Abstract
Objective:
To examine the performance of the Telephone Interview for Cognitive Status (TICS) for identifying participants appropriate for trials of physical activity and cognitive training interventions.
Methods:
Volunteers (N = 343), ages 70–85 years, who were being recruited for a pilot clinical trial on approaches to prevent cognitive decline, were administered TICS and required to score ≥31 prior to an invitation to attend clinic-based assessments. The frequencies of contraindications for physical activity and cognitive training interventions were tallied for individuals grouped by TICS scores. Relationships between TICS scores and other measures of cognitive function were described by scatterplots and correlation coefficients.
Results:
Eligibility criteria to identify candidates who were appropriate candidates for the trial interventions excluded 51.7% of the volunteers with TICS<31. TICS scores above this range were not strongly related to cognition or attendance at screening visits, however overall enrollment yields were approximately half for participants with TICS = 31 versus TICS = 41, and increased in a graded fashion throughout the range of scores.
Conclusions:
Use of TICS to define eligibility criteria in trials of physical activity and cognitive training interventions may not be worthwhile in that many individuals with low scores would already be eliminated by intervention-specific criteria and the relationship of TICS with clinic-based tests of cognitive function among appropriate candidates for these interventions may be weak. TICS may be most useful in these trials to identify candidates for oversampling in order to obtain a balanced cohort of participants at risk for cognitive decline.
Keywords: clinical trial design, cognitive interventions, eligibility criteria
Introduction
The prevalence of older individuals who have experienced cognitive decline continues to rise and the psychological, social, and financial costs of cognitive disorders to the individual and society are extraordinary (Plassman et al., 2007, 2008). It is increasingly urgent to develop strategies to delay or prevent age-associated cognitive decline (Elias and Wagster, 2007). Optimal approaches are likely to vary depending on many characteristics of individuals, including their current level of cognitive function. Because of this, assessment of cognitive function during the screening process for clinical trials is necessary to identify appropriate candidates for the therapies being tested. Clinic-based cognitive assessment may be costly and burdensome; there is a growing interest in using telephone-based interviews for this initial cognitive screen (Barber and Stott, 2004; Moylan et al., 2004; Yaari et al., 2006; van Uffelen et al., 2007).
Leading candidates for telephone-base screening are the Telephone Interview for Cognitive Status (TICS) and various modifications of this instrument, often referred to as TICS-m (Brandt et al., 1988; Jarvenpaa et al., 2002; Hogervorst et al., 2004; Barber and Stott, 2004), although other instruments have been proposed (Hill et al., 2005; Rabin et al., 2007, Kiddoe et al., 2008). TICS and TICS-m have been widely used with great success as measures of cognitive function (Grodstein et al. 2001; de Jager et al., 2003; Rankin et al., 2005; Xiong et al., 2006; Debling et al., 2006; King et al., 2006; Arnold et al., 2009) and as screeners for cognitive impairment and dementia (Petitti et al., 2002; dal Forno et al., 2006; Rocca et al., 2007; Cook et al., 2009; Smith et al., 2009; Duff et al., 2009).
TICS-based instruments are also being used to identify participants for clinical trials who are within specific ranges of cognitive function: mild cognitive impairment (Lines et al., 2003; Yaari et al., 2006; van Uffelen et al., 2008), free of memory complaints and cognitive impairment (Graff-Radford et al., 2006), and at enhanced risk for cognitive impairment (DeKosky et al., 2006). They have also been used to identify suitable participants for a trial of cognitive training and physical activity interventions (O’Dwyer et al., 2007), for which the most appropriate candidates may be individuals with memory concerns who are free of cognitive impairment (i.e., mild cognitive impairment or dementia) and for whom these interventions are appropriate. The properties of TICS-based screening for this latter use, however, have not been reported and cannot be inferred from more general settings.
We examine two potential uses of the TICS for screening individuals for trials of physical activity and cognitive training: to identify participants who have deficits in cognitive function but are free of cognitive impairment and to identify groups of individuals for whom oversampling may be warranted. The data we describe come from a pilot trial designed to provide information for designing and conducting full-scale trials of promising interventions, including how to improve recruitment efficiency.
Methods
The Seniors Health and Activity Research Program Pilot trial (SHARP-P) was a single-blinded pilot randomized controlled trial that involved the delivery of a physical activity training intervention and/or a cognitive training intervention in a 2 × 2 factorial design. Physical activity training consisted of center-based and home-based sessions to include aerobic, strength, flexibility, and balance training with a targeted duration of 150 min/week. The cognitive training intervention was developed to improve consciously controlled memory processing or recall of episodic memory information and to produce changes in cognitive performance that transfer to untrained domains of cognitive abilities such as executive function, working memory, planning and memory monitoring, long-term item memory, and cognitive processing speed (Jennings et al., 2005).
SHARP-P targeted the enrollment of 80 community-dwelling persons, who were at risk for cognitive decline by being aged 70–85 years and having subclinical cognitive deficits (Winblad et al., 2004). Inclusion/exclusion criteria were selected to identify individuals who were appropriate candidates for physical activity and cognitive training, who did not have neurological conditions or current medications likely to affect cognitive functioning, and who appeared likely to adhere to study protocols. Table 1 summarizes exclusion criteria, grouping them by their relationship to physical activity, cognition, adherence, and trial objectives.
Table 1.
Exclusion criteria for the Seniors Health and Activity Research Program Pilot Trial
| Exclusion Criteria Related to Physical Activity |
Telephone Screening Visits
|
Clinic Visits
|
| Exclusion Criteria Related to Cognition |
Telephone Screening Visits
|
Clinic Visits
|
| Exclusion Criteria Related to Trial Design or Adherence |
Telephone Screening Visits
|
Clinic Visits
|
Enrollment proceeded in four steps. Mailing to targeted zip codes from lists purchased from a local newspaper and presentations at health education meetings were used to identify interested volunteers. After an initial contact was made to confirm age, concerns about memory loss, and self-reported physical activity levels, a phone call was used to query regarding some major sources of exclusions. At this time, TICS was administered. TICS items, which briefly assess various cognitive functions including orientation, concentration, memory, naming, comprehension, calculation, reasoning, judgment, and praxis, provide scores ranging from 0 to 41, with higher scores indicating better overall cognitive function (see Brandt et al., 1988). Volunteers for SHARP-P were required to have TICS≥31 to be invited to clinic visit for further screening. This cutoff has been used previously to identify individuals with possible clinically significant cognitive impairment including dementia (Grodstein et al., 2001; Desmond et al., 1994). During the clinic-screening visit, additional cognitive testing was administered and used to rule out those with significant cognitive deficits (e.g., MCI, dementia) not identified by the TICS. To rule out significant global cognitive deficits suggestive of MCI or dementia, scores on the Modified Mini Mental State Exam (Teng and Chui, 1987), a 100-point measure of global cognitive functioning similar to the TICS, were required to be ≥88 (≥80 if fewer than nine years of education). These cutoffs were projected to be roughly equivalent to the TICS cut-point and thus represent a second screening of cognitive functioning. Also, pairs of more sensitive domain-specific cognitive tests were used to rule out further significant deficits. For episodic memory, the delayed recall scores from the Hopkins Verbal Learning Test (HVLT) (Brandt, 1991) and the Logical Memory (LM) subtest from the Wechsler Memory Scale-III (WMS-III) (Wechsler, 1997) were used. For speed of mental processing, the Trail Making Test-Part A (Reitan, 1958) and the Digit Symbol Coding subtest of the Wechsler Adult Intelligence Scale-III test (Wechsler, 1996) were used. For verbal fluency, the Category and Letter Fluency Tests (Strauss et al., 2006) were used. For each of these three domains, participants were determined to have a significant cognitive deficit, and therefore excluded, if the score on any test was ≥2.0 standard deviations below age- and education-specific norms or if two tests in the same domain were both ≥1.5 standard deviations below mean expected scores, criteria commonly used by clinicians when evaluating individuals for dementia and MCI. At this visit, are view of current medications was conducted. Use within the prior 4 weeks of the following excluded volunteers: anticholinergic agents, tricyclic antidepressants, clonidine, anti-Parkinsonian agents, narcotic analgesics, neuroleptics, sedatives/benzodiazepines (selected short acting benzodiazepines were allowed if not used >3 days/week and on days of testing), and dementia drugs. Selective serotonin reuptake inhibitor antidepressants were allowed if the dose was stable for 8 weeks. Volunteers who remained eligible and received clearance from their personal physicians were invited to a final visit for collection of baseline measures and were then randomly assigned with equal probability among the four experimental conditions. All participants signed an informed consent document; the study protocol was approved by an Institutional Review Board. Participants received a small honarium ($25) for completing study visits.
Cognitive testing
The TICS and clinic-based assessments of cognitive function were administered by trained and certified staff. Training was didactic and experiential and required certification, which was overseen by a geropsychologist experienced in multicenter studies. The approximate times of administration were 8 min for TICS and no more than 45 min for the clinic-based cognitive assessments we describe.
Statistical methods
Rates that individuals were ineligible were tallied. Associations that TICS had with other tests of cognitive function were described with scatterplots and correlation coefficients. Logistic regression was used to develop smoothed estimates of overall yields by TICS scores.
Results
Figure 1 summarizes the enrollment process of SHARP-P, during which 349 participants were administered an initial telephone screen, 143 attended a clinic screening visit, and 73 were ultimately randomized. Of those initially screened, 343 completed the TICS. Their mean (SD) age was 76.8 (4.3) years; 58.3% were women; 0.5% self-identified as Native American, 13.2% as African American, 84.4% as Caucasian, and 1.2% as other or multiple ethnicities. The mean TICS (SD) score was 33.2 (3.1). Scores ranged from 22 to 41; 58 (16.9%) individuals scored <31 and were therefore not eligible for further screening. We grouped individuals with TICS scores 35–41 (i.e., within 2 standard deviations of a ‘perfect’ score), 31–34, and <31 (ineligible for SHARP-P) to represent no global cognitive deficits, minimal cognitive deficits, and moderate deficits, respectively.
Figure 1.
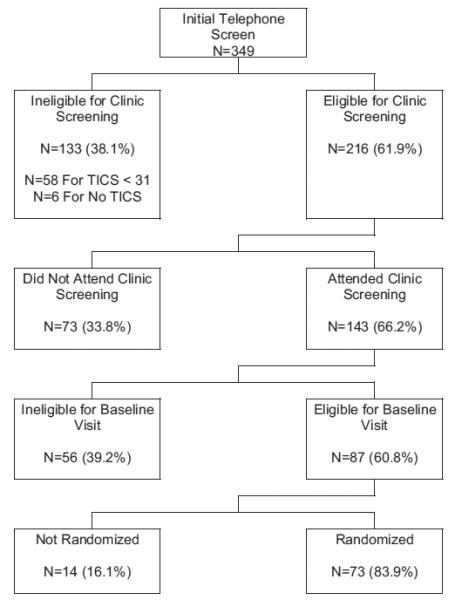
Enrollment process of SHARP-P from initial telephone screen to randomization.
Table 2 examines how performance in TICS is related to other eligibility criteria assessed during the initial telephone screening, which are grouped according to their relationships with physical activity, cognitive training, or other trial objectives. The rates that screenees met each of these exclusion criteria are listed, overall and for ranges of TICS scores. Many individuals were excluded for several reasons. Criteria related to physical activity excluded 18.4% of those interviewed. These, in a graded fashion, culled individuals with lower TICS scores at increasing rates, excluding 29.3% of those with scores <31. Criteria related to cognition excluded 14.0% of those screened. Not surprisingly, these too had a graded relationship with lower TICS scores. Exclusions related to adherence or other aspects of the trial design affected 4.4% of individuals and also were more prevalent among those with TICS <31 (13.8%). Overall, 51.7% of those with TICS <31 also were also excluded for at least one of the criteria listed in Table 2. The exclusion rate for individuals with TICS 31–34 was 26.2%; for those with TICS 35–41, the rate was 21.6%.
Table 2.
Most prevalent sources of exclusions during telephone screening—overall and for individuals grouped by TICS score
| Ineligibility criteria other than TICS | Number (percent) ineligible |
|||
|---|---|---|---|---|
| All screenees N=343 |
No global cognitive deficit TICS 35–41 N=125 |
Mild cognitive deficit TICS 31–34 N=160 |
Moderate cognitive deficit TICS<31 N=58 |
|
| Exclusions related to physical activity | ||||
| Exercise ≥ 30min > 1 per week | 32 (9.3) | 6 (4.8) | 18 (11.2) | 8 (13.8) |
| History of severe chest pain | 15 (4.4) | 4 (3.2) | 5 (3.1) | 6 (10.3) |
| Congestive heart failure | 14 (4.1) | 3 (2.4) | 4 (2.5) | 7 (12.1) |
| Undergoing physical therapy | 5 (1.5) | 2 (1.6) | 3 (1.9) | 0 (0.0) |
| Severe joint problems | 4 (1.2) | 0 (0.0) | 4 (2.5) | 0 (0.0) |
| Aortic stenosis | 4 (1.2) | 2 (1.6) | 0 (0.0) | 2 (3.4) |
| Cardiac arrest | 3 (0.9) | 0 (0.0) | 2 (1.2) | 1 (1.7) |
| Other exclusions related to physical activity | 19 (5.5) | 6 (4.8) | 6 (3.7) | 7 (12.1) |
| Any of above | 63 (18.4) | 17 (13.6) | 29 (18.1) | 17 (29.3) |
| Exclusions related to cognition | ||||
| Hospitalization for stroke | 14 (4.1) | 3 (2.4) | 5 (3.1) | 6 (10.3) |
| Head injury with loss of consciousness and hosp | 13 (3.8) | 5 (4.0) | 5 (3.1) | 3 (5.2) |
| Medications for memory | 8 (2.3) | 1 (0.8) | 3 (1.9) | 4 (6.9) |
| Depression symptomsa | 7 (2.0) | 2 (1.6) | 3 (1.9) | 2 (3.4) |
| Other research study on memory | 6 (1.8) | 3 (2.4) | 3 (1.9) | 0 (0.0) |
| Hospitalization for TIA in past 6 months | 4 (1.2) | 0 (0.0) | 1 (0.6) | 3 (5.2) |
| Prior diagnosis of MCI | 3 (0.9) | 0 (0.0) | 2 (1.1) | 1 (1.7) |
| Other exclusions related to cognition | 11 (3.2) | 0 (0.0) | 6 (3.8) | 5 (8.6) |
| Any of above | 48 (14.0) | 13 (10.4) | 20 (12.5) | 15 (25.9) |
| Exclusions related to trial design of adherence | ||||
| No primary care physician | 4 (1.2) | 1 (0.8) | 0 (0.0) | 3 (5.2) |
| Alcohol drinks > 14/week | 3 (0.9) | 1 (0.8) | 1 (0.6) | 1 (1.7) |
| Unwilling to accept randomization | 3 (0.9) | 2 (1.6) | 1 (0.6) | 0 (0.0) |
| Other exclusions related to adherence or protocol | 5 (1.5) | 0 (0.0) | 1 (0.6) | 4 (6.9) |
| Any of above | 15 (4.4) | 4 (3.2) | 3 (1.9) | 8 (13.8) |
| Any | 99 (28.9) | 27 (21.6) | 42 (26.2) | 30 (51.7) |
Geriatric Depression Scale score ≥8.
Table 3 describes findings from the volunteers who attended clinic screening. Overall, 24.5% were ineligible due to low cognitive test scores at this visit: 20.0% of those with TICS 35 and 28.8% of those with TICS 31–34. Among those with relatively higher TICS scores, the most common exclusions were based on tests of episodic memory or verbal function. Among those with TICS 31–34, the most common sources of exclusions were tests of global cognitive function and episodic memory. Current use of medications that may affect cognitive function or interfere with cognitive training excluded 20.3% of individuals at this clinic visit, and was slightly more common among individuals with TICS ≥35 (24.3%) compared to those with TICS 31–34 (16.4%). Overall, 38.6% of screenees with TICS ≥35 were eliminated by the criteria in Table 3, compared to 39.7% of these with TICS 31–34.
Table 3.
Most prevalent sources of exclusions during clinic visit—overall and for individuals grouped by TICS Score
| Ineligibility criteria | Number (percent) ineligible |
||
|---|---|---|---|
| All screenees N = 143 |
No global cognitive deficit TICS 35–41 N=70 |
Mild cognitive deficit TICS 31–34 N=73 |
|
| Exclusions related to cognitive tests | |||
| Global cognitive function deficit | 13 (9.1) | 3 (4.3) | 10 (13.7) |
| Episodic memory deficit | 18 (13.1) | 5 (7.5) | 13 (18.6) |
| Speed of processing and attention deficit | 1 (0.7) | 1 (1.5) | 0 (0.0) |
| Verbal function deficit | 15 (11.0) | 8 (11.9) | 7 (10.0) |
| Any of above | 35 (24.5) | 14 (20.0) | 21 (28.8) |
| Medicationsa | 29 (20.3) | 17 (24.3) | 12 (16.4) |
| Any | 56 (39.2) | 27 (38.6) | 29 (39.7) |
Use of the following medications within 4 weeks prior to screening in the following classes excluded participants: anticholinergic agents, tricyclic antidepressants, clonidine, anti-Parkinsonian agents, narcotic analgesics, neuroleptics, sedatives/benzodiazepines (selected short acting benzodiazepines were allowed if not used on more than 3 days/week or days of testing), and dementia drugs. Selective serotonin reuptake inhibitor antidepressants were allowed as long as the dose was stable for 8 weeks.
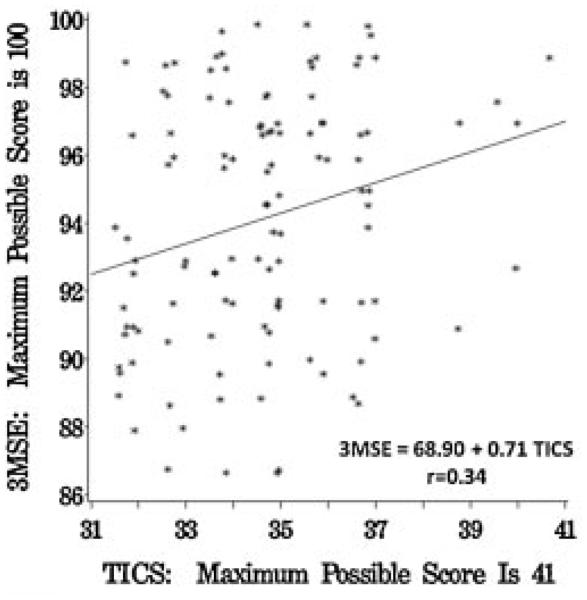
The mean scores of the clinic-based cognitive assessments appear in Table 4. There was a moderate correlation between 3MSE and TICS: r = 0.34 (p<0.001). As seen in Figure 2, across the range confined to TICS scores from 31 to 41 there was a graded positive relationship between the two measures, however there was considerable variability surrounding the regression line throughout much of this range. The correlation between TICS and the other cognitive tests ranged from r = 0.35 for the Hopkins Verbal Learning Test to r = 0.00 for the Trails A test (Table 4).
Table 4.
Correlations that TICS scores had with scores from other tests of cognitive function used in determining eligibility. Composites of domain specific and overall tests were created by averaging z-transformed scores from individual tests
| Cognitive tests | Mean (SD) | Correlation with TICS | p-value |
|---|---|---|---|
| Global cognitive function | |||
| 3MSE | 93.4 (4.4) | 0.34 | <0.001 |
| Episodic memory | |||
| Hopkins verbal learning | 6.5 (3.1) | 0.35 | <0.001 |
| Logical memory | 23.0 (6.2) | 0.29 | 0.002 |
| Composite of two tests | 0.37 | <0.001 | |
| Speed of processing and attention | |||
| Trails A | 40.5 (14.1) | 0.00 | 0.98 |
| Digit symbol coding test | 48.4 (11.0) | 0.14 | 0.16 |
| Composite of two tests | 0.03 | 0.73 | |
| Verbal fluency | |||
| Category fluency | 15.8 (4.4) | 0.06 | 0.51 |
| Letter fluency | 37.0 (12.3) | 0.21 | 0.03 |
| Composite of two tests | 0.17 | 0.06 | |
| Composite of all tests | 0.18 | 0.03 |
Figure 2.
Relationship between TICS and 3MSE scores. (Small random perturbations have been added to avoid overlapping data points.)
Of 118 individuals with TICS 31–34 who were eligible, 73 (61.9%) attended the screening visit, compared to 70 of 98 (71.4%) with TICS 35–41 (p = 0.14). Of the 44 individuals with lower TICS scores who remained eligible, 36 (81.8%) were ultimately randomized, compared to 37 of 43 (86.0%) with higher TICS scores (p = 0.59).
The ineligibility criteria and attrition combined to produce the yields that were related to TICS scores in a graded fashion. Logistic regression was used to estimate yields; these ranged from 19% for individuals with TICS scores of 31 to over 41% for individuals with TICS scores of 41.
Discussion
Behavioral interventions hold great promise as strategies to decrease cognitive decline and risk of cognitive impairment (Elias and Wagster, 2007; Acevedo and Loewenstein, 2007; Angevaren et al., 2008; Scarmeas et al., 2009). Conducting trials in older cohorts, particularly those at increased risk for age-related deficits in cognitive and physical function, faces many challenges (Ellenberg, 2004; Ferucci et al., 2004; Lebowitz, 2004). Efficient screening algorithms for identifying appropriate candidates for interventions are important to reduce costs and accelerate the pace of trials. Pilot studies are often used to identify ways in to enhance screening and recruitment approaches.
In SHARP-P, a cutpoint of 31 was chosen to include individuals within the lower end of the normal range for cognitive functioning and to rule out almost all cases of dementia (Lipton et al., 2003; Barber and Stott, 2004; Smith et al., 2009). While relieving the distress and dysfunction of demented persons is an important goal, cognitive and behavioral interventions are not well suited for these individuals because of their substantial degree of cognitive and functional impairment. Older individuals who score in the middle to low normal range on tests of global cognitive functioning are at significantly greater risk of significant cognitive decline over a 5-year period compared with persons who score higher (Espeland et al., 2006). Because the TICS cutoff would not necessarily reliably exclude participants with mild cognitive impairment (Jarvenpaa et al., 2002; van Uffelen et al., 2007), additional cognitive testing was required. Overall, 58 (16.9%) of volunteers fell below this cutpoint and were excluded from further enrollment. Had TICS not been administered, 51.7% of these 58 participants would have been excluded for other contra-indications for the interventions that were queried during the telephone interview. It is not possible for us to project accurately how many of the remaining 28 participants with TICS<31 may have attended the screening visit and met requirements for randomization. However, among those with TICS 31–41 who remained eligible after the initial telephone screen i.e., who appeared to be appropriate candidates for the interventions, TICS scores were not strongly related to eligibility rates and the rates at which eligible participants returned for additional enrollment visits. As we discuss below, the relationships that TICS scores in this range had with the clinic-based cognitive assessment tests were only moderate. Thus it is likely that many of the 28 participants who were otherwise eligible but had TICS<31 at the telephone screen may have successfully been enrolled in SHARP-P.
The performance of TICS-based instruments to identify individuals within bands of cognitive function has been variable. Graff-Radford, et al. (2006) found TICS-m to be very useful, among individuals reporting no memory problems, to screen out those with dementia or mild cognitive impairment. Some have found the performance of TICS-based instruments to identify clinical trial participants with cognitive impairment to be successful (Lines et al., 2003; van Uffelen et al., 2007), but others have not (Yaari et al., 2006). In general populations, TICS and TICS-m have been found to have correlations with 3MSE scores ranging from r = 0.44 to r = 0.94 (Brandt et al., 1988; de Jager et al., 2003; Rankin et al., 2005). Arnold, et al. (2009) report that the relationship between TICS and 3MSE is non-linear; they used a quadratic regression equation to account for 67% of the 3MSE variability. Across higher scores, the relationship is relatively flat, however the slope becomes much steeper for TICS <31. The lower correlation we found may be due to sampling only within the range of TICS scores for which the association was weakest. It may also be affected by other eligibility criteria: limiting the cohort to individuals who expressed concerns about their memory further compressed the range of TICS scores and targeting appropriate candidates for the SHARP-P interventions eliminated many individuals with strong risk factors for cognitive impairment.
Crooks, et al. (2006) report that TICS-m had modest correlations with domain-specific cognitive function, inline with what we found. Like us, they also reported that the Trails A test was essentially uncorrelated with TICS-m. TICS and TICS-m have no measures of speed of processing and, as coarse measures of global cognitive functioning, may not perform well if deficits are domain-specific.
While the TICS may not have been an efficient means to identify appropriate candidates for SHARP-P, it may serve a useful purpose in larger recruitment efforts to identify cohorts for oversampling. We found that overall recruitment yields were inversely related to TICS scores; estimates from logistic regression varied by twofold over the range adopted by SHARP-P. Compared to those with high TICS scores, individuals who scored relatively low were much less likely to meet eligibility criteria related to their suitability as candidate for physical activity and cognitive training interventions. While our sample sizes are modest, low TICS scores appeared to be associated with higher prevalence of comorbities such as congestive heart failure and chest pain. Not surprisingly lower TICS scores were also associated with conditions that might interfere with cognition and cognitive training, such as stroke, TIA, head injury, and medication use. Unexpectedly, lower TICS scores were associated with more frequent reports of regular exercise, which precluded enrollment. Whether this reflects adoption of physical activity as an attempt to combat cognitive deficits or biases in self-report is unknown. Thus, while TICS scores ≥31 were not strongly related to other measures of cognitive function within the SHARP-P cohort, they still may be useful to identify cohorts that require oversampling if a uniform distribution of cognitive function is to be achieved. Because individuals with lower TICS scores are more likely to be excluded for other criteria, it may be necessary to allocate greater resources toward recruiting them to develop a cohort of participants that is balanced across a range of cognitive function. If this approach is used, TICS should be administered after other telephone-based criteria have been established and used only to identify individuals for oversampling to enhance the full representation targeted cognitive function ranges.
Limitations
The TICS was administered to individuals interested in volunteering for a clinical trial of physical activity and cognitive training, who may not represent well other populations. The staged enrollment process limited segments of data collection to individuals eligible to proceed to successive stages. SHARP-P primarily used mailings to advertise the study—other approaches may attract cohorts with different characteristics. How our findings from the TICS generalize to its various modifications is not known. We are unable to project enrollment rates for TICS <31.
Key points.
Using low TICS scores to exclude individuals who may be inappropriate candidates for trials of physical activity and cognitive training interventions may be inefficient: many of the volunteers excluded for low TICS scores would be eliminated by other criteria and others may remain appropriate candidates.
Enrollment rates increase in a graded fashion across the range of TICS scores 31–40, so that TICS may be used to target volunteers for oversampling. When used for this purpose, TICS should be administered only to individuals once other telephone-based eligibility criteria have been confirmed.
Acknowledgements
The SHARP-P Study Group includes:
Wake Forest University: Robert Amoroso, Lee Ann Andrews; Jerry Barnes, MA; Mary Barr; Dale Dagenbach, PhD; Patricia Davis; Meredith Dobrosielski, MS; Robin Dove; Mark A. Espeland, PhD; Deborah Felton; Sarah A. Jaramillo, MS; Janine M. Jennings, PhD; Jeff A. Katula, PhD; Mark D. King; Claudine Legault, PhD; Abbie Prescott, MS; Stephen Rapp, PhD; W. Jack Rejeski, PhD; Wes Roberson; Kaycee M. Sink, MD, MAS; Shannon Sharp; Sally A. Shumaker, PhD; and Terri Windham.
University of California at San Francisco: Deborah E. Barnes, PhD, MPH
Stanford University: Victor W. Henderson, MD, MS; Abby C. King, PhD; and Marcia L. Stefanick, PhD.
The Seniors Health and Activity Research Program Pilot Study was funded by the Department of Health and Human Services, National Institutes of Health (1R01AG029285—01A1) and the General Clinical Research Center of Wake Forest University Baptist Medical Center (M01-RR07122).
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT00688155.
Conflict of interest
None known.
Publisher's Disclaimer: Copyright of International Journal of Geriatric Psychiatry is the property of John Wiley & Sons, Inc. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
References
- Acevedo A, Loewenstein DA. Nonpharmacologic cognitive interventions in aging and dementia. J Geriatr Psychiatry Neurol. 2007;20:239–249. doi: 10.1177/0891988707308808. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verharr HJJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;3:1–103. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- Arnold AM, Newman AB, Desmond N, Haan H, Fitzpatrick A. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. Neuroepidemiol. 2009;33:55–65. doi: 10.1159/000215830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M, Stott DJ. Validity of the Telephone Interview for Cognitive Status (TICS) in post-stroke subjects. Int J Geriatric Psychiatry. 2004;14:75–79. doi: 10.1002/gps.1041. [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsych. 1991;5:125–142. [Google Scholar]
- Cook SE, Mariske M, McCoy KJM. The use of the Modified Telephone Interview for Cognitive Status (TICS-m) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:103–109. doi: 10.1177/0891988708328214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks VC, Petitti DB, Robins SB, Buckwalter JG. Cognitive domains associated with performance on the Telephone Interview for Cognitive Status – modified. Am J Alzheimer’s Dis Other Demen. 2006;21:45–53. doi: 10.1177/153331750602100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dal Forno G, Chivenda P, Bressi F, et al. Use of an Italian version of the telephone interview for cognitive status in Alzheimer’s disease. Int J Geriatric Psychiatry. 2006;21:126–133. doi: 10.1002/gps.1435. [DOI] [PubMed] [Google Scholar]
- de Jager CA, Budge MM, Clarke R. Utility of the TICS-m for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18:318–324. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- Debling D, Amelan M, Hasselback P, Sturmer T. Diabetes and cognitive function in a population-based study of elderly women and men. J Diab Complications. 2006;20:238–245. doi: 10.1016/j.jdiacomp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of ginkgo biloba extract in prevention of dementia. Cont Clin Trials. 2006;27:238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Tatemichi TK, Hanzawa L. The Telephone Interview for Cognitive Status (TICS): reliability and validity in a stroke sample. Int J Geriatr Psychiatry. 1994;9:803–807. [Google Scholar]
- Duff K, Beglinger LJ, Adams WH. Validation of the Modified Telephone Interview for Cognitive Status in anmestic mild cognitive impairment and intact elders. Alzheimer Dis Assoc Disord. 2009;23:38–43. doi: 10.1097/WAD.0b013e3181802c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JW, Wagster MV. Developing context and background underlying cognitive intervention / training studies in older populations. J Gerontol. 2007;62B:5–10. doi: 10.1093/geronb/62.special_issue_1.5. [DOI] [PubMed] [Google Scholar]
- Ellenberg SS. Analytical, practical and regulatory issues in prevention studies. Statist Med. 2004;23:297–303. doi: 10.1002/sim.1717. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Robertson J, et al. Modified Mini-Mental State Examinations in two-stage study designs: results from the Women’s Health Initiative Memory Study. Clin Trials. 2006;3:99–106. doi: 10.1191/1740774506cn140oa. [DOI] [PubMed] [Google Scholar]
- Ferucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR, Ferman TJ, Lucas JA, et al. A cost effective method of identifying and recruiting persons over 80 free of dementia or mild cognitive impairment. Alzheimer Dis Assoc Disord. 2006;20:101–104. doi: 10.1097/01.wad.0000213813.35424.d2. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Chen J, Wilson RS, Manson JE. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diab Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- Hill J, McVay JM, Walter-Ginzburg A, et al. Dis Management. 2005;8:223–234. doi: 10.1089/dis.2005.8.223. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Hart J, Henderson VW. Telephone word-list recall tested in the Rural Aging and Memory Study: two parallel versions for the TICS-m. Int J Geriatr Psychiatry. 2004;19:875–880. doi: 10.1002/gps.1170. [DOI] [PubMed] [Google Scholar]
- Jarvenpaa T, Rinne J, Raiha I, et al. Characteristics of two telephone screens for cognitive impairment. Dement Geriatr Cog Disord. 2002;13:149–155. doi: 10.1159/000048646. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Webster LM, Kleykamp BA, et al. Recollection training and transfer effects in older adults: successful use of a repetition-lag procedure. Aging Neuropsychol C. 2005;12:278–298. doi: 10.1080/138255890968312. [DOI] [PubMed] [Google Scholar]
- Kiddoe JM, Whitfield KE, Andel R, Edwards CL. Evaluatng brief cognitive screening instruments among African-Americans. Aging Ment Health. 2008;12:488–493. doi: 10.1080/13607860802224383. [DOI] [PubMed] [Google Scholar]
- King JT, DiLuna ML, Cicchetti DV, Tsevat J, Roberts MS. Cognitive functioning in patients with cerebral aneurysms measures with the Mini Mental State Examination and the telephonce interview for cognitive status. Neurosurgery. 2006;59:803–811. doi: 10.1227/01.NEU.0000232666.67779.41. [DOI] [PubMed] [Google Scholar]
- Lebowitz BD. Clinical trials in late life: new science in old paradigms. Gerontologist. 2004;44:452–458. doi: 10.1093/geront/44.4.452. [DOI] [PubMed] [Google Scholar]
- Lines CR, McCarroll KA, Lipton RB, Block GA. Telephone screening for amnestic mild cognitive impairment. Neurology. 2003;60:261–266. doi: 10.1212/01.wnl.0000042481.34899.13. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Katz MJ, Kuslanky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- Moylan T, Das K, Gibb A, et al. Assessment of cognitive function in older hospital inpatients: is the Telephone Interview for Cognitive Function (TICS-m) a useful alternative to the MiniMental State Examination? Int J Geriatr Psychiatry. 2004;19:1008–1019. doi: 10.1002/gps.1181. [DOI] [PubMed] [Google Scholar]
- O’Dwyer ST, Burton NW, Pachana NA, Brown WJ. Protocol for the fit bodies, fit minds: a randomized controlled trial on the affect of exercise and cognitive training on cognitive functioning in older adults. BMC Geriatr. 2007;7:23. doi: 10.1186/1471-2318-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti DB, Buckwalter G, Crooks VC, Shui V. Prevlance of dementia in users of hormone replacement therapy as defined by prescription data. J Gerontology. 2002;57A:M532–M538. doi: 10.1093/gerona/57.8.m532. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Saykin AJ, Wishart HA, et al. The Memory and Aging Telephone Screen (MAATS): development and preliminary validation. Alzheimers Dement. 2007;3:109–121. doi: 10.1016/j.jalz.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin MW, Clemons TE, McBee WL. Correlation analysis of the in-clinic and telephone batteries from the AREDS Cognitive Function Ancillary Study. Ophthalmic Epidemiol. 2005;12:271–277. doi: 10.1080/09286580591003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Mayeaux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Tremont G, Ott BR. A review of telephone-administered screening tests for dementia diagnosis. Am J Alzheimer’s Dis Other Dementias. 2009;24:58–69. doi: 10.1177/1533317508327586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd Oxford Press; New York: 2006. [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- van Uffelen JGZ, Chinapaw MJM, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults. A randomised controlled trial. Br J Sports Med. 2008;42:344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- van Uffelen JGZ, Pa MJ, Kelin M, van Mechelen W, Hopman-Rock M. Detection of memory impairment in the general population: screening by questionnaire and telephone compared to face-to-face assessment. Int J Geriatr Psychiatry. 2007;22:203–210. doi: 10.1002/gps.1661. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Adult Intelligence Scale-III (WAIS-III) Psychological Corporation, Harcourt, Inc; New York: 1996. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale-3rd Edition (WHM-III) Psychological Corporation, Harcourt, Inc; San Antonio: 1997. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Xiong GL, Plassman BL, Helms MJ, Steffens DC. Vascular risk factors and cognitive decline among elderly male twins. Neurology. 2006;67:1586–1591. doi: 10.1212/01.wnl.0000242730.44003.1d. [DOI] [PubMed] [Google Scholar]
- Yaari R, Fleisher AS, Gamst AC, Bagwell VP, Thal LJ. Use of the Telephone Interview for Cognitive Status for enrollment in clinical trials. Alzheimer’s Dement. 2006;2:104–109. doi: 10.1016/j.jalz.2006.02.004. [DOI] [PubMed] [Google Scholar]