Abstract
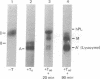
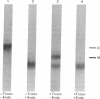
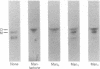
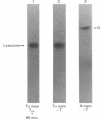
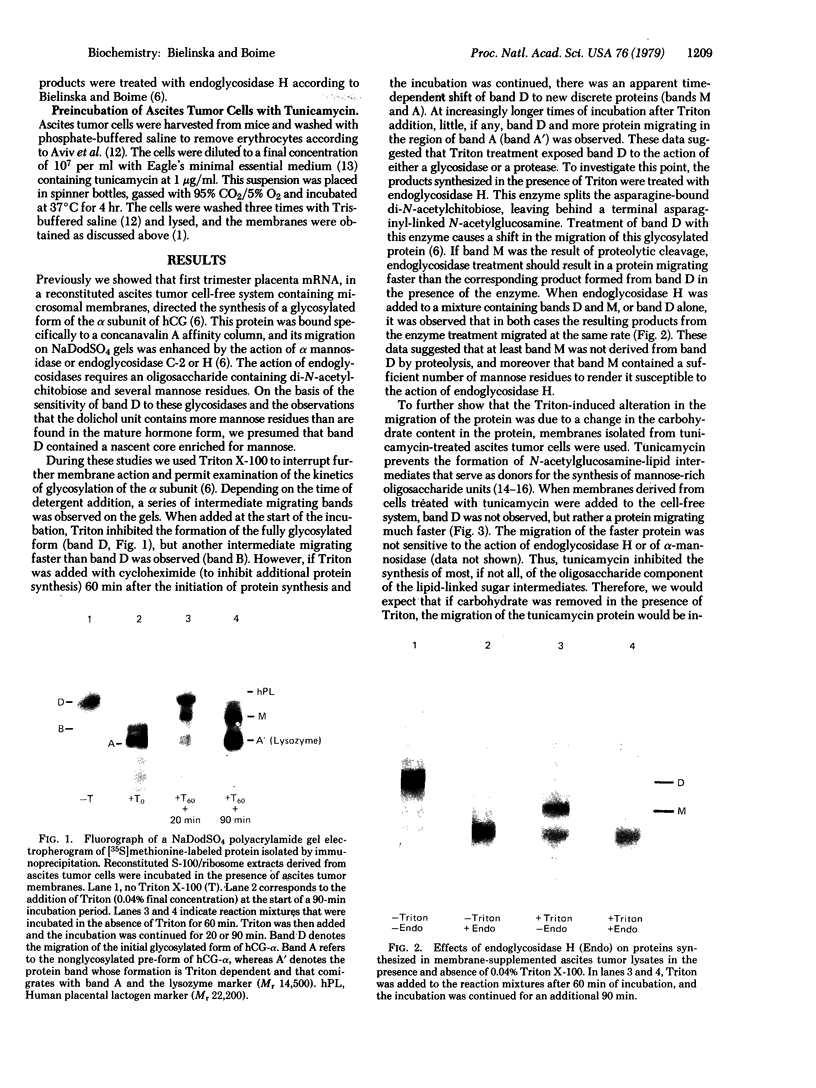
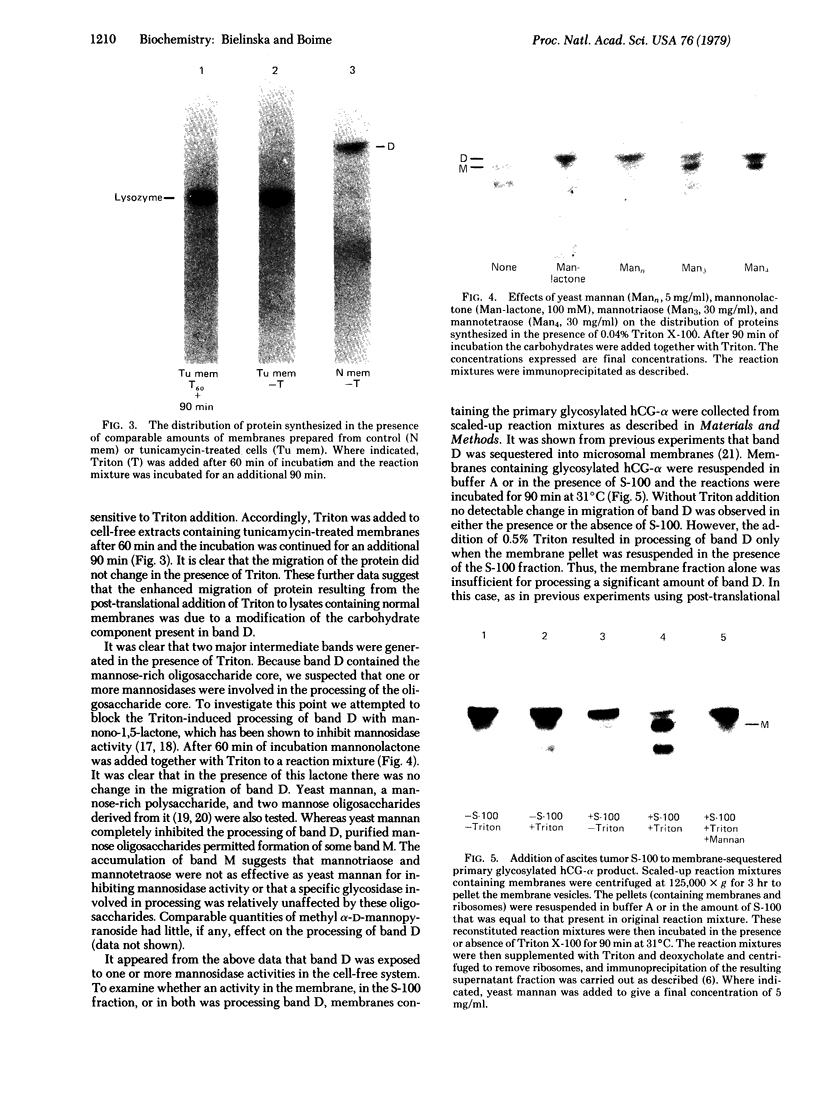
Placental RNA has previously been shown to direct the synthesis of an asparagine-linked mannose-rich glycosylated form of the α subunit of human chorionic gonadotropin (hCG-α) in lysates derived from mouse ascites tumor cells. Glycosylation was dependent on the presence of homologous microsomal membranes, and the glycosylated protein was sequestered into the microsomal vesicles. Here we show that when Triton X-100 is added after 60 min of translation and the incubation is continued, there is a shift of this glycosylated form to new discrete lower molecular weight proteins. The formation of these new proteins was not the apparent result of proteolysis because (i) treatment of the fully glycosylated protein or the proteins formed in the presence of Triton with endoglycosidase H resulted in the formation of a single protein migrating at the same rate on sodium dodecyl sulfate gels; (ii) the migration of nonglycosylated hCG-α synthesized in the presence of membranes isolated from tunicamycin-pretreated ascites tumor cells was not changed upon Triton addition; and (iii) the Triton-induced change was inhibited by mannonolactone, yeast mannan, and purified mannose oligosaccharides. It was also shown that little processing of the mannose-rich glycoprotein occurred in the presence of microsomal membranes alone. However, addition of the ribosome-free supernatant fraction to the glycoprotein resulted in processing. These data suggest that processing of the oligosaccharide core is a compartmentalized process in which removal of sugar, presumably mannose, requires a transfer of the glycoprotein from the endoplasmic reticulum to another component of the secretory cascade.
Keywords: carbohydrate processing in vitro, glycoprotein hormone, cell-free protein synthesis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M., Boime I. mRNA-dependent synthesis of a glycosylated subunit of human chorionic gonadotropin in cell-free extracts derived from ascites tumor cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1768–1772. doi: 10.1073/pnas.75.4.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M., Grant G. A., Boime I. Processing of placental peptide hormones synthesized in lysates containing membranes derived from tunicamycin-treated ascites tumor cells. J Biol Chem. 1978 Oct 25;253(20):7117–7119. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. W., Lennarz W. J. Enzymatic excision of glucosyl units linked to the oligosaccharide chains of glycoproteins. J Biol Chem. 1978 Aug 25;253(16):5780–5785. [PubMed] [Google Scholar]
- Chen W. W., Lennarz W. J. Enzymatic synthesis of a glucose-containing oligosaccharide-lipid involved in glycosylation of proteins. J Biol Chem. 1978 Aug 25;253(16):5774–5779. [PubMed] [Google Scholar]
- Daniels-McQueen S., McWillians D., Birken S., Canfield R., Landefeld T., Boime I. Identification of mRNAs encoding the alpha and beta subunits of human choriogonadotropin. J Biol Chem. 1978 Oct 10;253(19):7109–7114. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely M. L., McKnight G. S., Schimke R. T. Studies on the attachment of carbohydrate to ovalbumin nascent chains in hen oviduct. J Biol Chem. 1976 Sep 25;251(18):5490–5495. [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Krag S. S., Cifone M., Robbins P. W., Baker R. M. Reduced synthesis of [14C]mannosyl oligosaccharide-lipid by membranes prepared from concanavalin A-resistant Chinese hamster ovary cells. J Biol Chem. 1977 May 25;252(10):3561–3564. [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Synthesis and glycosylation in vitro of glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1516–1520. doi: 10.1073/pnas.74.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Opheim D. J., Touster O. Purification and characterization of alpha-D-mannosidase from rat liver golgi membranes. J Biol Chem. 1977 May 25;252(10):3227–3233. [PubMed] [Google Scholar]
- Turco S. J., Stetson B., Robbins P. W. Comparative rates of transfer of lipid-linked oligosaccharides to endogenous glycoprotein acceptors in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4411–4414. doi: 10.1073/pnas.74.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Action of glycosidases on the saccharide moiety of the glucose--containing dolichyl diphosphate oligosaccharide. FEBS Lett. 1978 Jul 15;91(2):209–212. doi: 10.1016/0014-5793(78)81174-0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Ballou C. E. Partial characterization of the sexual agglutination factor from Hansenula wingei Y-2340 type 5 cells. Biochemistry. 1974 May 21;13(11):2428–2437. doi: 10.1021/bi00708a030. [DOI] [PubMed] [Google Scholar]