Abstract
Malaria is one of the worldwide parasitic diseases which threaten the life of hundreds of millions of people at the malarious areas each year. The emergence of chloroquine-resistant strains of Plasmodium falciparum in most of the malarious areas has encountered the relevant countries with some difficulties about treating the acute cases of the disease particulary if the monotherapy regimen has been used. Because of many advantages for the combination therapy, the effectiveness of chloroquine (CQ) and Otostegia persica (OP), a medicinal plant in combination form, was tested against the chloroquine-sensitive and chloroquine-resistant strains of Plasmodium berghei in sourian mouse using in-vivo adapted fixed ratios method in this study.
At the first step, ED50s (50% effective dose) of chloroquine and O. persica against both CQ-sensitive and CQ-resistant strains of P. berghei were calculated using in-vivo test in the mice. Ratios of 0, 10, 30, 50, 70, 90 and100% from each ED50 were prepared and contrarily combined together to make the following fixed ratios of 0/100, 10/90, 30/70, 50/50, 70/30, 90/10, and 100/0 of CQ/OP and the parasites were exposed to the combined ratios. Determination of ED50s showed 1.1 mg/Kg and 2.4 mg/Kg of mouse body weight for chloroquine in CQ-sensitive and CQ-resistant strains respectively and 450 mg/Kg for O. persica in both strains. The results also showed that the combinations of “50% CQ + 50% OP”, “30% CQ + 70% O.P” and “70% CQ + 30% OP” were more effective than other combinations against CQ-sensitive strain. The fixed ratio combinations of chloroquine and O. persica showed an additive in CQ-resistant strain. Toxicity consideration showed no toxic effect of the combinations on the mice. Otostegia persica potentiated the effectiveness of chloroquine against the chloroquine-sensitive strain of P. berghei but not on chloroquine-resistant P. berghei. Moreover, the greatly modified fixed ratios method in this study can be considered as useful methods for in-vivo combination tests in murine malaria parasites.
Key Words: Otostegia persica, Plasmodium berghei, Drug combination, Fixed ratios, Chloroquine
Introduction
Malaria, the most important parasitic life threating disease in the malaria-affected areas, is a hematoprotozoan infection caused by four species of plasmodium. Although malaria is cured by some effective antimalarial drugs, at present, the medicinal treatment of the infection has encountered with an immense obstacle due to the emergence of drug resistance phenomenon in some species of plasmodium. Plasmodium falciparum is highly resistant to chloroquine (CQ) in Iran and many of the malarious countries and more or less to a number of other antimalarials in some malaria endemic areas (1-3). Moreover, some reports indicate the emergence of chloroquine-resistant strains of Plasmodium vivax in a number of malarious countries (4-7). In view of the increasing incidence of Plasmodium falciparum and Plasmodium vivax drug-resistant strains besides preparing new antimalarials, effective approaches are also needed that might delay the emergence of the resistance. Combination therapy is a highly recommended pathway for delaying the emergence of drug resistance or potentiating the effectiveness of antimalarials against the resistant strains in Plasmodia (8). Such demand is more emphasized when the combination takes place between medicinal herbal extracts and synthetic antimalarials. Otostegia persica (OP) is an endemic medicinal plant growing in south and southeast parts of Iran. The equeous extract of the plant is traditionally used as antispasmodic, anti-arthritis and antipyretic (9) through the local habitants.
Otostegia persica has a modest action against the sexual forms of CQ-sensitive Plasmodium berghei and is non-toxic to the mouse at antiparasitic doses (10). Despite many uses of O. persica, little data can be found about the biological activity of the plant. Producing an experimental CQ-resistant strain of Plasmodium berghei derived from CQ-sensitive parasite in our malaria laboratory (11), excited us to consider the effect of Otostegia persica in combination with chloroquine against the CQ-resistant and CQ-sensitive strains of Plasmodium berghei in sourian mice using fixed ratio method as a first study among its category.
Experimental
Experiments were conducted on sourian male mice weighing between 20 and 25 g. Animals were kept in plastic and comfortable cages under a normal light-dark cycle, fed on mice pellets and received tap water.
Parasites
Chloroquine-sensitive NICD strain of Plasmodium berghei stored in liquid nitrogen (originally from Haffkine Institue, India) was maintained in mice through a syringe passage, a couple of weeks before the experiments.
Drugs
Otostegia persica (Labiatea) plant was collected from Hormozgan province, a malarious area in southeast of Iran, and the aerial parts of the plant were powdered for the extraction. Powdered parts were extracted with 96˚ ethanol and the alcohol was evaporated under the pressure of vacuum. The extract was made up as a stabilized suspension to produce concentrations of 100, 200, 350 and 450 mg/Kg. For achieving the stabilized suspension, the extract was dissolved in 2.5% Tween 80 (diluted in normal saline) under the sonicating situation. Chloroquine diphosphate (sigma chemical Co.) was dissolved in distilled water to make up 1, 3, 10, 20 and 30 mg/Kg concentrations.
Methods
The cryopreserved parasites were thawed and suspended in physiological saline to be made up a volume of 0.2 mL suspension. A number of mice were infected via the intraperitoneal route and allowed parasitemia to develop up to approximately 10% (by day 4 after the infection). The infected blood was then collected into the heparinized tubes directly from heart via the cardiac puncture under the ether anesthesia. The blood was diluted in physiological saline at the ratio of 106 parasitized erythrocytes in 0.2 mL of the dilution. The prepared suspension was injected subcutaneously in mice. To determine the drug doses for producing 50% suppression of parasitemia for chloroquine and O. persica, a 4-day suppressive test was conducted (12). For each previously mentioned concentrations of chloroquine and O. persica, five mice were appropriated. P. berghei-infected erythrocytes were injected on day 0 and the drugs were given during the days 0 to 3. The first dose was administered 2 h after the injection of the infected erythrocytes on day 0. On the 4th day, the parasitemia of the mice was estimated using Giemsa-stained thin tail-blood smears. For each compound, ED50 was calculated from the graphs drawn on semi-log papers against both chloroquine-sensitive and chloroquine-resistant P. berghei. Five uninfected and five untreated mice were allocated for each line of the tests as the control groups. The in-vitro fixed ratios technique of determining the influence of one drug on the activity of another based on the predetermined inhibitory concentration 50% (IC50) values as described by Chawira and Warhurst (13) with considerable modifications, was adapted for in-vivo tests in this study as followes. Aliquots of 0.2 mL containing fifty percent of effective dose (ED50) concentrations of chloroquine and O. persica alone (in the ratios of 90%:10%, 70%:30%, 50%:50%, 30%:70% and 10%:90% respectively) of the first and second drug solutions were injected subcutaneously into the relevant groups of mice. The number of mice in each group and control sets were similar to that described for ED50s determination. Percentage inhibition values and standard deviation (SD) for each ratio were calculated. The results of each combination were plotted on a figure containing two vertical axes for percentage inhibition values of the ED50 concentrations of the individual drugs and a horizontal axis for fixed ratios of combined concentrations. The points of ED50 values on the vertical axes were joined through a straight line. The influence of O. persica on the effectiveness of chloroquine against the parasites was shown as a point according to the relevant fixed ratios. When the points take place above the line, it is considered as the potentiation of the activity between O. persica and chloroquine against the parasites. When they fall below the line, it is assumed as an antagonistic activity. Points lying on the line indicate an additive effect. The treated mice were reassessed through the thin smears on days 7, 14 and 21 to be noted weather the recrudescence had been occurred. Toxicity of the combination at the combined ratios was assessed against the mice using 3 mice for each fixed ratio of the combination that received a subcutaneous injection daily for 14 days. Subsequently, at the end of the injection period, clinical situations of the mice were followed up until day 50.
Results and Discussion
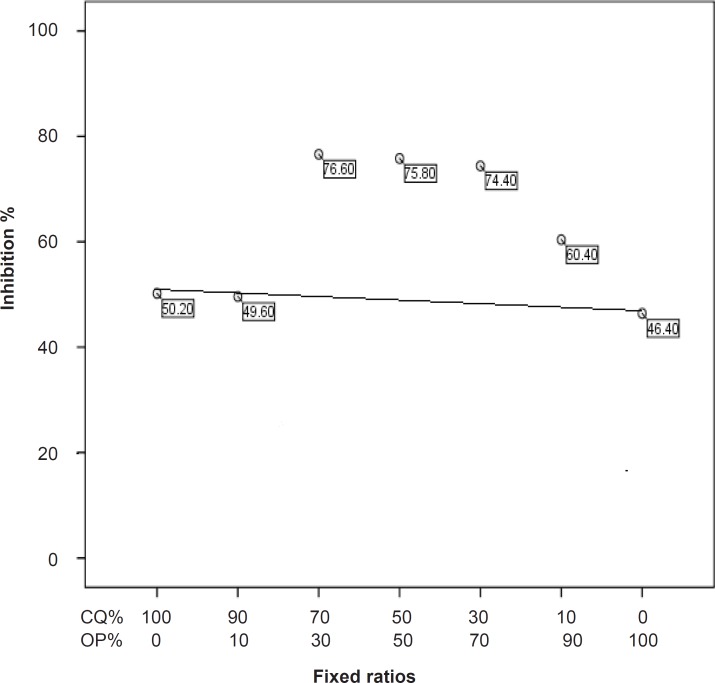
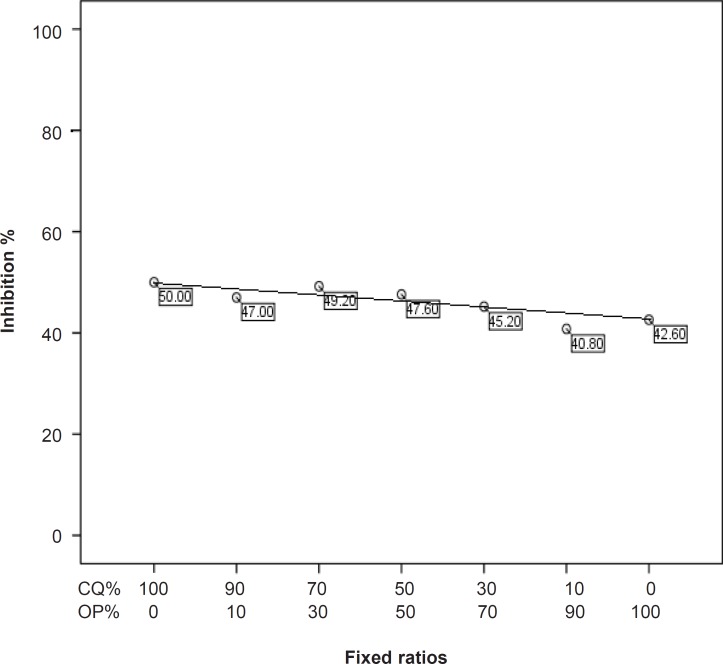
Fifty percent of effective dose values of chloroquine and O. persica tested against chloroquine-sensitive and chloroquine-resistant strains of P. berghei have been tabulated in Table 1. The results of the interaction between chloroquine and O. persica on chloroquine-sensitive and chloroquine-resistant strains of P. berghei are graphically shown in Figures 1 and 2 and given in Tables 2 and 3. The combination between chloroquine and O. persica demonstrated marked potentiating effects on chloroquine-sensitive strain (NICD strain ) in ratios of 70% CQ + 30% OP, 50% CQ + 50% OP and 30% CQ + 70% OP but not in other ratios (Table 2). Additive effects were seen in combination between chloroquine and O. persica against the chloroquine-resistant strain (TUMS/PB/R strain) in 90% CQ + 10% OP, 70% CQ + 30% OP, 50% CQ + 50% OP and 30% CQ + 70% OP ratios, but an antagonism was detected in the ratio of 10% CQ + 90% OP (Table 3). The results showed that the average survival time of the five mice (for each concentration) infected with chloroquine-sensitive parasites after treating with combined doses of 70% CQ + 30% OP, 50% CQ + 50% OP and 30% CQ + 70% OP ratios with 20.4, 21.2 and 21 days respectively, was longer than those treated mice infected with chloroquine-resistant strain (p < 0.05). The result obtained from the toxicity considerations did not reveal any clinical deleterious manifestation until fifty days follow up. Otostegia persica, originally an Iranian plant, is usually employed as an antipyretic traditional remedy in malarious south and southeastern areas of Iran. Little data can be found about O. persica in the literatures. Some pharmacokinetics, antibacterial , antiparasitic and antiplasmodial descriptions were reported by a number of authors (10, 14- 16). Combination therapy in malaria is considerably recommended both in drug-sensitive and drug-resistant infections particularly in falciparum malaria (8, 17). The interaction between chloroquine and O. persica against the two strains of P. berghei, the murine malaria parasites, was considered in this study. Plasmodium berghei is an acceptable animal model for the chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum malaria. In fact, although the combination therapy could not interrupt the establishment of drug-resistance in malaria parasites, it could considerably delay the extension of phenomenon (17). Our findings showed that O. persica potentiated the effect of chloroquine on the chloroquine-sensitive P. berghei whilst an additive effect was observed against the chloroquine-resistant strain of the parasite. The interaction between chloroquine and O. persica in the ratio of 30% CQ + 70% OP implied that even lower amount of chloroquine can potentiate the effect of O. persica on chloroquine-sensitive P. berghei. This may be interpreted that such native herb with low side-effects can fill more portion than chemical compound in combined drugs. Potentiating the effect of chloroquine through O. persica in combination form against the chloroquine-sensitive P. berghei implies that the drugs may share the similar mechanisms of the action on parasites. On the other hand, some authors obtained additive results between chloroquine and artemisinin, originally a Chinese traditional plant, because of their different modes of action on chloroquine-sensitive P. berghei and P. falciparum strains (17-19). The additive result of the chloroquine and O. persica combination on the chloroquine-resistant P. berghei obtained in this study confirms the mentioned hypothesis. In other words, the establishment of resistance to chloroquine in P. berghei aborts effective interaction of the plant with chloroquine on the parasite.
Table 1.
ED50 concentration of chloroquine and O.persica against the chloroquine-sensitive and chloroquine-resistant strains of P.berghei.
| Parasites |
Drugs
|
|||
|---|---|---|---|---|
|
Chloroquine
|
O.persica
|
|||
| MeanED50 (mg/Kg) |
Inhibition% (± SD) |
MeanED50 (mg/Kg) |
Inhibition% (± SD) |
|
| Chloroquine | 1.1 | 50.2 | 450 | 46.2 |
| -sensitive P.berghei | (± 5.3) | (± 2.4) | ||
| Chloroquine | 24 | 50 | 450 | 42.6 |
| -resistant P.berghei | (± 4.7) | (± 3.7) | ||
Figure 1.
Interaction between chloroquine and O.persica on the chloroquine-sensitive strain of P.berghei.
Figure 2.
Interaction between chloroquine and O.persica on the chloroquine-resistant strain of P.berghei
Table 2.
Fixed ratios of chloroquine and O.persica combination and their relevant effective doses on the chloroquine-sensitive strain of P.berghei.
| Groups | Fixed ratios | Mean effective doses ( X ± SD) |
|---|---|---|
| 1 | 100% CQ | 50.2 ± 5.3 |
| 2 | 90% CQ + 1O% OP | 49.6 ± 3.5 |
| 3 | 70% CQ + 30% OP | 76.6 ± 4.6 |
| 4 | 50% CQ + 50% OP | 75.8 ± 5.9 |
| 5 | 30% CQ + 70% OP | 74.4 ± 3.7 |
| 6 | 10% CQ + 90%OP | 60.4 ± 2.8 |
| 7 | 100% OP | 46.4 ± 9.1 |
Table 3.
Fixed ratios of chloroquine and O.persica combination and their relevant effective doses on chloroquine-resistant strain of P.berghei.
| Groups | Fixed ratios | Mean effective doses ( X ± SD) |
|---|---|---|
| 1 | 100% CQ | 50 ± 4.7 |
| 2 | 90% CQ + 1O% OP | 47 ± 4.7 |
| 3 | 70% CQ + 30% OP | 49.2 ± 8.7 |
| 4 | 50% CQ + 50% OP | 47.6 ± 6.8 |
| 5 | 30% CQ + 70% OP | 45.2 ± 6.7 |
| 6 | 10% CQ + 90%OP | 40.8 ± 8 |
| 7 | 100% OP | 42.6 ± 3.7 |
Otostegia persica potentiated the effectiveness of chloroquine against the chloroquine-sensitive strain of P. berghei but not on chloroquine-resistant P. berghei. Moreover, the greatly modified fixed ratios method in this study can be considered as a useful method for in-vivo combination tests in murine malaria parasites.
Acknowledgment
The authors would like to express their gratitude to Dr.S Rezaei, Miss N Ghobakhloo, Mrs S Charedar and Mr R Eskandary for their useful collaboration. This study received full financial support from the School of Public Health, Tehran University of medical sciences.
References
- 1.Edrissian G, Nateghpour M, Afshar A, Mohseni G. In-vivo monitoring of the response of falciparum and vivax Plasmodia to chloroquine in Bandar-Abbas , Kahnoudj, South-East Iran. Med. J. Iran Hosp. 2001;3:30–33. [Google Scholar]
- 2.Raeisi A, Ringwald P, Safa O, Shahbazi A, Ranjbar M, Keshavarz H, Nateghpour M, Faraji L. Monitoring of the therapeutic efficacy of chloroquine for the treatment of uncomplicated, Plasmodium falciparum malaria in Iran. Ann. Trop. Med. Parasitol. 2006;100:11–16. doi: 10.1179/136485906X86220. [DOI] [PubMed] [Google Scholar]
- 3.White NJ. Antimalarial drug resistance. J. Clin. Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird JK, Basri H, Purnomo , Bangs MJ, Subianto B, Patchen LC, Hoffman SL. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 1991;44:547–552. doi: 10.4269/ajtmh.1991.44.547. [DOI] [PubMed] [Google Scholar]
- 5.Canessa A, Mazzarello G, Cruciani M, Bassetti D. Chloroquine-resistant Plasmodium vivax in Brazil. Trans R. Soc. Trop. Med. Hyg. 1992;86:570–571. doi: 10.1016/0035-9203(92)90120-2. [DOI] [PubMed] [Google Scholar]
- 6.Marlar T, Myat PhoneK, Aye YuS, Khaing KhaingG, Ma S, Myint O. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R. Soc. Trop. Med. Hyg. 1995;89:307–308. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 7.Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am. J. Trop. Med. Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Assessment of Therapeutic Efficacy of Antimalarial Drugs for Uncomplicated Falciparum Malaria. Working draft, Version 5. Geneva: The Organization; 2002. [Google Scholar]
- 9.Ghahraman A. Color Atlas of Iranian Flora. Vol. 9. Research Institute of forests and Rangelands Publishing; 1996. p. 3071. [Google Scholar]
- 10.Nateghpour M, Miahipour A, Edrissian G, Souri E, Motevalli HaghiA. Effectiveness of ethanolic extract of Otostegia persica against Plasmodium berghei in comparison with chloroquine in white mice using in-vivo test. J. School Public Health and Inst. Public Health Res. 2008;6:57–62. [Google Scholar]
- 11.Ghobakhloo N, Nateghpour M, Rezaee S, Hajjaran H, Mohebali M, Abedkhojasteh H. Variation of the chloroquine resistance transporter (Crt) gene in chloroquine-resistant and chloroquine-sensitive Plasmodium berghei. Iranian J. Parasitol. 2008;3:39–44. [Google Scholar]
- 12.Peters W. Chemotherapy and Drug Resistance in Malaria. London: Academic Press; 1970. p. 876. [Google Scholar]
- 13.Chawira AN, Warhurst DC, Robinson BL, Peters W. The effect of combination of quinghaosu (artemisinin) with standard antimalarial drugs in the suppressive treatment of malaria in mice. J. Trans R. Soc. Trop. Med. Hyg. 1987;81:554–558. doi: 10.1016/0035-9203(87)90404-4. [DOI] [PubMed] [Google Scholar]
- 14.Shariffar F, Yassa N, Shafiei A. Antioxidant activity of Otostegia persica (Labiatae) and its constituents. Iranian J. Pharm. Res. 2003;2:235–239. [Google Scholar]
- 15.Asghari G, Nourallahi H, Havaie SA, Issa L. Antimicrobial activity of Otostegia persica Bioss. extracts. Res. Pharm. Sci. 2006;1:53–58. [Google Scholar]
- 16.Esmaeili S, Naghibi F, Mosaddegh M, Sahranavard Sh, Ghafari S, Abdullah NR. Screening of antiplasmodial properties among some traditionally used Iranian plants. J. Ethnopharmacol. 2009;121:400–04. doi: 10.1016/j.jep.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Wernsdorfer WH. Drug resistant malaria. Endeavour. 1984;8:166–171. doi: 10.1016/0160-9327(84)90080-2. [DOI] [PubMed] [Google Scholar]
- 18.McChesney EW, Fitch CD. 4-Aminoquinolines. In: Peters ED, Richrds W, editors. Antimalarial Drugs. Spring-Verlag: Berlin ; 1984. pp. 3–60. [Google Scholar]
- 19.Chawira AN, Warhurst DC. The effect of artemisinin combined with standard chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in-vitro. J. Trop. Med. Hyg. 1987;90:1–8. [PubMed] [Google Scholar]