.7). Decreased protein synthesis in Oca2-null cells may reflect a slowing of proliferation, with recovery observed at 8 days (figure 2a).
Rapid eIF2α dephosphorylation upon induction of ER stress in Oca2-null melanocytes is mediated by the Gadd34-PP1 complex
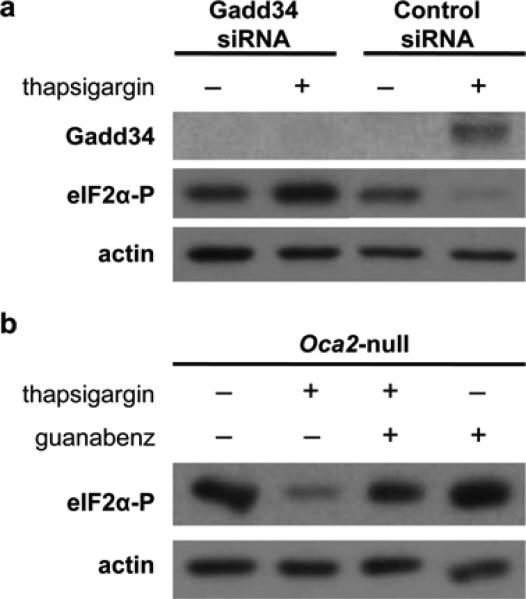
We observed activation of the UPR-induced expression of Gadd34 in both melanocyte cell lines, although the increase was not as dramatic in Oca2-null melanocytes as compared to wildtype. Typically, Gadd34 recruits the PP1α catalytic subunit to the ER membrane where it forms a potent phosphatase that dephosphorylates eIF2α over time and allows resumption of protein synthesis necessary for cell survival (Brush et al., 2003). To investigate the role of Gadd34 in Oca2-null melanocytes, cells were transfected with siRNA targeting Gadd34, and subsequently treated with 75 nM thapsigargin for 3 hours. A lower thapsigargin dose was used in these experiments as the transfection process itself reduced viability. A 75 nM dose can induce UPR activation without causing additional cell death within the 3 hour treatment period. Successful knockdown was achieved as Gadd34 protein expression was not induced in siRNA transfected cells upon acute ER stress. Moreover, Gadd34 siRNA prevented eIF2α dephosphorylation in Oca2-null melanocytes during thapsigargin treatment, indicating that Gadd34 mediates this process (figure 3a). Next, we treated Oca2-null melanocytes with thapsigargin in combination with guanabenz, a specific inhibitor of the Gadd34-PP1α complex (Tsaytler et al., 2011). Oca2-null melanocytes were pre-treated with 50 μM guanabenz for 30 minutes, and then exposed to 75 nM thapsigargin and a second dose of 50 μM guanabenz for 2.5 hours. Immunoblot analysis revealed that the combined treatment blocked dephosphorylation of eIF2α in response to ER stress, restoring phospho-eIF2α levels to close to normal (figure 3b). Thus, eIF2α is rapidly dephosphorylated by Gadd34 during thapsigargin-induced ER stress in Oca2-null melanocytes.
Figure 3. The Gadd34-PP1α complex mediates eIF2α dephosphorylation upon ER stress in Oca2-null melanocytes.
(a) Immunoblot analysis of Oca2-null melanocytes transfected with either Gadd34 or control siRNA for 48 h, and treated with 75 nM thapsigargin for 3 h. (b) Oca2-null melanocytes were pre-treated with 50 μM guanabenz for 30 min, then dosed with 75 nM thapsigargin and an additional dose of guanabenz for 2.5 h. Phospho-eIF2α levels were determined by immunoblot analysis.
Thapsigargin resistance in Oca2-null melanocytes is mediated by eIF2α dephosphorylation
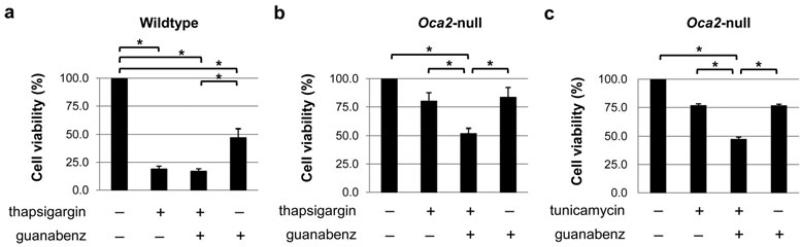
Next, we examined whether guanabenz could decrease Oca2-null melanocyte resistance to thapsigargin, since blocking of rapid eIF2α dephosphorylation would restore pro-apoptotic Perk signaling. Melanocytes were pre-treated with 50 μM guanabenz for 30 minutes, followed by 75 nM thapsigargin for 24 hours. Additional doses of 25 μM guanabenz were added at 0 and 6 hours of thapsigargin treatment to prevent early eIF2α dephosphorylation. As expected, addition of guanabenz in combination with thapsigargin did not further reduce viability of wildtype melanocytes beyond that seen with thapsigargin treatment alone despite the fact that guanabenz alone is cytotoxic (figure 4a). Oca2-null melanocytes were much less sensitive to either thapsigargin or guanabenz alone, showing similar survival rates (80.1% and 83.5%, respectively) that were not significantly different from the vehicle-treated control cells. However, viability of Oca2-null melanocytes treated with thapsigargin and guanabenz in combination was significantly decreased compared to cells treated with thapsigargin alone, indicating that thapsigargin resistance in Oca2-null melanocytes is mediated by eIF2α dephosphorylation (figure 4b). The combination of thapsigargin and guanabenz exhibited a synergistic effect on cell viability (51.9%) since the predicted viability for an additive effect is 66.9% according to the Bliss independence model (Bliss, 1939). A similar synergistic effect was observed when cells were treated with tunicamycin in combination with guanabenz (figure 4c).
Figure 4. Guanabenz increases sensitivity of Oca2-null melanocytes to thapsigargin.
(a-c) Viability of wildtype and Oca2-null melanocytes after pre-treatment with 50 μM guanabenz for 30 min, followed by either 75 nM thapsigargin treatment or 5 μg/ml tunicamycin for 24 h. Additional doses of 25 μM guanabenz were added at 0 and 6 h of treatment. Average viability ± SEM is shown as percentage compared to vehicle-treated controls (n = 4). *P<0.05.
Discussion
In this study we compared UPR activation in wildtype and Oca2-null melanocytes to elucidate adaptive mechanisms to ER stress that promote cell survival. The UPR was not activated at baseline in Oca2-null melanocytes despite sustained ER stress due to accumulation of tyrosinase in the ER. However, expression of pro-survival Ire1, although not active, was upregulated, while expression of pro-apoptotic Perk was downregulated in Oca2-null melanocytes. Thus our data suggest that chronic accumulation of tyrosinase in the ER of melanocytes did cause activation of the UPR, which was attenuated, allowing the cells to survive both in the skin and in culture. Chemical ER stressors, which were more toxic to wildtype melanocytes, activated Ire1 signaling in both wildtype and Oca2-null lines. Pro-apoptotic Perk signaling was however disrupted in Oca2-null melanocytes through eIF2α dephosphorylation (figure 2e). To our knowledge, our current study is the first to demonstrate rapid eIF2α dephosphorylation as an adaptive response to ER stress.
Under routine culture conditions, key UPR signaling proteins were differentially expressed in Oca2-null melanocytes, where ER stress is chronic, compared to wildtype cells. Although expression of Ire1 was increased in Oca2-null melanocytes, Xbp1 mRNA was not spliced. Thus, this UPR pathway was not activated, suggesting that activated or prolonged Ire1 signaling, which primarily promotes cell survival, does not contribute to the adaptation of Oca2-null melanocytes. Oca2-null melanocytes did however express decreased baseline levels of Perk and Atf4, which may favor survival.
Oca2-null melanocytes were less sensitive to thapsigargin compared to wildtype cells, indicating that Oca2-null melanocytes not only adapted to chronic ER stress to escape UPR-induced apoptosis, but are also more resistant to additional acute stress. Preconditioning of cells with sub-lethal levels of ER stress have been shown to increase resistance to additional ER stress (Goldfinger et al., 2011), suggesting a potential mechanism by which Oca2-null melanocytes escape thapsigargin-induced death.
Thapsigargin activated Ire1 signaling in a similar fashion in both wildtype and mutant cell lines. Given that Ire1 is expressed at higher levels in Oca2-null melanocytes, signaling of this pro-survival pathway may be more robust, contributing to survival following exposure to thapsigargin. Remarkably, while acute ER stress resulted in phosphorylation and activation of Perk in Oca2-null melanocytes, Perk signaling was disrupted by rapid dephosphorylation of eIF2α which prevented preferential expression of the Atf4 transcription factor.
Our data suggest that Gadd34 rapidly dephosphorylates eIF2α during acute ER stress, but Gadd34 protein levels are not increased in Oca2-null melanocytes compared to wildtype cells (figure 2c), suggesting that expression of Gadd34 is not the only factor required for the rapid dephosphorylation of eIF2α. Rapid formation of the Gadd34-PP1α complex upon UPR activation in Oca2-null melanocytes could explain this phenomenon, as Gadd34 recruits PP1α to the ER where it facilitates eIF2α dephosphorylation during ER stress (Brush et al., 2003). PP1 is a serine/threonine phosphatase that comprises a group of holoenzymes. Each functional PP1 enzyme complex consists of a PP1 catalytic subunit (α, β, γ1 or γ2) and a regulatory subunit (e.g. Gadd34). Subcellular localization as well as substrate specificity is directed by the regulatory subunit. Furthermore, inhibitory subunits prevent accumulation of free unbound PP1 catalytic subunits (Cohen, 2002). Gadd34 has been shown to recruit PP1α to the ER membrane where it forms a potent eIF2α phosphatase (Brush et al., 2003). Both Gadd34 siRNA and guanabenz (specific Gadd34-PP1α eIF2α phosphatase inhibitor) blocked eIF2α dephosphorylation during thapsigargin-induced ER stress in Oca2-null melanocytes. Since Gadd34 specifically binds PP1α (Brush et al., 2003), it is unlikely that increased phosphatase activity in Oca2-null melanocytes is due to binding of other PP1 catalytic subunit isoforms. However, PP1α may be less tightly controlled by inhibitory subunits in Oca2-null melanocytes, lowering the threshold for Gadd34-PP1α complex formation - and thus eIF2α dephosphorylation - upon ER stress. Another possibility is the existence of a yet unknown mediator protein that promotes recruitment of Gadd34-PP1α to phospho-eIF2α. For example, Smad7 and CUEDC2 interact directly with Gadd34 to recruit the Gadd34-PP1α complex, which in turn dephosphorylates TGF-β type 1 receptor and IκB kinase (IKK), respectively (Shi et al., 2004, Li et al., 2008). An in-depth study of the interactions between PP1α, PP1 inhibitory subunits, Gadd34, eIF2α, and potentially other proteins may provide more clarity on this subject.
Despite the rapid dephosphorylation of eIF2α, Oca2-null melanocytes underwent a significant drop in the rate of protein synthesis and cell viability was compromised following thapsigargin treatment. The cells did however recover after 8 days, while the wildtype cells did not. It has been shown that UPR activation can cause cell cycle arrest in melanoma, thus the slow recovery of Oca2-null melanocytes may be dependent on additional mechanisms (Han et al., 2013). The duration of each UPR signaling pathway after stress induction varies, with early attenuation of Ire1 and Atf6 responses and sustained Perk signaling during prolonged ER stress (Lin et al., 2007). Although Perk signaling triggers apoptosis, Perk also directly activates the cytoprotective NF-E2 related factor-2 (Nrf2) pathway, which promotes an antioxidant response and has been implicated in chemoresistance (Cullinan and Diehl, 2004). We have shown recently that this mechanism is activated in melanocytes in response to vitiligo-inducing agents that promote production of reactive oxygen species (Toosi et al., 2012). Thus, pro-survival Perk signaling may remain intact, while pro-apoptotic signaling is disrupted at the eIF2α level by reducing the Atf4 response. Given that OCA2 polymorphisms modulate risk of developing vitiligo (Jin et al., 2012), modulation of PERK signaling due to OCA2 may play a role in vitiligo.
Despite the disruption of Perk signaling, Chop expression continues to increase in Oca2-null melanocytes upon thapsigargin-induced UPR activation. Although primarily induced via Perk and Atf4, Chop is also regulated by the Ire1 and Atf6 pathways. While Chop has been shown to promote cell death in some cells (such as oligodendrocytes), Chop expression can prevent cell death in other cell types (Gow and Wrabetz, 2009) and may represent another pro-survival pathway. Furthermore, Chop promotes apoptosis by downregulating expression of Bcl-2. In melanocytes, Bcl-2 expression is regulated by the microphthalmia transcription factor (MITF) (McGill et al., 2002), which also induces expression of many of the melanocyte-specific proteins including tyrosinase. Thus, this mechanism of UPR-induced apoptosis may be less effective despite Chop expression.
In this study, we have identified eIF2α dephosphorylation as a novel, innate adaptive response to ER stress. Dephosphorylation of eIF2α was previously observed in B cells stimulated to differentiate by LPS treatment. However, in contrast to our observation in melanocytes, the reduction in phospho-eIF2α in B cells was slow, occurring only after 3 days of LPS stimulation. Loss of phospho-eIF2α may contribute to plasma cell survival through the reorganization of the ER that is required to facilitate the production of immunoglobulins (Goldfinger et al., 2011).
Guanabenz increases Oca2-null melanocyte sensitivity to thapsigargin by preventing eIF2α dephosphorylation. Guanabenz is a marketed α2-adrenergic receptor agonist that is indicated for treatment of hypertension, but also shows anti-prion activity (Holmes et al., 1983, Tribouillard-Tanvier et al., 2008). It was recently discovered that guanabenz targeted Gadd34 and inhibited the formation of the Gadd34-PP1α complex that has eIF2α phosphatase activity. Guanabenz specifically targets Gadd34; it does not affect the Gadd34 homolog constitutive repressor of eIF2α phosphorylation (CReP), nor does it inhibit PP1α directly (Tsaytler et al., 2011). In our current study, guanabenz inhibited eIF2α dephosphorylation in Oca2-null melanocytes upon acute ER stress thereby increasing the sensitivity of Oca2-null cells to thapsigargin. Despite the significant synergistic effect of guanabenz and 75 nM thapsigargin, the combined treatment only resulted in a 48.1% reduction in viability in Oca2-null cells, similar to the reduction in viability seen with a 150 nM dose of thapsigargin alone. By contrast, wildtype cell viability was reduced by 81% with thapsigargin alone and there was no significant increase with addition of guanabenz. Thus, additional adaptive mechanisms, such as modulation of glutathione mediated detoxification (Staleva et al., 2002), may contribute to Oca2-null melanocyte robustness.
We have shown previously that Oca1 melanocytes also adapt to ER stress. However these mutants fail to increase expression of Ire1, Xbp-1 splicing or phosphorylation of Perk in response to thapsigargin treatment (Manga et al., 2010). We have now demonstrated that Perk signaling is disrupted in Oca2-null melanocytes at a step subsequent to Perk phosphorylation, suggesting that melanocytes can adapt to the UPR by multiple mechanisms.
Methods
Antibodies
Primary antibodies that were used in this study: rabbit anti-Atf4 (C-20), rabbit anti-BiP/Grp78 (H-129), mouse anti-CHOP/Gadd153 (B-3), rabbit anti-Gadd34 (C-19), goat anti-PP1α (N-19), and rabbit anti-PDI (H-160) from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-eIF2α (L57A5), rabbit anti-phospho-eIF2α (Ser51, D9G8), rabbit anti-Ire1 (14C10), rabbit anti-PARP (46D11), rabbit anti-Cleaved PARP (Asp214,D64E10) and rabbit anti-Perk (C33E10) from Cell Signaling (Danvers, MA). Horseradish peroxidase-conjugated goat anti-mouse antibody (Thermo Fisher Scientific, Waltham, MA), goat anti-rabbit antibody (Cell Signaling), and donkey anti-goat antibody (Santa Cruz Biotechnology) were used for immunoblot analysis.
Cell culture
Wildtype (melan-a) (Bennett et al., 1987) and Oca2-null (melan-p1) (Sviderskaya et al., 1997) mouse melanocyte cell lines were cultured in a mixture of 80% RPMI/20% DMEM supplemented as previously reported (Chen et al., 2002). Cells were cultured until full confluency followed by RNA and protein extraction to determine differential UPR expression between the two cell lines. To test the effects of acute ER stress and guanabenz, cells were cultured until 80-90% confluent. The culture medium was then replaced with medium containing vehicle only (dimethyl sulfoxide (DMSO), Thermo Fisher Scientific) as control, 75 or 150 nM thapsigargin (Sigma, St. Louis, MO), 10 μg/ml tunicamycin (Sigma), and/or 50 μM guanabenz (Santa Cruz Biotechnology) for the indicated times. Additional doses of 25 μM guanabenz were added to the existing medium at the indicated times.
Protein extraction and immunoblot analysis
Protein extraction and immunoblot analysis were performed as described previously (Manga et al., 2010). Protein content/cell was determined by dividing total yield of protein extraction by total cell count. Densitometry was performed using Image J software and was calculated relative to intensity of actin, which was used as a loading control.
RT-PCR analysis of Xbp-1 mRNA splicing
RNA extraction and semi-quantitative RT-PCR analysis of Xbp-1 mRNA splicing was performed as described previously (Manga et al., 2010). The unspliced Xbp-1 (Xbp-1-u) produced a 289-bp fragment, while the spliced Xbp-1 (Xbp-1-s) generated a 263-bp fragment.
Protein synthesis assay
A protein synthesis assay was performed using the Click-IT system (Life Technologies, Grand Island, NY). Briefly, cells were treated with vehicle (DMSO) or 150 nM thapsigargin for 2 h, then transferred to methionine-free medium for 1 hour. Cells were transferred to medium containing a fluorescently labeled methionine analog for 3 hours then fixed and fluorescence measured as per manufacturer's instructions.
Cell viability assay and statistical analysis
Cell viability was assessed by crystal violet assay. Cells were washed with ice-cold Dulbecco's phosphate buffered saline (DPBS) with calcium and magnesium (Mediatech, Manassas, VA), and subsequently fixed with ice-cold 50% methanol in DPBS for 15 min on ice followed by another 10 min with ice-cold 100% methanol. Cells were then stained with 0.5% crystal violet (Sigma) in 25% methanol for 10 min at room temperature. The culture dishes were thoroughly rinsed with water to clear excess crystal violet and air-dried. Cell-bound crystal violet was then released by adding 15% acetic acid, and transferred into a 96-well plate; absorbance at 595 nm was measured using a plate reader. A stained empty culture dish was added as a blank control. Data from independent experiments were normalized to vehicle-treated controls, pooled, and analyzed using the paired Student's t-test. Data were regarded as statistically significant if P<0.05. The combined additive effect was predicted according to the Bliss independence model to determine synergistic effects of combination treatments (Bliss, 1939).
siRNA assay
Oca2-null melanocytes were transfected with 200 nM mouse Gadd34 siRNA or control siRNA-A (Santa Cruz Biotechnology) by electroporation using the Amaxa Nucleofector Cell Line Kit L (Lonza, Basel Switzerland) as described previously (Manga et al., 2010). Cells were also transfected with pmaxGFP plasmid (Lonza) to determine transfection efficiency, which was 90%, although a large number of cells did not survive the procedure (data not shown). 48 h after transfection cells were treated with either vehicle (DMSO) as control or 75 nM thapsigargin for 3 h, and subsequently collected for further analysis.
Significance.
The unfolded protein response (UPR) may play a role in disorders of melanocyte viability, including vitiligo and melanoma, where OCA2 polymorphisms have been associated with an increased risk of developing either of these conditions. Our findings suggest that modulation of UPR signaling pathways mediated by loss of OCA2 may play an important role in melanocyte adaptation to endoplasmic reticulum stress and therefore melanocyte viability. Understanding the UPR mechanisms in melanocytes is crucial for the development of effective agents that target UPR-induced apoptosis for treatment of vitiligo and melanoma.
Acknowledgements
The authors thank Martha Vega, Genevieve Torres, Nazanin Roudiani, Sabina Bis and Khushboo Abhichandani for technical assistance. Melan-a and melan-p1 melanocyte lines were obtained from the Wellcome Trust Functional Genomics Cell Bank. These studies were supported in part by grant AR041880 from the NIH/NIAMS (SJO) and a Dermatology Foundation Career Development award (PM).
References
- Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi: 10.1016/j.ymeth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–8. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Bliss CI. The toxicity of poisons applied jointly. Ann Appl Biol. 1939;26:585–615. [Google Scholar]
- Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13:1953–64. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Jiang CC, Kiejda KA, Wang YF, Thorne RF, Zhang XD, Hersey P. Thapsigargin sensitizes human melanoma cells to TRAIL-induced apoptosis by up-regulation of TRAIL-R2 through the unfolded protein response. Carcinogenesis. 2007;28:2328–36. doi: 10.1093/carcin/bgm173. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–50. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–17. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Goldfinger M, Shmuel M, Benhamron S, Tirosh B. Protein synthesis in plasma cells is regulated by crosstalk between endoplasmic reticulum stress and mTOR signaling. Eur J Immunol. 2011;41:491–502. doi: 10.1002/eji.201040677. [DOI] [PubMed] [Google Scholar]
- Gow A, Wrabetz L. CHOP and the endoplasmic reticulum stress response in myelinating glia. Curr Opin Neurobiol. 2009;19:505–10. doi: 10.1016/j.conb.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Jin L, Mei Y, Wu M. Endoplasmic reticulum stress inhibits cell cycle progression via induction of p27 in melanoma cells. Cell Signal. 2013;25:144–9. doi: 10.1016/j.cellsig.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res. 2008;21:358–67. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS. Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs. 1983;26:212–29. doi: 10.2165/00003495-198326030-00003. [DOI] [PubMed] [Google Scholar]
- Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, Cole JB, Gowan K, Holland PJ, Bennett DC, Luiten RM, Wolkerstorfer A, Van Der Veen JP, Hartmann A, Eichner S, Schuler G, Van Geel N, Lambert J, Kemp EH, Gawkrodger DJ, Weetman AP, Taieb A, Jouary T, Ezzedine K, Wallace MR, Mccormack WT, Picardo M, Leone G, Overbeck A, Silverberg NB, Spritz RA. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676–80. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994;330:529–34. doi: 10.1056/NEJM199402243300803. [DOI] [PubMed] [Google Scholar]
- Li HY, Liu H, Wang CH, Zhang JY, Man JH, Gao YF, Zhang PJ, Li WH, Zhao J, Pan X, Zhou T, Gong WL, Li AL, Zhang XM. Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol. 2008;9:533–41. doi: 10.1038/ni.1600. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga P, Bis S, Knoll K, Perez B, Orlow SJ. The unfolded protein response in melanocytes: activation in response to chemical stressors of the endoplasmic reticulum and tyrosinase misfolding. Pigment Cell Melanoma Res. 2010;23:627–34. doi: 10.1111/j.1755-148X.2010.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Shi W, Sun C, He B, Xiong W, Shi X, Yao D, Cao X. GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor. J Cell Biol. 2004;164:291–300. doi: 10.1083/jcb.200307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staleva L, Manga P, Orlow SJ. Pink-eyed dilution protein modulates arsenic sensitivity and intracellular glutathione metabolism. Mol Biol Cell. 2002;13:4206–20. doi: 10.1091/mbc.E02-05-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskaya EV, Bennett DC, Ho L, Bailin T, Lee ST, Spritz RA. Complementation of hypopigmentation in p-mutant (pink-eyed dilution) mouse melanocytes by normal human P cDNA, and defective complementation by OCA2 mutant sequences. J Invest Dermatol. 1997;108:30–4. doi: 10.1111/1523-1747.ep12285621. [DOI] [PubMed] [Google Scholar]
- Toosi S, Orlow SJ, Manga P. Vitiligo-Inducing Phenols Activate the Unfolded Protein Response in Melanocytes Resulting in Upregulation of IL6 and IL8. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.181. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku K, Valencia JC, Kushimoto T, Costin GE, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res. 2002;15:217–24. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Valencia JC, Kushimoto T, Ferrans VJ, Hearing VJ. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. FASEB J. 2001;15:2149–61. doi: 10.1096/fj.01-0216com. [DOI] [PubMed] [Google Scholar]
- Tribouillard-Tanvier D, Beringue V, Desban N, Gug F, Bach S, Voisset C, Galons H, Laude H, Vilette D, Blondel M. Antihypertensive drug guanabenz is active in vivo against both yeast and mammalian prions. PLoS One. 2008;3:e1981. doi: 10.1371/journal.pone.0001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–4. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–74. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–67. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Scolyer RA, Lee CS, Mccarthy SW, Cooper WA, Zhang XD, Thompson JF, Hersey P. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–70. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]