Abstract
Metastasis begins with the escape, or dissemination, of cancer cells from the primary tumor. We recently demonstrated that tumors preferentially disseminate into collagen I and not into basement membrane protein gels (Matrigel). In this study, we used synthetic polymer systems to define material properties that could induce dissemination into Matrigel. We first specifically varied rigidity by varying the crosslinking density of poly(ethylene glycol) (PEG) networks within Matrigel scaffolds. Increased microenvironmental rigidity limited epithelial growth but did not promote dissemination. We next incorporated adhesive signals into the PEG network using peptide-conjugated cyclodextrin (α-CDYRGDS) rings. The α-CDYRGDS rings threaded along the PEG polymers, enabling independent control of matrix mechanics, adhesive peptide composition, and adhesive density. Adhesive PEG networks induced dissemination of normal and malignant mammary epithelial cells at intermediate values of adhesion and rigidity. Our data reveal that microenvironmental signals can induce dissemination of normal and malignant epithelial cells without requiring the fibrillar structure of collagen I or containing collagen I-specific adhesion sequences. Finally, the nanobiomaterials and assays developed in this study are generally useful both in 3D culture of primary mammalian tissues and in the systematic evaluation of the specific role of mechanical and adhesive inputs on 3D tumor growth, invasion, and dissemination.
Keywords: Breast cancer, Invasion, Dissemination, Poly(ethylene) glycol hydrogels, Mechanical properties, Cell adhesion
Introduction
Normal mammary epithelial ducts consist of a bilayered tube with a single layer of luminal epithelial cells surrounded by a basally positioned myoepithelial cell layer and a basement membrane [1]. When epithelial cancer cells escape, or disseminate, past the basement membrane to colonize distant sites in the body, patient prognosis is greatly reduced [2]. Dissemination involves a profound change in the structure and function of the epithelial cancer cell and requires an exchange of specialized cell-cell adhesions for adhesive interactions with the extracellular matrix (ECM) [3–5]. However, the molecular triggers for dissemination remain incompletely understood. During metastatic progression the cancer cells acquire additional genetic mutations and the microenvironment around the tumor changes in parallel [6–10]. Carcinoma cells therefore experience a very different microenvironment than cells in a normal duct and these microenvironmental alterations could play a critical role in regulation of both invasion and dissemination [6, 7, 11, 12]. Our goal in this study was to understand the contribution of specific microenvironmental factors to the disseminative phenotype. Our approach was to isolate primary murine epithelial tissues and present them with microenvironments that differ in features known to vary during malignant progression. Work from multiple laboratories has revealed that the density and alignment of the stromal collagen I changes characteristically during breast cancer progression [7, 13, 14], that a higher density of collagen I at the tumor-stromal interface correlates with invasion [15, 16], and that the alignment of collagen I at the stromal border provides independent prognostic information regarding patient outcomes [17, 18].
We previously used gels composed of natural ECM proteins to expose tumors to a microenvironment with proteins characteristic of the basement membrane (Matrigel) or of the stromal ECM surrounding breast tumors (fibrillar collagen I). In each comparison the starting point was freshly isolated primary epithelial tissues that were explanted into 3D culture as coherent groups of 100–500 cells. Tissues from the same animal were explanted into multiple different ECM microenvironments and cell behavior correlated with the current, local ECM environment. We found that simply varying the composition of the ECM in this way induced rapid and profound changes in tissue architecture and epithelial cell behavior. Metastatic human breast tumors preferentially disseminated into collagen I rich microenvironments and not into basement membrane protein gels (Figure 1A and [19]). Importantly, normal epithelia also disseminated cells into collagen I, while exhibiting an organotypic program of branching morphogenesis in basement membrane gels (Fig 1B and [19]). The major difference we observed was that dissemination of normal cells into collagen I is transient and they rapidly reestablished epithelial organization inside a basement membrane, while tumors persistently disseminated [19].
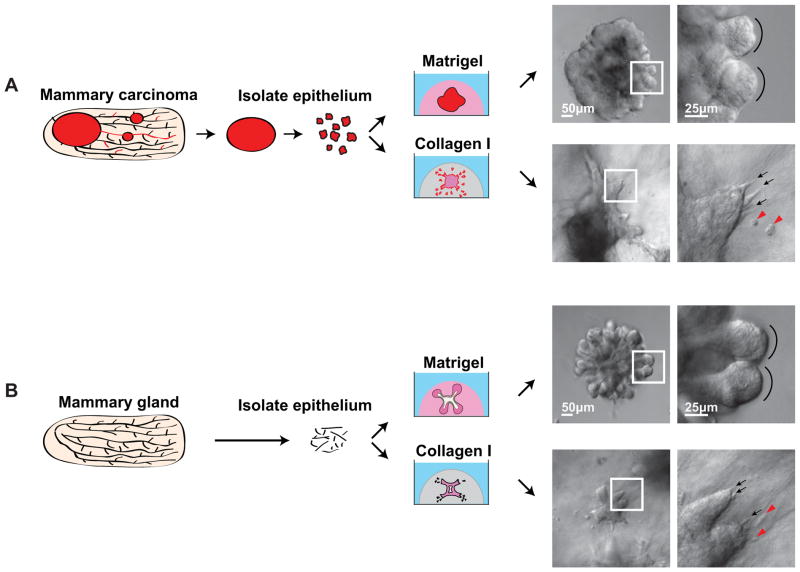
Fig. 1. Primary epithelial organoids as a model of tumor microenvironment induced dissemination.
(A) Mouse mammary carcinomas were surgically isolated and then epithelial organoids were derived from the carcinoma through a combination of mechanical disruption and enzymatic digestion. (B) Normal epithelial organoids were similarly derived from normal mouse mammary glands. In both cases 1000s of organoids are recovered and so organoids from the same mouse were embedded in a series of microenvironments. Both normal and tumor organoids exhibited restrained epithelial growth in Matrigel and protrusive, disseminative growth in collagen I. DIC images show the organoid morphology in the different conditions. Black arrows highlight cellular protrusions, red arrowheads mark disseminated cells, black arcs highlight the rounded epithelial edge of mammary buds.
Given the central importance of dissemination to the metastatic process, we sought to understand the nature of the dissemination promoting signals in collagen I rich microenvironments. However, Matrigel and collagen I gels differ in protein composition, supramolecular organization of the protein components, and in the material and mechanical properties of the resulting gel [20–22]. We hypothesized that collagen I induced dissemination might reflect a response to collagen I dependent changes in the mechanical properties of the microenvironment [20]. In support of this hypothesis matrix rigidity has been shown to be a powerful regulator of cell behavior [13, 14, 23–27]. Furthermore, both the rigidity of the tumor microenvironment and the abundance of collagen I increase during breast cancer progression in vivo [13–17].
A major challenge to studying the role of mechanical factors in tumor progression is that epithelial tumors exist as multicellular tissues whose architecture is not recapitulated in conventional 2D cultures [6, 11, 28]. In contrast, techniques for systematically varying the mechanical properties of a cell’s microenvironment were typically developed for cells cultured on top of a thin gel [24, 29]. The most frequently utilized technique relies on varying the crosslinking of acrylamide and then plating cells on top of the resulting thin gels. This approach is not easily generalizable to a 3D embedded culture format, as acrylamide is highly toxic to cells prior to polymerization. Alternate approaches that are suited to 3D culture have frequently relied on enzymatic crosslinking of native ECM proteins, such as collagen I [13]. The challenge in that context is that crosslinking can alter biologically important properties of the matrix other than rigidity. For example, crosslinking of collagen I can lead to recruitment of signaling molecules or to increased PI3K signaling [13]. We therefore developed tunable, poly(ethylene glycol) (PEG) based synthetic scaffolds to vary the rigidity of the non-permissive microenvironment (Matrigel), to test the hypothesis that increased microenvironmental rigidity would induce epithelial dissemination. We then combined our PEG scaffolds with cyclodextrin (CD) conjugated adhesive peptides to enable systematic variation of rigidity, protein composition, and the average concentration of adhesive sites in 3D formats.
Materials and Methods
Isolation of primary murine mammary organoids
All mouse work was done in accordance with protocols approved by the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC). Mouse mammary organoids from MMTV-PyMT tumor mice and their normal FVB counterparts (JAX Mice 002374, 001800) were harvested using previously described techniques [19, 30]. We removed the #3 and #4 mammary glands from FVB mice age 8–12 weeks, digested the tissue in a collagenase/trypsin (Sigma C2139/Gibco 27250-018) solution, which also contained Insulin (Sigma I9278) and Gentamicin (Gibco 15750060). The collagenase treated epithelial tissue was then treated with DNase (Sigma D4527) and isolated from single cells using a series of differential centrifugation steps. The resulting pellet contained small epithelial clusters consisting of a few hundred cells each, which have been termed “organoids.” Tumors were collected from 12–16 week old MMTV-PyMT mice. The largest tumor was harvested and prepared similarly to the FVB mammary glands. The tumor organoids were separated from undigested pieces after the collagenase digestion step. All solutions were made in DMEM/F-12, GlutaMAX (Gibco 10565). All tubes and pipets were coated with 2.46% BSA-DPBS solution to avoid tissue adhering to the plastic (Sigma A9576 and Gibco 14040182 or Sigma D8662).
Embedding organoids in hybrid PEG-Matrigel hydrogels
Organoids from either FVB or MMTV-PyMT mice were embedded in Growth Factor Reduced BD Matrigel Matrix (BD 354230). PEG-Matrigel composite hydrogels were synthesized as described in the following example: PEGDA (Mw ~ 3400 Da, pdi 1.1, SunBio) was dissolved in PBS (pH 7.4) to make a final concentration of 15% (w/v). This solution was added directly to either α-CD or α-CDpeptide with a final concentration of 1.25% (w/v). If needed, varying concentrations of PEGMA (PEGMA, Laysan Bio MW 5000 Da) were added to the above PEGDA solution. The solution was mixed on a shaker overnight and UV sterilized for 10–15 min. To this solution, Matrigel was added for a final PEGDA concentration of 3% (w/v) and α-CD concentration to 0.25% (w/v). Cultures were set up in 24-well non-TC treated plates (Nunc 144530) or 24-well non-TC treated movie plates (Greiner #662892). Transwell inserts (BD Falcon 353095) were used to help maintain the gel integrity. Before embedding the organoids, a photoinitiator, Irgacure® 2959 (Ciba®) (1.0 %, w/v) in 70 % (v/v) ethanol was dissolved and added to the PEGDA/Matrigel solution with a final concentration of (0.05 %, w/v). To this solution, organoids were mixed to give a final concentration of 2–3 orgs/μL, plated (100 μL) into the 24-well plate and immediately brought under a UV lamp (365 nm, 5 mW/cm2) and kept on a heating block (at 37 °C) for polymerization. After 20–30 min, growth media with DMEM/F12 GlutaMAX, Insulin-Transferrin-Selenium-X Supplement (100X) (Gibco 51500-056), Penicillin-Streptomycin (Sigma P4333), and FGF2 (2.5 nM, Sigma F0291) were added to the constructs. The media was changed on day 1 to remove any unthreaded α-CD or α-CDpeptide. The samples were cultured for 7 days and then analyzed.
Synthesis of α-CDYRGDS and α-CDYRDGS
The peptides conjugated to α-CD were synthesized according to the method as described in the published results [31].
Mechanical properties of the hydrogels
The storage and loss shear moduli of the PEGDA/PEGMA hydrogels were measured using a parallel-plate method of RFS-3 rheometer (Rheometric Scientific Inc.). The experiment was conducted using an 8 mm diameter plate within the linear viscoelastic region of the samples (n =3) at a frequency of 1 rad/s.
Bright field microscopy
Images were taken of these samples at 7 days using a Zeiss Primo Vert Inverted microscope with 5 Megapixel CMOS camera. Pictures were taken post fixation of 4% PFA.
Differential interference contrast (DIC) microscopy
Images of the samples were taken at 7 days using a Zeiss Cell Observer system with a Zeiss AxioObserver Z1 and an AxioCam MRM camera. Images were taken post fixation with 4% PFA. Movie images were taken using the microscope chamber for 7 days and then fixed in 4% PFA.
Confocal microscopy
Confocal images were collected using a Solamere Technology Group spinning-disk confocal microscope with a Zeiss 40× C-Apochromat objective lens (details in [32, 33]).
Antibody staining
Post 4% PFA fixation, the samples were permeabilized with 0.2% Triton X-100 for 20 min, blocked with PBS+1%BSA for 1–2 h and stained with DAPI (Molecular Probes D3571), phalloidin (Molecular Probes A22287), rat anti-cytokeratin 8 (Developmental Studies Hybridoma Bank TROMA-I-c), and goat anti-rat Alexa Fluor 568 (Molecular Probes A11077). After incubating overnight at 4°C, the samples were washed with PBS and kept at 4 °C until imaging. Images were taken from a Zeiss 710 confocal microscope within one week after staining.
Results
3D Microenvironments with Defined Rigidity
We sought to isolate the specific consequences of altering the rigidity of the 3D microenvironment on epithelial cell behavior. To ensure that the crosslinking strategy did not also send specific molecular signals to the cells we selected a bioinert synthetic polymer, poly(ethylene glycol) (PEG). PEG hydrogel systems have been successfully employed for a wide range of tissue engineering and therapeutic purposes [31, 34–39]. We utilized photopolymerization to crosslink PEG hydrogels and varied the elastic modulus of the hydrogel through systematic manipulation of the relative concentration of PEG-Diacrylate (DA) to PEG-Monoacrylate (MA) (Fig. 2A). PEG-DA polymerizes through acrylate bonds on both sides of the PEG chain. This allows PEG-DA to form a net-like structure when cross-linked. However, PEG-MA only has an acrylate bond on one side of the PEG chain. Because of this feature, it only forms strands when cross-linked. Normal or tumor organoids embedded in hydrogels composed of 3–10% PEG-DA alone or in combinations of PEG-DA and PEG-MA did not undergo epithelial morphogenesis. The epithelial tissue in these pure PEG gels appeared disorganized, with extensive cell death. We therefore chose to vary the crosslinking of PEG networks within Matrigel scaffolds, as Matrigel is known to provide a ligand environment sufficient for epithelial growth and branching of both normal and tumor tissue [19, 30, 40]. When Matrigel is combined with pure PEG-MA, the resulting strands cannot increase the stiffness of the Matrigel. Therefore, by varying the ratio of PEG-DA to PEG-MA, the crosslinking density can be changed proportionally to the rigidity of the hydrogel.
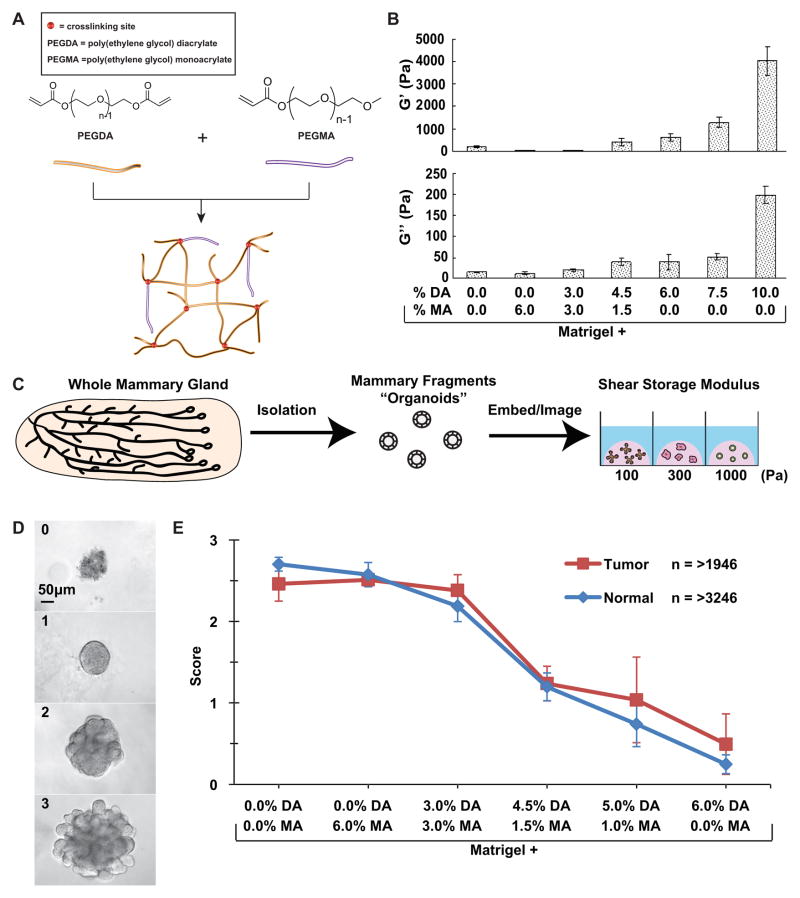
Fig. 2. PEG-Matrigel composite hydrogels enable specific control of rigidity in 3D microenvironments.
(A) Diagram describing the polymer system used to increase the rigidity of Matrigel. Poly(ethylene glycol) diacrylate (PEGDA) can crosslink PEG and increase the stiffness of the hydrogel whereas poly(ethylene glycol) monoacrylate (PEGMA) cannot crosslink and so limits the rigidity of the hydrogel. The total concentration of PEGDA+PEGMA was held constant at 6% so that the mechanics were varied independently of Matrigel or PEG concentration. (B) Parallel plate rheometer measurements of the shear storage modulus (G′) and shear loss modulus (G″) of a series of PEG-Matrigel composite hydrogels. Matrigel is the base polymer into which varying amounts of PEGDA and PEGMA were dissolved and photocrosslinked. Increasing concentrations of PEGDA correlated with increased rigidity of the hydrogel. (C) Schematic illustrating organoid extraction, isolation and embedding in multiple microenvironments of varying rigidity. (D) Phenotypic scores given to quantify growth of organoids. 0 = no growth, 1 = cyst-like structure, 2 = branching initiated, 3 = fully branched. Scale bar = 50 μm. (E) Graph of the trend in organoid development in microenvironments of varying rigidity, scored after 7 days of culture. Both tumor and normal displayed a negative correlation between rigidity and organoid development. Error bars = standard error of the mean.
We used a parallel plate rheometer to determine the shear storage modulus (G′), which represents the elastic properties of the gel, and the shear loss modulus (G″), which represents the viscous properties of the gel, of the resulting PEG-Matrigel composite hydrogels (Fig. 2B). Matrigel, Matrigel + (6% MA), and Matrigel + (3% DA / 3% MA) all formed highly compliant gels with G′ and G″ under 100 Pa (Fig. 2B). As the ratio of DA to MA increased we observed increases in both G′ and G″ (Fig. 2B). Our main analysis set was limited to DA/MA ratios that constituted total PEG concentrations of 6%, as 6% was the minimum concentration of PEG-MA that resulted in a stable gel when combined with Matrigel. We also determined the elastic modulus of combinations of Matrigel with either 7.5% or 10% PEG-DA (Fig. 2B). Our PEG-Matrigel composite hydrogel approach was sufficient to enable specific variation of elastic modulus over two orders of magnitude in a 3D environment.
Microenvironmental Rigidity and Epithelial Morphogenesis
We next tested the effect of increased shear elastic modulus on the behavior of epithelial organoids derived from either normal mammary glands or from advanced mammary carcinomas. We isolated carcinomas from a genetically engineered mouse tumor model (MMTV-PyMT [41]) that has been validated to recapitulate the cellular and molecular events in human breast cancer [42]. Briefly, we isolated the epithelial organoids through a combination of mechanical disruption and enzymatic digestion [43]. Each organoid initially consists of approximately 200–500 cells and we acquire 2–5,000 per normal mouse and 10–50,000 per mammary tumor. Due to the high number of organoids available we explanted tissue from the same mouse into a series of gels and therefore were able to test the impact of the rigidity of the microenvironment on organoids with the same genetic background (Fig. 2C).
We evaluated epithelial response to the hybrid gels using a four point scale: 0 = no growth and rough, disorganized tissue edges, 1 = smooth epithelial edges but no branching, 2 = smooth edges and initiation of new epithelial buds, 3 = initiation and elongation of a minimum of three new epithelial buds (Fig. 2D). Consistent with their similar mechanical properties, organoids embedded in pure Matrigel, Matrigel + 6% MA, and Matrigel + (3% DA / 3% MA) exhibited robust branching morphogenesis and were indistinguishable from each other (Fig. 2E). These data enable us to conclude that the presence of PEG does not interfere with epithelial morphogenesis. However, as the ratio of DA to MA increased we observed a strong decrease in the extent of branching morphogenesis. Matrigel + 6% DA was the highest rigidity environment in which we regularly observed organoids with rounded, epithelial organization. Organoids embedded within Matrigel + 7.5% DA or Matrigel + 10% DA did not survive. The clear trend was of decreased epithelial morphogenesis with increased microenvironmental rigidity, with no evidence of branching morphogenesis in microenvironments with a shear storage modulus above 1500 Pa. We did not observe a difference in mechanical responsiveness between normal and tumor organoids (Fig. 2E).
Microenvironmental Rigidity and Epithelial Dissemination
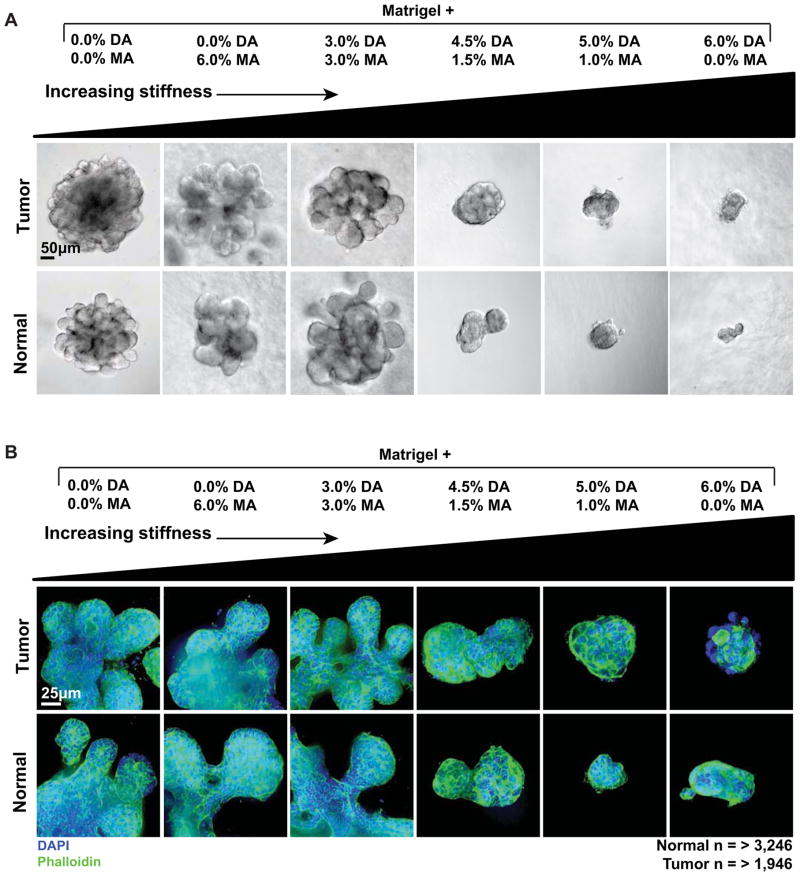
We next sought to determine the effect of high microenvironmental rigidity on protrusive and disseminative cell behaviors. Normal and tumor organoids rapidly induce ECM-directed, actin-based protrusions and disseminate viable epithelial cells when explanted into gels of collagen I [19]. We therefore used both differential interference contrast (DIC) and spinning disk confocal microscopy to evaluate the organization of epithelial organoids in microenvironments with varying rigidity. Consistent with the reduction in epithelial morphogenesis (Fig. 2E), organoids in more rigid microenvironments (Matrigel + (4.5% DA / 1.5% MA), Matrigel + (5% DA / 1% MA), and Matrigel + 6% DA) were both smaller and rounder than those cultured in the less rigid microenvironments (Matrigel alone, Matrigel + 6% MA, and Matrigel + (3% DA / 3% MA) (Fig. 3A,B). DIC imaging did not reveal protrusions into the ECM or disseminated cells in any of the microenvironments (Fig. 3A). Similarly, spinning disk confocal micrographs of F-actin organization did not reveal ECM-directed actin-based protrusions in any microenvironment (Fig. 3B). We therefore conclude that the rigidity of the microenvironment functions as a permissive and not an instructive signal. Within an acceptable range of rigidity values, we observed a similar degree of branching morphogenesis. As the rigidity increased we observed a progressive reduction in the extent of epithelial morphogenesis. However, we did not observe a transition to protrusive or disseminative epithelial cell behaviors as a function of increasing microenvironmental rigidity.
Fig. 3. Negative correlation between microenvironmental rigidity and epithelial morphogenesis.
(A) DIC (scale bar = 50 μm) and (B) confocal (scale bar = 25 μm) images showing representative growth of tumor and normal organoids in microenvironments of increasing rigidity. The concentration of ECM ligands and the concentration of PEG were both held constant. Normal and tumor organoids were larger and more developed in the less rigid microenvironments. Blue = DAPI DNA stain, marking nuclei. Green = Phalloidin, marking F-actin.
Biomaterials Strategy to Control Rigidity and Adhesion
PEG-Matrigel composite hydrogels enabled independent control over the elastic modulus of the microenvironment while holding both the concentration of PEG and ECM proteins constant. However, PEG is an bioinert polymer and does not provide specific molecular sites for receptor-mediated interaction with embedded cells. Accordingly, while the epithelial cells can sense the increased resistance against displacement of the Matrigel-PEGDA gels they cannot directly transduce these signals through integrins. Therefore, it was possible that high rigidity would induce protrusive or disseminative behaviors if we provided adhesive signals within the PEG network. To test this hypothesis, we relied on recent innovations in cyclodextrin (α-CD) PEG hydrogel systems. Cyclodextrins are cyclic oligosaccharides that can be conjugated with adhesive signals and incorporated into PEG networks (Fig. 4A) [44, 45]. To enable specific adhesion, RGD adhesion peptides were covalently incorporated onto one of the hydroxyl groups of the α-CD ring structure (α-CDYRGDS) [31]. The mechanics of the microenvironment were controlled by varying the concentration of PEG-DA and the average number of adhesive groups was controlled by varying the ratio of α-CD-YRGDS in the PEG-DA threading solution. Increasing the PEG-DA concentration from 3% to 6% resulted in an approximately 10 fold increase in G′ (Fig. 4B).
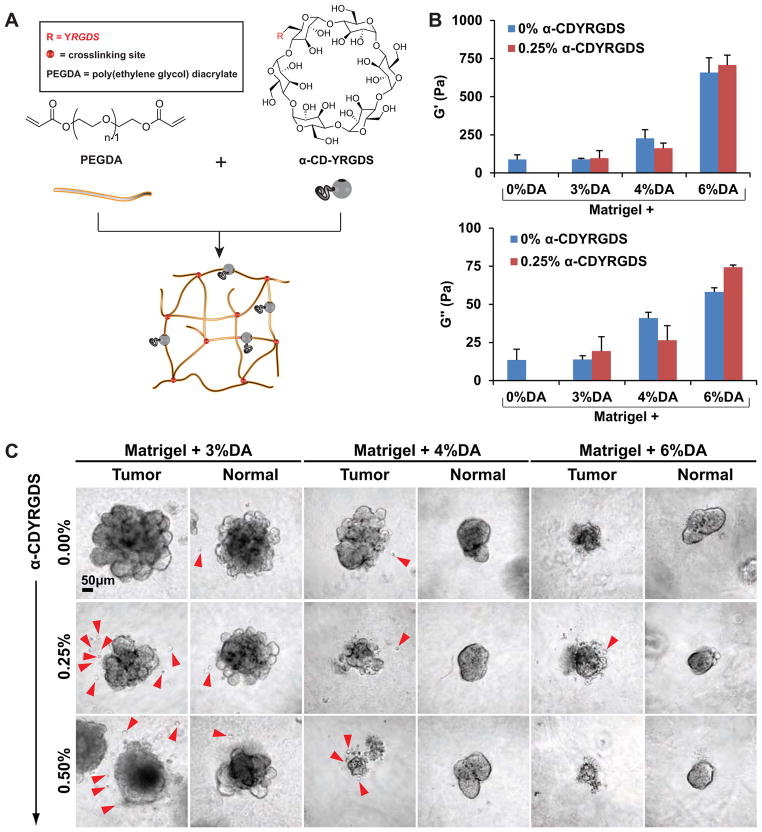
Fig. 4. Adhesive PEG-Matrigel composite hydrogels induce dissemination of epithelial cells.
(A) We incorporated cyclodextrin rings with adhesive peptides (α-CDYRGDS) into the PEG networks to enable independent control over polymer mechanics and adhesivity. (B) Shear storage modulus (G′) and shear loss modulus (G″) measurements of PEG-Matrigel composite hydrogels revealed progressively increased rigidity with increased PEG concentration. The presence of α-CDYRGDS had a comparatively small effect on matrix mechanics. (C) Normal and tumor organoids were explanted into a range of microenvironments with different mechanics and adhesivity. We inferred cellular dissemination from the presence of single cells adjacent to organoids. Microenvironments with lower rigidity and intermediate concentrations of adhesive peptides displayed maximal dissemination (3%DA and 0.25% CDYRGDS) in both tumor and normal conditions. Scale bar = 50 μm
Regulation of Dissemination by Microenvironmental Adhesion and Rigidity
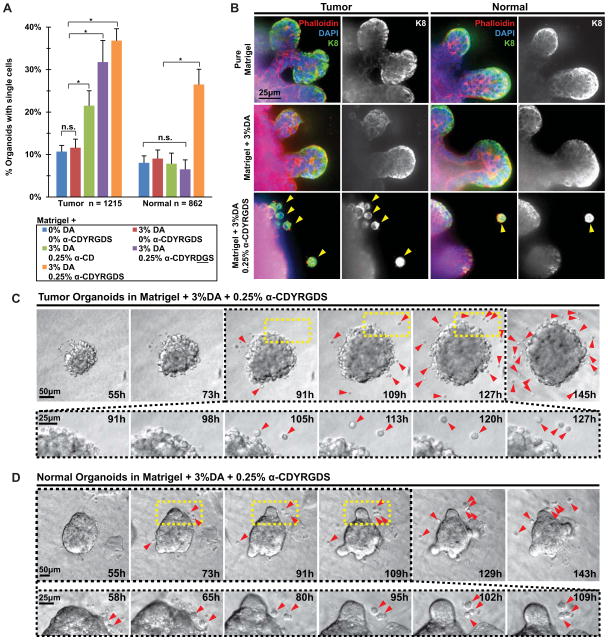
As a surrogate for dissemination, we first assayed for single cells surrounding epithelial organoids in hybrid hydrogels on day 7 of culture. We used Matrigel as the base environment and tested all combinations of: 3% PEGDA, 4% PEGDA and 6% PEGDA and 0, 0.25, and 0.5% α-CDYRGDS (Fig. 4C). The appearance of single cells correlated negatively with microenvironmental rigidity, with the greatest frequency of single cells observed in the 3% DA gels (red arrowheads, Fig. 4C). We also varied the concentration of α-CDYRGDS in the threading solution and observed a maximum level of dissemination at an intermediate α-CDYRGDS concentration (0.25%, Fig. 4C). Consistent with our experience with the DA/MA ratio experiments we observed a negative correlation between microenvironmental rigidity and the extent of epithelial morphogenesis (Fig. 4C).
We next quantified the frequency with which individual cells were observed adjacent to epithelial organoids in the Matrigel + 3% DA, 0.25% α-CDYRGDS condition (experimental condition, orange, Fig. 5A). As controls we utilized pure Matrigel (blue), Matrigel + 3%DA (red), Matrigel + 3%DA,0.25% α-CD (green), and Matrigel + 3%DA,0.25% α-CDYRDGS (scrambled peptide, purple). Tumor organoids displayed background levels of single cell accumulation in the Matrigel and Matrigel + 3% DA control. Tumors organoids exhibited a progressively increasing frequency of single cells in the Matrigel + 3% DA,0.25% α-CD control, Matrigel + 3% DA,0.25% α-CDYRDGS scrambled peptide control, and the Matrigel + 3%DA,0.25% α-CDYRGDS experimental conditions. Each of these conditions was statistically significantly different from the Matrigel and Matrigel + 3%DA controls (Mixed Model, p<0.05, Fig. 5A). The accumulation of single cells near tumor organoids in the Matrigel + 3%DA,0.25% α-CD, Matrigel + 3%DA,0.25% α-CDYRDGS may be attributed to adhesion associated with non-specific protein adsorption to the CD ring and to the adhesive properties of the RDG peptide motif, as has been previously observed [46].
Fig. 5. Normal and tumor organoids disseminate epithelial cells in response to synthetic microenvironmental signals.
(A) Quantification of the fraction of organoids with adjacent single cells in each microenvironmental condition. Normal n = 862, tumor n = 1215 organoids counted. Statistical significance was tested using a generalized linear model to enable us to isolate the variation in single cell abundance from any possible batch effects in the 3 independent experiments. Conditions that were significantly different (P<0.05) are indicated. (B) Confocal images of tumor and normal organoids grown in Matrigel, Matrigel + 3% DA, and Matrigel + 3% DA,0.25% CDYRGDS. The single cells adjacent to epithelial organoids stained positive for antibodies targeting epithelial-specific cytokeratin 8, confirming their epithelial identity. Scale bar = 25 μm. We directly observed single cells disseminating from both tumor (C) and normal (D) organoids embedded within Matrigel + 3% DA,0.25% CDYRGDS using time-lapse DIC microscopy. Scale bar = 50 μm and 25 μm. The magnified portion shows more detail of dissemination as well as proliferation of disseminated cells.
Normal organoids exhibited background levels of single cell accumulation for the Matrigel + 3% DA, Matrigel + 3% DA,0.25% α-CD control, and Matrigel + 3% DA,0.25% α-CDYRDGS scrambled peptide controls. We observed a statistically significant increase in single cell accumulation adjacent to normal organoids only in the experimental condition with Matrigel + 3% DA,0.25% α-CDYRGDS (Mixed Model P<0.05, Fig. 5A). Taken together these data demonstrate both that extracellular signals in the synthetic polymer networks are able to induce single cell accumulation and that tumor organoids display both a higher frequency of single cell accumulation and accumulate single cells under a broader range of experimental conditions than normal organoids.
Organoids are freshly isolated from intact organs, which themselves contain a mixture of epithelial and stromal cell populations. We therefore next used epithelial specific molecular markers to determine the cellular identity of the single cells that accumulate in the Matrigel + 3% DA, 0.25% α-CDYRGDS gels. The single cells accumulating in the Matrigel + 3% DA, 0.25% α-CDYRGDS condition stained positive for the epithelial-specific cell marker cytokeratin 8, leading us to conclude that they represent isolated epithelial cells in the ECM. We next used time-lapse differential interference contrast microscopy to visualize cell and tissue dynamics in the hybrid PEG-Matrigel hydrogels. We specifically observed dissemination of cells from both tumor (Fig. 5C) and normal organoids (Fig. 5D). There were typically a larger number of cells disseminating from tumor organoids and disseminated tumor cells typically migrated a greater distance through the ECM (Fig. 5C,D). We therefore conclude that epithelial cells disseminate in response to the adhesive PEG polymer networks and that dissemination is dependent on the presence of adhesive signals within those networks.
Discussion
We previously demonstrated that breast tumor cells are highly invasive and disseminative in collagen I and not in Matrigel [19]. These data motivated our hypothesis that increased microenvironmental rigidity could induce protrusive migration and dissemination in epithelial cells. To test this hypothesis we first utilized PEG-Matrigel composite hydrogels and varied the extent of crosslinking of the PEG network by varying the ratio of PEG-DA and PEG-MA. This approach enabled us to vary the rigidity of the microenvironment while holding the concentration of both ECM ligands and PEG constant. Organoids explanted into a series of PEG-Matrigel composite hydrogels with progressively increasing rigidity exhibited a strong negative correlation between microenvironmental rigidity and the extent of epithelial morphogenesis in both normal and tumor tissue. We observed only minimal epithelial growth in microenvironments with a shear elastic modulus above 1.0 kPa. Furthermore, we did not observe protrusive migration or local dissemination of normal or neoplastic epithelial cells in PEG-Matrigel composite hydrogels through the range from 50 – 4,000 Pa. However, PEG is a bioinert polymer and cells lack receptors through which to interact with PEG in a molecularly-specific fashion. Accordingly, we developed a second series of materials in which we non-covalently incorporated adhesive peptides into the PEG network in varying concentration. We observed frequent epithelial dissemination into adhesive PEG-α-CDYRGDS-Matrigel composite hydrogels, demonstrating that chemically defined extracellular signals can induce dissemination of cancer cells despite the presence of differentiating signals from Matrigel.
Mechanotransduction in 3D
Cells cultured on top of thin 2D substrates of varying rigidity can respond to differences in rigidity from kPa through GPa [14, 23, 24, 26, 27, 29]. In 2D experiments, the cells can freely migrate over the surface and the increased rigidity does not provide a mechanical barrier to forward migration. In contrast, in our experiments the multicellular epithelial organoids were fully embedded within the PEG-Matrigel composite hydrogel and there were no sites in the PEG network that could be enzymatically degraded. Accordingly, the epithelial cells can displace, but cannot degrade, the PEG network to make space for migration and growth. We observed minimal growth or migration of normal or neoplastic epithelial groups in microenvironments more rigid than approximately 1.0 kPa. Growth in stiffer environments may require local proteolysis [47]. Since we observed dissemination specifically into adhesive PEG-Matrigel networks, our data further suggest that cells interact actively with the PEG network during dissemination.
Implications for tissue engineering
Our previous publications revealed that the protein composition [19] and the 3D organization [48] of natural ECM gels are each potent regulators of epithelial architecture and function. Specifically, native ECM signals are capable of inducing dissemination of normal epithelial cells [19, 48]. The present study reveals that even small changes in the mechanical or adhesive properties of synthetic polymer networks can also potently regulate epithelial organization and dissemination. Accordingly, scaffolds intended to support the growth and differentiation of engineered replacement epithelial tissues will require careful tuning of mechanics and adhesion to prevent the systemic distribution of epithelial cells through the body.
Implications for breast cancer dissemination in vivo
Previous publications demonstrated that collagen I accumulated at the interface between breast tumors and their surrounding stroma [7, 15, 16]. Studies utilizing natural ECM gels have revealed that collagen I strongly induces epithelial dissemination [7, 19, 49]. However, the microenvironment surrounding breast tumors is also more rigid than that surrounding normal ducts and increasing the lysl oxidase-mediated crosslinking of collagen I in the mammary gland accelerated breast cancer progression [7, 13, 14, 25]. These data raised the possibility that cancer cells were specifically responding to the rigidity of the 3D environment. However, while lysl oxidase based crosslinking of collagen I increases the rigidity of the collagen I network, it can also increase integrin clustering or activate PI3K signaling [13]. Our work with PEG-Matrigel composite hydrogels demonstrates that increased microenvironmental rigidity does not necessarily induce dissemination. Even within adhesive PEG-α-CDYRGDS-Matrigel composite hydrogels the maximal dissemination was observed at relatively low rigidity (G′~100–200 Pa).
Conclusions
Our combination of nanobiomaterials and primary organotypic cultures enabled us to study the cellular response to the decoupled mechanical and adhesive properties of 3D microenvironments. Both normal and malignant epithelial cells disseminated into adhesive PEG-α-CDYRGDS-Matrigel composite hydrogels despite the fact that they lack the triple helical fibrillar organization of collagen I and did not contain collagen I-specific adhesion sequences. We therefore conclude that there are collagen I-independent extracellular signals that can induce epithelial dissemination. Our data further suggest that there could be molecular routes to breast cancer dissemination that do not depend on collagen I and that dissemination could occur into regions with low or moderate rigidity. The nanobiomaterials developed for this study enable the independent modulation of microenvironmental mechanics, ECM protein composition, and density of adhesive sites within the PEG-ECM scaffolds. Our approaches should be generally useful both in developing optimum 3D culture conditions for engineered tissues and in the systematic evaluation of the role of mechanical and adhesive inputs on 3D tissue growth. The nanobiomaterials and 3D organotypic assays developed in this study can also directly enable the identification of molecular regulators of epithelial cancer dissemination.
Acknowledgments
The authors thank Alison Smith, Dr. Ryan S. Gray, and Soe Htet for assistance in early experiments. The authors thank Dr. Peng Huang of the SKCCC for biostatistics support. The authors gratefully acknowledge the Maryland Stem Cell Research Foundation for a postdoctoral research fellowship (A.S.), the National Science Foundation (CMMI 0948053 to JHE), the Safeway Foundation Award for Breast Cancer Research to JHE and AJE, and the National Institutes of Health (1F31AG034016 to ARR, NIDCR R01DE016887 to JHE, NCI U01 CA155758 to AJE, and NCI U54 CA151838 to JHE and AJE). AJE was supported by a Research Scholar Grant, RSG-12-141-01-CSM from the American Cancer Society.
Footnotes
Disclosure Statements
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8(1):201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polyak K. Molecular markers for the diagnosis and management of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):210–3. doi: 10.1093/jncimonographs/lgq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22(42):6524–36. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 4.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 5.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27(55):6920–9. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–52. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 9.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagios C, Lochter A, Bissell MJ. Tissue architecture: the ultimate regulator of epithelial function? Philos Trans R Soc Lond B Biol Sci. 1998;353(1370):857–70. doi: 10.1098/rstb.1998.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–32. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conklin MW, Keely PJ. Why the stroma matters in breast cancer: insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh Migr. 2012;6(3):249–60. doi: 10.4161/cam.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, et al. The ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. PNAS. 2012;109(39):E2595–E604. doi: 10.1073/pnas.1212834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreger ST, Bell BJ, Bailey J, Stites E, Kuske J, Waisner B, et al. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers. 2010;93(8):690–707. doi: 10.1002/bip.21431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20(8):931–41. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YL, Motte S, Kaufman LJ. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials. 2010;31(21):5678–88. doi: 10.1016/j.biomaterials.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Discher DEJP, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;18(310):1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 24.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Paszek MJW, VM The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–42. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 26.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121(Pt 20):3285–92. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 28.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parekh A, Ruppender NS, Branch KM, Sewell-Loftin MK, Lin J, Boyer PD, et al. Sensing and modulation of invadopodia across a wide range of rigidities. Biophys J. 2011;100(3):573–82. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14(4):570–81. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, Zhan J, Ye Z, Elisseeff JH. Modular Multifunctional Poly(ethylene glycol) Hydrogels for Stem Cell Differentiation. Advanced Functional Materials. 2013;23(5):575–82. doi: 10.1002/adfm.201201902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewald AJ. Practical considerations for long-term time-lapse imaging of epithelial morphogenesis in three-dimensional organotypic cultures. Cold Spring Harb Protoc. 2013;2013(2) doi: 10.1101/pdb.top072884. [DOI] [PubMed] [Google Scholar]
- 33.Ewald AJ, Werb Z, Egeblad M. Dynamic, long-term in vivo imaging of tumor-stroma interactions in mouse models of breast cancer using spinning-disk confocal microscopy. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.top97. pdb top97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–56. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 36.Hwang NS, Varghese S, Li H, Elisseeff J. Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res. 2011;344(3):499–509. doi: 10.1007/s00441-011-1153-2. [DOI] [PubMed] [Google Scholar]
- 37.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 38.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15(2):205–19. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh A, Zhan J, Elisseeff J. Directed Stem Cell Differentiation Using PEG/alpha-CD-derived Biomaterials. Molecular Biology of the Cell. 2011:22. [Google Scholar]
- 40.Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, et al. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J Cell Sci. 2012;125(11):2638–54. doi: 10.1242/jcs.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163(5):2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen-Ngoc KV, Shamir ER, Huebner RJ, Beck JN, Cheung KJ, Ewald AJ. 3D Culture Assays of Murine Mammary Branching Morphogenesis and Epithelial Invasion. Methods in Molecular Biology. 2013 doi: 10.1007/978-1-4939-1164-6_10. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harada A, Li J, Kamachi M. The Molecular Necklace - a Rotaxane Containing Many Threaded Alpha-Cyclodextrins. Nature. 1992;356(6367):325–7. [Google Scholar]
- 45.van de Manakker F, Vermonden T, van Nostrum CF, Hennink WE. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules. 2009;10(12):3157–75. doi: 10.1021/bm901065f. [DOI] [PubMed] [Google Scholar]
- 46.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research. 1998;39(2):266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 47.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–65. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen-Ngoc KV, Ewald AJ. Mammary ductal elongation and myoepithelial migration are regulated by the composition of the extracellular matrix. J Microsc. 2013;251(3):212–23. doi: 10.1111/jmi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333–9. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]