Abstract
Essential tremor (ET) is a common tremor disorder affecting postural/action tremor of the upper extremities and midline. Recent research revealed a cerebellar-like deficit during tandem gait in persons with ET, though spatiotemporal variability during normal gait in ET has been relatively ignored. The first purpose of this study was to investigate gait variability magnitude and structure in ET as compared to healthy older adults (HOA). To address this issue, 11 ET and 11 age-matched HOAs walked on a treadmill for five minutes at preferred walking speeds. HOAs walked for an additional minute while speed-matched to an ET participant. The second purpose was to describe the clinical correlates of gait variability in this population. To address this aim, 31 persons with ET walked on a treadmill for five minutes and completed the Fahn-Tolosa-Marin Tremor Rating Scale. Gait variability magnitude was derived by calculating coefficients of variation in stride length, stride time, step length, step time, and step width. Gait variability structure was derived using a detrended fluctuation analysis technique. At preferred walking speeds, ET participants walked significantly slower with significantly increased variability magnitude in all five spatiotemporal gait parameters. At speed-matched walking, ET participants exhibited significantly higher step width variability. Gait variability structure was not different between groups. We also observed that gait variability magnitude was predicted by severity of upper extremity and midline tremors. This study revealed that self-selected gait in ET is characterized by high variability that is associated with tremor severity in the upper extremity and midline.
Keywords: gait, variability, essential tremor, motor control, cerebellum
Introduction
Essential tremor (ET) is one of the most prominent movement disorders in the adult population (Louis et al., 1998; Louis and Ferreira, 2010). Persons with ET have a syndrome typically characterized by an upper extremity action tremor which, in many patients, leads to increased difficulty in performing activities of daily living and a reduced quality of life (Chandran and Pal, 2013). As such, significant effort has been put into developing our understanding of upper extremity tremor and its implications on motor control in ET (Louis, 2005). Hence, motor control deficits in this population have been classically described as dysfunctional control of the upper extremities, trunk, head, and neck.
More recent findings have expanded upon the traditional understanding of motor control in persons with ET and have suggested that lower extremity function is also abnormal in ET. Midline tremors experienced by persons with ET may disrupt control of axial movements involved with locomotor and postural control. Several studies have described deficits during normal and tandem gait in persons with ET (Kronenbuerger et al., 2009; Louis et al., 2010; Rao et al., 2011; Stolze et al., 2001). Rao and colleagues documented that persons with ET spent an increased percentage of the gait cycle in double limb support, exhibited greater step time asymmetry, and walked with a reduced cadence and slower velocity when compared to age-matched controls (Rao et al., 2011). Conversely, other studies have observed only subtle differences in traditional gait performance, but have revealed marked cerebellar-like dynamic balance disturbance during tandem walking (Hoskovcová et al., 2013; Louis et al., 2010; Stolze et al., 2001). Louis and colleagues have recently elaborated on the clinical correlates of these findings, demonstrating associations between severity of midline tremors and worsened performance on gait and balance tests (Louis et al., 2010; Louis et al., 2012). Parisi and colleagues also noted worsening of functional mobility in persons with ET who experienced head tremor as compared to those without head tremor (Parisi et al., 2006). Though it has been postulated that these associations between midline tremor and worsening of gait may be indicative of the presence of a midline cerebellar syndrome in ET (Louis et al., 2010), more work is needed to characterize disturbances in gait variability.
Classical spatiotemporal gait characteristics such as gait velocity and step length provide important information pertaining to global gait performance; however, these parameters suggest very little about the variability of gait patterns. To date, only a few studies have examined gait variability in persons with ET (Fasano et al., 2010; Rao et al., 2011; Stolze et al., 2001). This is surprising considering the well-known associations between increased gait variability, dynamic instability, and fall risk (Brach et al., 2005; Callisaya et al., 2011; Toebes et al., 2012). These studies have reported inconclusive results, perhaps due to the analysis of a relatively limited number of strides and the reliance upon clinical measures such as ataxia ratio. An ataxia ratio may be clinically-relevant, but confounds directional variability analyses by combining deviations in step length, step width, and step height into a singular measure (Stolze et al., 2001). Previous research on gait variability in ET has also been limited exclusively to linear measures of the magnitude of variability. Nonlinear measures have often supplemented these linear analyses by describing the structure of variability in gait in other populations (Hausdorff et al., 1997; Kaipust et al., 2012; Wuehr et al., 2013), including persons with cerebellar disease (Wuehr et al., 2013). Previous research by Hausdorff and colleagues has suggested that the spinal central pattern generators (CPGs) theorized to govern the regulation of steady-state gait possess a certain type of “memory” which regulates gait variability (Hausdorff et al., 1995). This work suggests the presence of long-range correlations between gait cycles over time (Hausdorff et al., 1995). However, in elderly and some pathological populations, this CPG “memory” deteriorates and gait variability patterns become less-correlated and sometimes more random (Hausdorff et al., 1997), perhaps indicating disruption of CPG control of locomotion. Given the difficulty frequently observed in ET during tandem gait (Louis et al., 2012; Rao et al., 2011; Stolze et al., 2001), research into the magnitude and structure of gait variability patterns may provide further insight into ET locomotor control deficits.
The goals of this study were two-fold. First, we aimed to compare both the magnitude and the structure of gait variability in persons with ET to neurologically-healthy older adults (HOA). Second, we investigated individual items on the Fahn-Tolosa-Marin Tremor Rating Scale (TRS) (Stacy et al., 2007) as predictors of gait variability parameters to examine potential relationships between gait variability and clinical measures of ET disease severity. Consistent with previous research on classic cerebellar disorders, we hypothesized that persons with ET would demonstrate increased step length, step width, and step time variability when compared to HOA (Serrao et al., 2012) while the structure of the variability would be similar between groups when walking at preferred speeds (Wuehr et al., 2012). Based on previous research suggesting a link between gait difficulty and midline tremor severity (Hoskovcová et al., 2013; Louis et al., 2010; Louis et al., 2012; Parisi et al., 2006), we also postulated that measures of gait variability magnitude would be predicted by the severity of midline tremors.
Methods
Participants
Thirty-one participants with ET (mean ± SD age : 66.5 ± 9.6 y, mean height: 175.6 ± 11.6 cm, mean body mass: 94.2 ± 23.4 kg, mean TRS motor (items 1–14) score: 32 ± 14, mean TRS ADL (items 15–21) score: 10 ± 7, mean TRS total score: 43 ± 19) were referred from the Center for Movement Disorders and Neurorestoration at the University of Florida and participated in this study. Thirteen ET participants were taking either a beta-adrenergic antagonist, an anticonvulsant, or both while the remaining 18 ET participants were not taking any medication specifically intended to reduce tremor. For the cross-sectional portion of the study, 11 healthy older adults (HOA; mean age: 63.6 ± 7.8 y, mean height: 170.9 ± 7.2 cm, mean body mass: 75.7 ± 13.3 kg) participated and were age-matched within one year of the persons with ET. Independent samples t-tests did not reveal any differences in mean age, height, or body mass between groups (all p>.05). All ET participants had been previously evaluated and diagnosed by a movement disorder neurologist. Six ET participants in the cross-sectional portion of the study were medicated while the remaining five ET participants were not. None of the participants had undergone deep brain stimulation implantation and all participants were unaffected by musculoskeletal and neurological impairment (with the exception of ET). HOA were enrolled from the university and neighboring community. Before participation in the study, all participants provided written informed consent that was approved by the University’s Institutional Review Board. The participants with ET also completed the TRS prior to performing the gait trials.
Fahn-Tolosa-Marin Tremor Rating Scale (TRS)
The TRS is composed of 21 individual items (many with sub-scores for assessment of resting, postural, and action/intention tremor) scored on a scale from 0 (normal) to 4 (most severe) such that the minimum score is zero and the maximum score is 144. The motor section (items 1–14) includes assessment of tremor at specific anatomical locations (face, tongue, voice, head, right and left upper extremity, trunk, right and left lower extremity) as well as during specific upper extremity motor tasks (handwriting, spiral drawing, pouring). The ADL section (items 15–21) includes assessment of tremor and its patient-reported interference with specific activities of daily living (speaking, feeding, bringing liquids to mouth, hygiene, dressing, writing, and working). Scores in each section are summed to motor and ADL scores, respectively. The total TRS score is the sum of the motor and ADL scores (or the sum of all TRS items). The TRS was scored by a movement disorders neurologist. For further details on the TRS, please see Stacy et al., 2007.
Treadmill gait analysis
All participants were fitted with 35 passive retroreflective markers placed in accordance with the Vicon Plug-in-Gait full body marker set. The participant’s preferred walking speed was determined by gradually speeding up the treadmill from a stop until the participant identified that the treadmill speed was similar to the pace at which they felt was similar to the pace at which they would typically walk down the sidewalk or down a hallway. Participants then walked on a treadmill for five minutes at their self-selected preferred walking speeds (mean number of strides: ET 235 ± 33.2, HOA 257 ± 24.3). After a short rest, HOA performed an additional minute of treadmill walking at a speed matched to a corresponding age-matched ET participant in order to examine any effect of walking speed on gait variability between the groups. The participants with ET did not walk at speeds matched to the HOA as many of the ET participants could not comfortably walk at these faster speeds. Kinematic data were collected throughout the duration of the five-minute treadmill walking trial using a 10-camera motion capture system (120 Hz; Vicon Nexus, Vicon, Oxford, UK). Heel-strikes and toe-offs were manually labeled based on marker trajectory profiles.
Modified stride length was calculated for the treadmill as the distance traveled by the ankle marker along the antero-posterior walking axis from heel-strike to toe-off (Reisman et al., 2005). Stride time was calculated as the time between two consecutive heel-strikes of the same limb. Step length was calculated as the distance between the ankle markers along the antero-posterior walking axis at heel-strike. Step time was calculated as the time between contralateral heel-strikes. Step width was calculated as the distance between the ankle markers along the medio-lateral axis at heel-strike. Mean and coefficient of variation (CV = standard deviation/mean * 100%) were calculated over all strides across the entire five-minute treadmill walking trial for all five spatiotemporal gait parameters.
Detrended fluctuation analysis
We then applied a detrended fluctuation analysis (DFA) technique to analyze the structure of the variability across all strides within the five-minute treadmill trial. DFA techniques have been previously applied to physiological signals (Peng et al., 1993) including stride-to-stride gait variability (Hausdorff et al., 1995). First, the spatiotemporal gait data is listed as a time-series. These time-series signals are then integrated and partitioned into data boxes with length ranging from 4 data points per box to N/4 points per box, where N is the total number of spatiotemporal gait data points collected over the five-minute treadmill trial. A linear least-squares line is fit to the data within each box of 4 to N/4 points and the average fluctuation of the physiological gait data around the least-squares line is calculated for each individual data box. The logs of the average fluctuation values for all data boxes are then plotted against the logs of the individual data box sizes. The ultimate output of the DFA is the slope of the linear least-squares line fit to this log-log plot, denoted by α. An α=0.5 indicates random white noise while α>0.5 but ≤ 1.0 indicates the presence of long-range correlations within the time-series.
Statistical analysis
We performed independent samples t-tests to compare the stride length, stride time, step length, step time, and step width mean, CV, and α values between HOA and ET during treadmill walking at preferred walking speeds. We performed additional independent samples t-tests to compare the stride length, stride time, step length, step time, and step width means and CVs between HOA and ET at matched walking speeds in order to analyze the effect of walking speed on the mean gait parameters and the magnitude of gait variability observed in HOA and ET. Multivariate hierarchical regression models with age and preferred walking speed included in the first block and stepwise regression with clinical measures of tremor severity on TRS items in the second block were applied to assess TRS items as predictors of stride length, stride time, step length, step time, and step width CVs in the 31 participants with ET. The level of significance was set at p<0.05 and all statistical analyses were performed using IBM SPSS Statistics 20 (IBM Corporation, Armonk, NY).
Results
Spatiotemporal gait parameters and variability during treadmill walking at a preferred speed
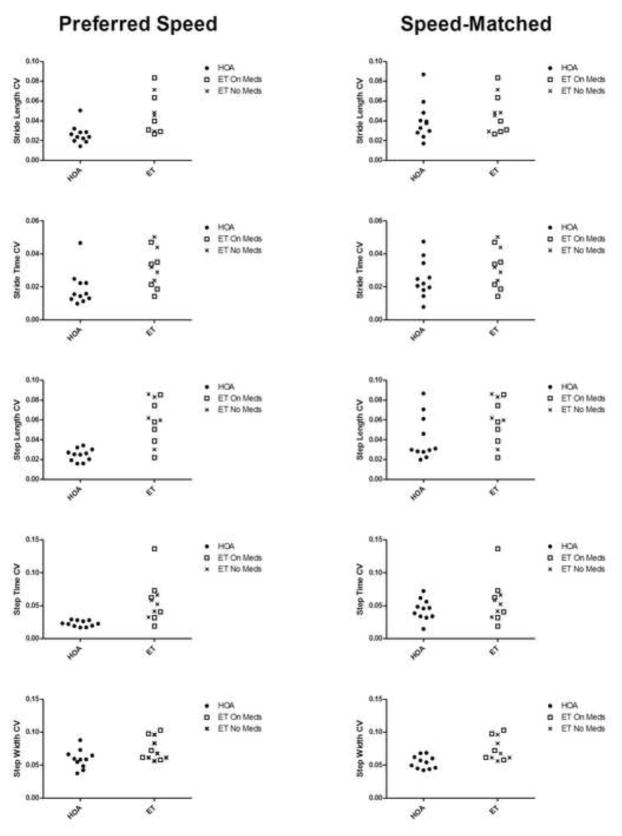
During the five minutes of treadmill walking at the participants’ preferred walking speeds, persons with ET exhibited significantly slower preferred walking speeds and shorter stride lengths, stride times, step lengths, and step times when compared to HOA (all p<0.05, Table 1). Persons with ET also demonstrated a higher magnitude of gait variability as stride length, stride time, step length, step time, and step width CVs were significantly larger in ET than HOA (all p<0.05, Figure 1). Medication did not appear to affect these parameters (Figure 2). However, the structure of the variability (indicated by the α value) was not different between groups in any of the spatiotemporal gait parameters (Figure 1).
Table 1. Spatiotemporal gait characteristics of healthy older adults (HOA) and persons with essential tremor (ET) (mean ± SD).
Mean ± standard deviation (SD) spatiotemporal gait characteristics for persons with essential tremor (ET) and age-matched healthy older adults (HOA). We did not observe differences between HOA and ET in the means of any spatiotemporal gait parameters during speed-matched treadmill walking.
| Preferred |
|
||
|---|---|---|---|
| HOA (n=11) | ET (n=11) | ||
| Walking speed (m/s) | 1.15±0.12 | 0.69±0.23 | p<0.001 |
| Stride length (m) | 0.64±0.06 | 0.45±0.08 | p<0.001 |
| Stride time (s) | 1.16±0.12 | 1.34±0.21 | p=0.027 |
| Step length (m) | 0.61±0.05 | 0.42±0.09 | p<0.001 |
| Step time (s) | 0.58±0.06 | 0.67±0.10 | p=0.027 |
| Step width (m) | 0.21±0.02 | 0.22±0.02 | p=0.397 |
|
Speed-Matched
| |||
| Walking speed (m/s) | 0.69±0.23 | 0.69±0.23 | p=0.963 |
| Stride length (m) | 0.51±0.08 | 0.45±0.08 | p=0.138 |
| Stride time (s) | 1.53±0.32 | 1.34±0.21 | p=0.108 |
| Step length (m) | 0.46±0.08 | 0.42±0.09 | p=0.259 |
| Step time (s) | 0.77±0.16 | 0.67±0.10 | p=0.107 |
| Step width (m) | 0.23±0.03 | 0.22±0.02 | p=0.232 |
Figure 1.
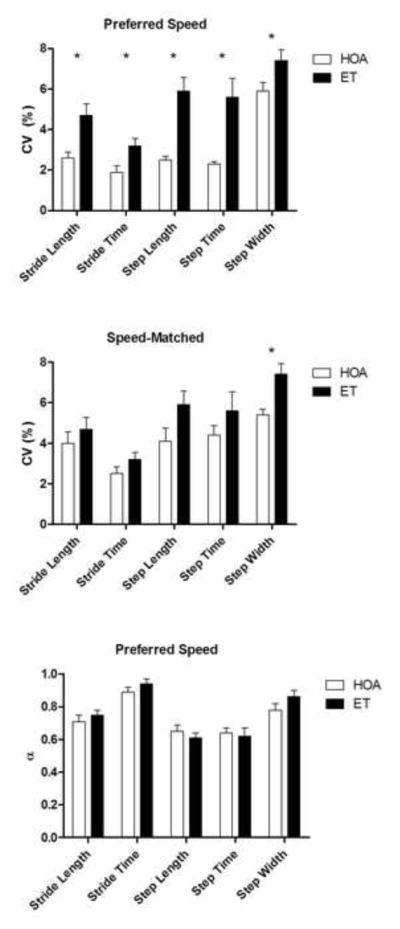
Coefficients of variation (CV) of stride length, stride time, step length, step time, and step with during preferred speed treadmill walking (top) and speed-matched treadmill walking (middle) in persons with essential tremor (ET) and age-matched healthy older adults (HOA). The α values resulting from the detrended fluctuation analyses for these same gait parameters during preferred speed treadmill walking is shown on bottom for both groups. * indicates p<0.05.
Figure 2.
Coefficients of variation (CV) of stride length, stride time, step length, step time, and step width during preferred speed treadmill walking (left) and speed-matched treadmill walking (right) for healthy older adults (HOA) as well as persons with essential tremor taking medication for tremor (ET On Meds) and those not taking medication for tremor (ET No Meds).
Spatiotemporal gait parameters and variability during speed-matched treadmill walking
During speed-matched treadmill walking, we did not observe any differences in mean stride length, stride time, step length, or step time between ET and HOA (all p>0.05, Table 1). However, we still observed a significantly higher step width CV (p=0.004) and a trend suggesting a higher step length CV (p=0.073) in ET compared to HOA. We did not observe any significant between-group differences in stride length, stride time, or step time CVs during speed-matched treadmill walking (Figure 1).
TRS scores as predictors of gait variability magnitude in ET
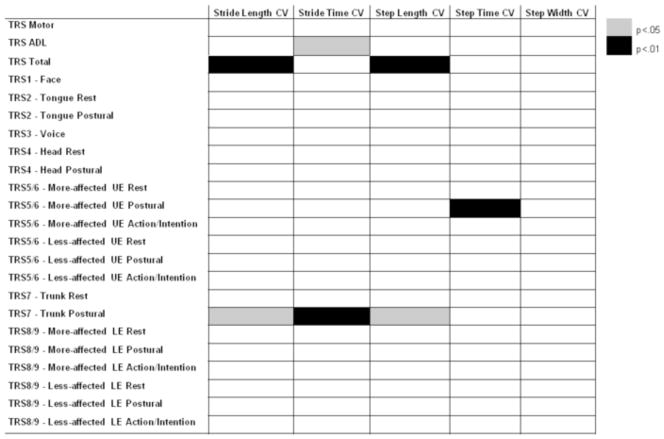
After controlling for age and preferred walking speed, TRS total score was a significant predictor of stride length and step length CVs (both p<0.01; Figure 3). TRS ADL score was a significant predictor of stride time CV (p<0.05; Figure 3). Among TRS items for midline tremors, postural trunk tremor severity was a significant predictor of stride length, stride time, and step length CVs (all p<0.05; Figure 3). Among TRS items for upper extremity tremor, postural tremor severity in the more-affected arm was a significant predictor of step time CV. Severity of lower extremity tremors did not significantly predict any measures of gait variability magnitude. Further, no items on the TRS were significant predictors of step width CV.
Figure 3.
Clinical measures of tremor severity as predictors of coefficients of variation (CV) of stride length, stride time, step length, step time, and step width after controlling for age and preferred walking speed in persons with essential tremor. Gray boxes indicate a beta value of p<0.05 while black boxes indicate a beta value of p<0.01. ADL – activities of daily living, UE – upper extremity, LE – lower extremity.
Discussion
The magnitude of gait variability was increased in persons with ET when compared to HOAs. Further, step width variability was higher in ET relative to controls even during speed-matched treadmill walking. This is an important finding as step width variability has previously been shown to be related to balance impairment (Brach et al., 2008). The presence of long-range correlations in relevant gait parameters was intact in persons with ET, as the structure of gait variability in this population was not different from HOAs despite the slower preferred walking speeds. Thus, the results of this study indicate that persons with ET walk with increased variability in several spatiotemporal gait parameters. This likely results, at least in part, from a slowness of preferred walking speed. Hence, interventions designed to improve walking speed may potentially improve global mobility and reduce gait variability in this population.
It is important to note that several measures of the magnitude of gait variability during preferred speed treadmill trials were predicted by clinical scores of midline and upper extremity tremor severity. Considering the established inverse relationship between gait velocity and gait variability (with the exception of step width variability, which is positively associated with gait speed) (Brach et al., 2001; Beauchet et al., 2009), it was necessary to control for gait velocity as well as age in our analyses. Specifically, we observed that postural tremor severity scores in the trunk were significant predictors of stride length, stride time, and step length variability in ET. Associations between gait dysfunction and midline tremor severity have also been previously noted in multiple studies of persons with ET (Hoskovcová et al., 2013; Louis et al., 2010; Louis et al., 2012; Parisi et al., 2006). In a previous study by Louis and colleagues (2010), the authors suggested that the association between gait dysfunction and midline tremors was likely due to shared pathophysiology between the two rather than mechanical factors. That is, the authors hypothesized that ET may consist of a midline cerebellar syndrome affecting gait and midline tremor and a cerebellar extremity syndrome affecting peripheral tremor (Louis et al., 2010). Indeed, our results further the idea that midline tremor severity is associated with gait dysfunction in persons with ET and thus may share similar pathophysiology (Louis et al., 2010). However, it is also interesting that upper extremity tremor severity appears to influence step time variability and this relationship should be further explored.
Though the magnitude of gait variability is higher in persons with ET, the structure of the variability (in terms of the presence of long-range correlations between gait cycles over time) remains unaffected. Though previous research on peripheral neuropathy suggested that long-range correlations in gait may be controlled by the central nervous system (Gates and Dingwell, 2007), our findings are in line with previous research suggesting that the structure of gait variability is relatively normal at preferred walking speeds in persons with cerebellar disorders (Wuehr et al., 2013). When coupled with findings in cerebellar patients (Wuehr et al., 2013), our results pose interesting questions about the role of the central nervous system in the regulation of long-range correlations during gait at preferred walking speeds. Specifically, while it appears that the cerebellum is likely not involved in this process, what are the roles of the spinal cord and other higher structures? Moreover, research into the structure of gait variability in persons with ET is needed since manipulation of gait speed can affect these parameters. For example, when studied across a broad spectrum of walking speeds, persons with cerebellar ataxia displayed the strongest long-range correlations at their preferred walking speeds while controls exhibited the weakest long-range correlations (Wuehr et al., 2013). Therefore, a similar study of persons with ET may provide further insight into the gait disturbances within this population.
By analyzing both the magnitude and the structure of gait variability, we were able to provide insight into multiple facets of locomotor control in persons with ET. First, the observed differences between HOA and ET in gait variability magnitude indicate that gait patterns exhibit greater stride-to-stride inconsistency in ET. These findings are in support of the growing body of literature describing gait ataxia within this population (Kronenbuerger et al., 2009; Louis et al., 2010; Rao et al., 2011; Stolze et al., 2001). Second, we observed that the structure of gait variability in ET is similar to HOA; thus, the complexity of gait variability appears to remain intact within this population as ET does not appear to impose additional constraints onto the regulation of gait variability patterns beyond those observed in normal aging. In summary, as the presence of long-range correlations is intact in ET during preferred-speed walking, stride characteristics remain dependent on previous strides in a fractal-like fashion to relatively the same degree as exists in HOA; however, ET does affect the magnitude of gait variability.
The present study is not without limitations. Our participants were asked to undergo the testing sessions in their typical everyday state, which in some cases meant the participant was taking medication for tremor. Therefore, some participants with ET were medicated while others were not. The analysis displayed in Figure 2 indicates that medication was not a driving factor influencing any of the variability measures. In addition, previous research has suggested that anti-tremor medications typically taken by persons with ET (propranolol and primidone, which were the only two medications taken by any participant in this study) have a minimal effect on gait (Stolze et al., 2001). As previously mentioned, our detrended fluctuation analyses were restricted to investigation of preferred walking speeds. When HOAs walked at slower speeds matched to those preferred by persons with ET, most measures of the magnitude of gait variability in ET were similar to HOA (with the exception of step width variability). Investigation of the effects of speed manipulation on the structure and magnitude of gait variability in persons with ET is warranted and may provide insight into the gait deficits within this population.
Conclusion
The magnitude but not structure of gait variability is affected in ET. Persons with ET walk at preferred speeds with increased spatiotemporal variability as compared to HOA. When HOA are slowed to match the preferred speeds of persons with ET, persons with ET still exhibit significant increases in step width variability which was not predicted by any of the clinical tremor severity measures. In persons with ET, clinical measures of global tremor severity were found to predict magnitudes of the variability in stride length, stride time, and step length. More specifically, postural trunk tremor severity was a significant predictor of stride length, stride time, and step length variability while postural tremor of the more-affected upper extremity was a significant predictor of step time variability. Our results further the ideas that gait is disrupted in persons with ET and that these locomotor difficulties may arise from a midline cerebellar disorder.
Acknowledgments
This work was supported in part by NIH grants R03HD054594, 1R21AG033284-01A2, and the UF National Parkinson’s Foundation Center of Excellence.
Footnotes
Conflict of interest statement
All authors report they have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) the work of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beauchet O, Annweiler C, Lecordroch Y, Allali G, Dubost V, Herrmann FR, et al. Walking speed-related changes in stride time variability: effects of decreased speed. Journal of Neuroengineering and Rehabilitation. 2009;6:32. doi: 10.1186/1743-0003-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB. Gait variability in community-dwelling older adults. Journal of the American Geriatrics Society. 2001;49(12):1646–50. doi: 10.1046/j.1532-5415.2001.t01-1-49274.x. [DOI] [PubMed] [Google Scholar]
- Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. Journal of Neuroengineering and Rehabilitation. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait & Posture. 2008;27(3):431–9. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age and Ageing. 2011;40(4):481–7. doi: 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- Chandran V, Pal PK. Quality of life and its determinants in essential tremor. Parkinsonism and Related Disorders. 2013;19(1):62–5. doi: 10.1016/j.parkreldis.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Fasano A, Herzog J, Raethjen J, Rose FE, Muthuraman M, Volkmann J, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain. 2010;133(Pt 12):3635–48. doi: 10.1093/brain/awq267. [DOI] [PubMed] [Google Scholar]
- Gates DH, Dingwell JB. Peripheral neuropathy does not alter the fractal dynamics of stride intervals of gait. Journal of Applied Physiology. 2007;102(3):965–71. doi: 10.1152/japplphysiol.00413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. Journal of Applied Physiology. 1995;78(1):349–58. doi: 10.1152/jappl.1995.78.1.349. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, et al. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. Journal of Applied Physiology. 1997;82(1):262–9. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hoskovcová M, Ulmanová O, Sprdlík O, Sieger T, Nováková J, Jech R, et al. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum. 2013;12(1):27–34. doi: 10.1007/s12311-012-0384-4. [DOI] [PubMed] [Google Scholar]
- Kaipust JP, Huisinga JM, Filipi M, Stergiou N. Gait variability measures reveal differences between multiple sclerosis patients and healthy controls. Motor Control. 2012;16(2):229–44. doi: 10.1123/mcj.16.2.229. [DOI] [PubMed] [Google Scholar]
- Kronenbuerger M, Konczak J, Ziegler W, Buderath P, Frank B, Coenen VA, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8(3):389–98. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ford B, Lee H, Andrews H, Cameron G. Diagnostic criteria for essential tremor: a population perspective. Archives of Neurology. 1998;55(6):823–8. doi: 10.1001/archneur.55.6.823. [DOI] [PubMed] [Google Scholar]
- Louis ED. Behavioral symptoms associated with essential tremor. Advances in Neurology. 2005;96:284–90. [PubMed] [Google Scholar]
- Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Movement Disorders. 2010;25(11):1633–8. doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders. 2010;25(5):534–41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: balance confidence, near misses and falls. Gait & Posture. 2012;35(1):43–7. doi: 10.1016/j.gaitpost.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi SL, Héroux ME, Culham EG, Norman KE. Functional mobility and postural control in essential tremor. Archives of Physical Medicine and Rehabilitation. 2006;87(10):1357–64. doi: 10.1016/j.apmr.2006.07.255. [DOI] [PubMed] [Google Scholar]
- Peng CK, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Physical Review Letters. 1993;70(9):1343–6. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait & Posture. 2011;34(1):65–70. doi: 10.1016/j.gaitpost.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? Journal of Neurophysiology. 2005;94(4):2403–15. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Serrao M, Pierelli F, Ranavolo A, Draicchio F, Conte C, Don R, et al. Gait pattern in inherited cerebellar ataxias. Cerebellum. 2012;11(1):194–211. doi: 10.1007/s12311-011-0296-8. [DOI] [PubMed] [Google Scholar]
- Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J TRS Study Group. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Movement Disorders. 2007;22(6):833–8. doi: 10.1002/mds.21412. [DOI] [PubMed] [Google Scholar]
- Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124(Pt 11):2278–86. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- Toebes MJ, Hoozemans MJ, Furrer R, Dekker J, van Dieën JH. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait & Posture. 2012;36(3):527–31. doi: 10.1016/j.gaitpost.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Wuehr M, Schniepp R, Ilmberger J, Brandt T, Jahn K. Speed-dependent temporospatial gait variability and long-range correlations in cerebellar ataxia. Gait & Posture. 2013;37(2):214–8. doi: 10.1016/j.gaitpost.2012.07.003. [DOI] [PubMed] [Google Scholar]