Abstract
Objective
Previous studies have reported that the prevalence of exercise-induced bronchoconstriction (EIB) in athletes is higher than that of the general population. There is increasing evidence that athletes fail to recognize and report symptoms of EIB. As a result, there has been debate whether athletes should be screened for EIB, particularly in high-risk sports.
Methods
We prospectively studied 144 athletes from six different varsity sports at a large National Collegiate Athletic Association Division I collegiate athletic program. Baseline demographics and medical history were obtained and the presence of asthma symptoms during exercise was documented. Each athlete subsequently underwent a eucapnic voluntary hyperventilation (EVH) test to document the presence of EIB. Exhaled nitric oxide (eNO) quantification was performed immediately before EVH testing. EIB was defined as a ≥10% decline in forced expiratory volume in 1 second compared with baseline.
Results
Only 4 of 144 (2.7%) athletes were EIB-positive after EVH testing. The presence of symptoms was not predictive of EIB as only 2 of the 64 symptomatic athletes (3%) were EIB-positive based on EVH testing. Two of the four athletes who were found to be EIB-positive denied such symptoms. The mean baseline eNO in the four EIB-positive athletes was 13.25 parts per billion (ppb) and 24.5 ppb in the EIB-negative athletes.
Conclusions
Our data argue that screening for EIB is not recommended given the surprisingly low prevalence of EIB in the population we studied. In addition, the presence or absence of symptoms was not predictive of EIB and eNO testing was not effective in predicting EIB.
Keywords: asthma, athletes, bronchoconstriction, exercise, screening
Introduction
Exercise-induced bronchoconstriction (EIB) describes airway narrowing that occurs in association with exercise. Exercise is one of the most common triggers of broncho-constriction and also occurs in approximately 10% of the general population who do not have a known history of chronic asthma (1, 2). Previous studies have reported that the prevalence of EIB in athletes is significantly higher than that of the general population (2–4). In addition, there is increasing evidence that athletes fail to recognize and report symptoms of EIB (5, 6). Based on these and more recent data, some authors advocate for routine screening of athletes for EIB (7).
We have published previous data demonstrating a high prevalence of undiagnosed EIB documented by eucapnic voluntary hyperventilation (EVH) testing in a cross section of college athletes from a large Division I athletic program (5). Our prior study was limited by the fact that we tested a cross section of the entire athletic department rather than specific teams; hence, despite the high prevalence of EIB, we were unable to draw reliable conclusions regarding which sports, if any, may benefit from routine screening for EIB. The goal of this study was to prospectively screen a specific group of athletic teams in a large Division I collegiate athletic program for EIB using EVH testing.
Methods
Potential participants in the study were members of six select male and female varsity athletic teams (Men’s and Women’s: Soccer, Lacrosse, and Ice Hockey) at The Ohio State University. Exclusion criteria included pregnancy, recent upper respiratory tract infection (within 2 weeks of study enrollment), or a history of >10 pack-years of tobacco use (calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked). History of asthma, defined as self-reported, prior diagnosis made by a physician, was not an exclusion criteria unless the participant was found to have a current forced expiratory volume in 1 second (FEV1) of <70% of the predicted value on baseline spirometry. Such a finding would identify baseline airway obstruction and possibly poor asthma control as well as increase the risk of further bronchoprovocation. Athletes were presented the proposal in a short oral presentation and then given the opportunity to participate in the trial. All participation was voluntary and written informed consent was obtained from every participant prior to enrollment. The study received the approval of the Institutional Review Board at The Ohio State University and the National Collegiate Athletic Association Compliance Office.
Participation in the study required one visit. At the study visit, athletes underwent baseline spirometry following American Thoracic Society (ATS) guidelines (8) followed by exhaled nitric oxide (eNO) quantification as per ATS guidelines (9). EVH testing was performed after eNO measurement per protocol outlined by Anderson et al. (10). Prior to testing, athletes completed both a baseline medical history questionnaire and an additional questionnaire addressing respiratory symptoms (e.g., wheezing, dyspnea, and chest pain) during exercise designed by the US Olympic Committee Sports Medicine Division (11).
Prior to objective lung function testing, all asthma medications were withheld as per standard clinical protocol. Restrictions included no short-acting bronchodilators for 8 hours prior, no long-acting bronchodilators for 48 hours prior, no leukotriene antagonists or inhaled steroids for 4 days prior, no caffeine or chocolate for 8 hours prior, and no vigorous exercise on the day of the test. Each athlete performed baseline spirometry according to ATS standards. eNO was then measured by chemoluminescence using an online nitric oxide monitor (Apieron, Inc., Menlo Park, CA, USA) according to the ATS guidelines published in 2005 (9). Seated subjects exhaled fully then inhaled ambient air through a nitric oxide scrubber to total lung capacity. Repeated exhalations were performed a maximum of six times to obtain three acceptable measurements that agreed within 10%. The average of these three measurements was used.
EVH bronchoprovocation challenge testing was then performed after spirometry and eNO. Participants breathed a mixture of dry compressed gas (5.0% CO2, 21.0% O2, and balance N2) at a target rate of 85% of their maximum voluntary ventilation (MVV) per minute (calculated as 30 times the baseline FEV1, which approximates 85% MVV) for 6 minutes. Gas was channeled from a cylinder into a calibrated rotameter (Brooks Model 1307, Brooks Instrument, Inc. Hatfield, PA, USA) and then through an inspiratory target balloon (medical research bladder 100 MRL) that was maintained half full (to ensure correct minute ventilation) and then to a two-way, low-resistance valve (Hans Rudolph; Model 2700 2-Way Non-Rebreathing Valve, Hans Rudolph, Inc, Shawnee, KS, USA) and mouthpiece (Medical Graphics #758301-001, Medical Graphics Corporation, St. Paul, MN, USA) for exercise. Ventilation was quantified using a flow sensor manufactured by SensorMedics, Inc. Spirometry was performed at 3, 5, 10, 15, and 20 minutes after the EVH challenge. EIB was defined by a fall ≥10% in FEV1 post-EVH testing. EVH testing was considered adequate if the MVV achieved was ≥60% of predicted MVV (10).
Results
Of the possible 185 (78%) athletes, 144 completed the study. Overall, participants were young, healthy, free of comorbidities, and predominantly Caucasian (Table 1). No athlete who consented for participation was excluded on the basis of any of the exclusion criteria. All athletes had normal baseline resting spirometry without any evidence of airway obstruction. Of the 144 (24%) athletes, 35 stated they had a history of either asthma or EIB prior to enrolling in the study, but none were excluded given the fact all had baseline FEV1 >70%. Only 14 of these 35 (40%) athletes had undergone spirometry previously and none had undergone formal bronchoprovocation testing specifically for EIB. Of the 35 patients with history of asthma or EIB, only 7 were currently prescribed a short-acting bronchodilator and only 3 were on any class of asthma controller medication.
Table 1.
Athlete demographics (n = 144).
| Age in years, mean (range) | 20 (18–23) |
| Male | 55% |
| Caucasian | 93% |
| Average FEV1/FVC baseline | 84% |
| Self-reported history of asthma or EIB | 35 of 144 (24%) |
| Undergone spirometry prior to study | 14 of 35 (40%) |
| Undergone bronchoprovocation testing prior to study | 0 of 35 (0%) |
| Presence of gastroesophageal reflux disease | <1% |
| Presence of seasonal allergies | <1% |
| Presence of eczema | <1% |
Note: EIB, exercise-induced bronchoconstriction; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
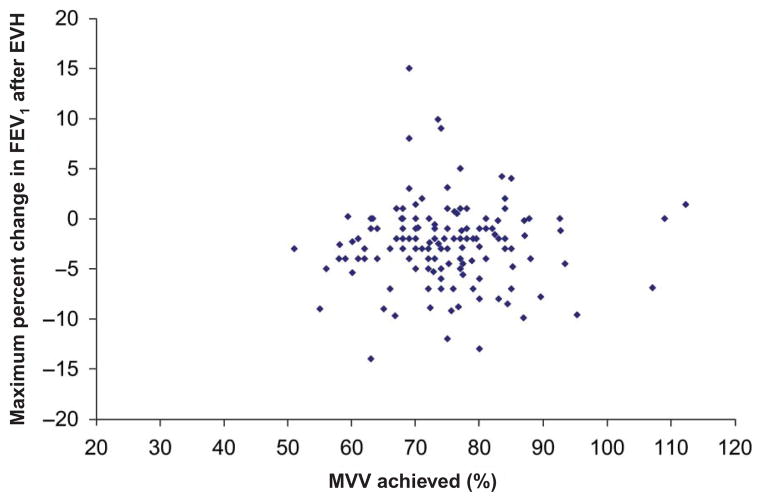
Based on the results of EVH testing, only 4 of the 144 (2.7%) athletes were EIB-positive (Table 2). All four EIB-positive athletes were female. Of the 144 (95%) athletes, 137 achieved ≥60% of predicted MVV during EVH testing (mean 74.5%, ±9.9%). Only 7 of the 144 athletes failed to meet the minimum MVV threshold for an adequate EVH test of 60% of predicted MVV. There were no significant differences between the athletes who were able to meet the 60% MVV threshold and the seven who did not. Figure 1 shows maximum MVV achieved during EVH plotted against maximum decline in FEV1 post-EVH (note many athletes actually bronchodilated after EVH and hence the smallest increase in FEV1 was used in lieu of a decline in FEV1).
Table 2.
Level of participation and prevalence of exercise-induced bronchoconstriction (EIB).
| Team | Number of athletes on team | Number of athletes who participated in study | Percentage of participation (%) | Number of EIB-positive athletes | Percentage of EIB-positive athletes (%) |
|---|---|---|---|---|---|
| Women’s Ice Hockey | 23 | 23 | 100 | 1 | 4.3 |
| Men’s Ice Hockey | 28 | 26 | 93 | 0 | 0 |
| Women’s Soccer | 27 | 17 | 63 | 1 | 5.8 |
| Men’s Soccer | 27 | 19 | 70 | 0 | 0 |
| Women’s Lacrosse | 32 | 23 | 72 | 2 | 8.7 |
| Men’s Lacrosse | 48 | 36 | 75 | 0 | 0 |
| Total | 185 | 144 | 78 | 4 | 2.7 |
Figure 1.
Decline in forced expiratory volume in 1 second (FEV1) post-eucapnic voluntary hyperventilation (EVH) testing plotted against percentage of maximum voluntary ventilation (MVV) achieved during EVH testing. Note: Many athletes actually bronchodilated after eucapnic voluntary hyperventilation (EVH) and hence the smallest increase in FEV1 was used in lieu of a decline in FEV1.
The average maximum decline in FEV1 for the four EIB-positive athletes was 12.7% (range 10–14%). Of the 144 (44%) athletes, 64 stated they experienced chest tightness, shortness of breath, or wheezing provoked by exercise periodically.
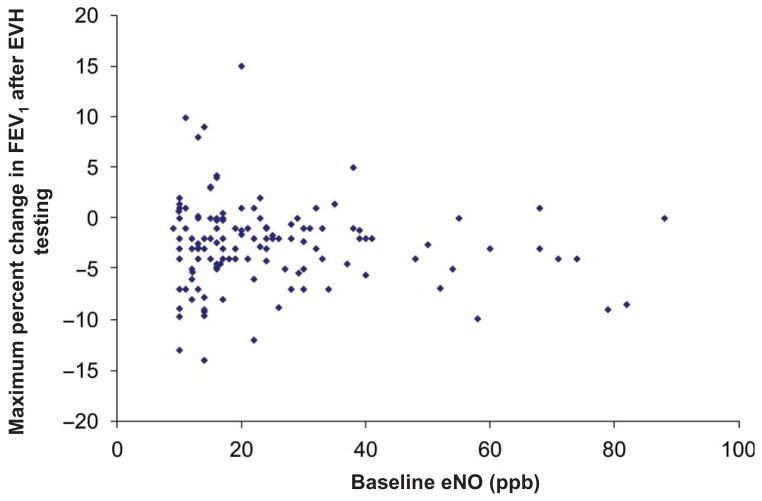
eNO was not predictive of EIB. In the four EIB-positive athletes, the mean baseline eNO was 13.25 ± 2.5 parts per billion (ppb) (range 10–16 ppb). The EIB-negative athletes had a mean baseline eNO of 25.50 ± 16.23 ppb (range 9–88 ppb). Figure 2 shows eNO plotted against maximum decline in FEV1 post-EVH (once again, where applicable, the smallest increase in FEV1 was used in lieu of a decline in FEV1).
Figure 2.
Decline in forced expiratory volume in 1 second (FEV1) post-eucapnic voluntary hyperventilation (EVH) testing plotted against baseline exhaled nitric oxide (eNO).
Note: Many athletes actually bronchodilated after EVH and hence the smallest increase in FEV1 was used in lieu of a decline in FEV1.
The presence of symptoms did not predict objectively confirmed EIB, as only 2 of 64 symptomatic athletes (3%) were found to be EIB-positive based on EVH testing. In addition, only 2 of 21 (5%) athletes stated that they had EIB, but had never undergone any type of lung function testing prior to enrolling in this study, and were EIB-positive after EVH testing. Comparison of EIB-positive athletes with EIB-negative athletes was not statistically reasonable given the low number of EIB-positive athletes.
Discussion
Previous studies, including from our group, have demonstrated a high prevalence of undiagnosed EIB documented by EVH testing in athletes (5, 7, 12–14). It is also clear that many athletes are unaware they have EIB as respiratory symptoms with exercise have poor specificity and sensitivity for this problem. Because symptoms alone have a very low positive predictive value for EIB, and previous studies have demonstrated a substantial burden of undiagnosed EIB (5, 7), some have advocated that populations of athletes should be screened with objective testing (7). The goal of this study was to determine whether all athletes, irrespective of symptoms, on select athletic teams in a collegiate athletic program should be screened for EIB.
Recently, Dickinson et al. (7) studied 228 elite athletes and performed EVH testing to determine the prevalence of EIB. The study found 34% of the cohort were EIB-positive and that 73% of those EIB-positive athletes had never been diagnosed with EIB. Based on the results of their study, Dickinson et al. proposed that elite athletes should be screened routinely for EIB using a suitable bronchoprovocation challenge. Surprisingly, in this study, despite the fact that we used the same bronchoprovocation technique as in the Dickinson study, we found a very low prevalence of EIB (2.7%) on the six collegiate athletic teams screened. Only seven athletes did not reach the minimum accepted threshold of MVV for EVH testing to be considered adequate (60% of predicted MVV) (10).
It is unclear why our data differ so greatly from the Dickinson et al. study, but some differences in the populations studied are apparent. Their study involved elite athletes versus our study of collegiate athletes. Furthermore, we tested athletes in different sports than they did, although we did choose sports that have high ventilatory requirements and that have been suggested to be more prone to have EIB (7, 15, 16). The two populations of athletes were similar in age and size, but athletes in their study had a much higher prevalence of self-reported atopy/ allergies, which has been implicated as an independent risk factor for EIB (17). The differences between the two populations could account, in part, for the widely discrepant findings and raise significant questions about the value of large-scale screening among populations of athletes. In addition, data from this study and our previous study (5) argue traditional stratification of high versus low ventilation sports does not seem to be of value in terms of efficacy of EIB screening.
Hull et al. (18) specifically examined the issue of screening athletes for EIB and concluded there are substantial barriers that would likely make large-scale screening difficult. From an epidemiological perspective, screening for any disease requires the disease be well understood and clearly defined, the prevalence of the disease be well known, and there should be a gold standard diagnostic technique with well-defined diagnostic thresholds. It would be reasonable to state that none of the above applies to EIB, at least in the populations studied herein.
Studies examining the prevalence of EIB in athletes have demonstrated prevalence widely ranging between approximately 10% and 50% (2–4). As discussed, our prevalence of EIB in this study is vastly different than Dickinson et al. We even found vast differences in the prevalence of EIB in this study compared with our previous data 5 years ago using identical testing methods on a similar athletic population (3% vs. 39%) (5). The reasons for the differences in our two studies are unclear. It may simply be that the incidence rate of EIB, although likely substantial overall, can vary widely from year to year. Whatever the true explanations for the differences are, the variable prevalence of EIB in our own data and in the literature overall suggest that widespread screening for EIB may not be of value.
In addition to the variable prevalence of EIB in different studies, the multiple potential testing methods available for diagnosis of EIB further complicate screening for EIB. Multiple bronchoprovocation techniques including traditional field-based exercise testing, laboratory-based exercise testing, EVH testing, and mannitol inhalation are all widely used both clinically and in research settings. Although each test alone is acceptable for diagnosing EIB, sensitivities, specificities, and predictive values differ among the tests. In addition, there remains a controversy on the appropriate threshold for making a diagnosis of EIB clinically and in research trials, with thresholds of 10–25% being used in different studies (19). Taken together, the lack of consensus about testing methods and diagnostic thresholds combined with the broad range of prevalence of EIB across different athletic populations argue that screening for EIB on a broad scale at the present time is unlikely to be successful.
In contrast to the variability in prevalence of EIB in athletic populations observed in different studies, one theme that has been consistently demonstrated is that the presence of exercise-induced respiratory symptoms is a very poor predictor of objectively confirmed EIB (5, 6, 20). In our previous study (5), the prevalence of EIB was 36% in athletes who denied exercise-induced symptoms and 35% in those who complained of symptoms. These data suggested that there was a large group of athletes that were EIB-positive but lacked awareness of their condition and a similar group that complained of symptoms provoked by exercise, but the symptoms were not a result of EIB. Although we did not find a high prevalence of EIB in this study, our current data continue to strongly support that symptoms alone are not of diagnostic value. Almost half of our cohort stated they were symptomatic periodically during exercise, but only two athletes who were symptomatic during exercise actually had EIB documented by EVH testing. Furthermore, only 2 of the 21 athletes who had been diagnosed with EIB previously based on symptoms alone were actually found to have EIB currently. Our findings continue to underscore the importance of objective confirmation of EIB that is suspected based on history alone and again demonstrate that diagnosis of EIB based on history is extremely poor in accurately making a diagnosis. These data have particular clinical relevance because physicians commonly diagnose and treat suspected EIB empirically according to symptoms alone, without objective testing, which may result in many inaccurate diagnoses of EIB, particularly in athletes (21).
eNO quantification has been used as a diagnostic adjunct in the management of asthma (22), but the role of eNO in EIB is not well established. Data are conflicting with eNO levels reported to both decrease (23) and increase (24) after exercise in asthmatic patients with known EIB. Others have suggested that low pre-exercise eNO levels (<12 ppb) have significant negative predictive value and may obviate the need for bronchoprovocation testing in patients who complain of exertional dyspnea (25). Our data do not support eNO as a useful diagnostic tool for EIB. None of the EIB-positive athletes in our study had elevated baseline eNO levels (≥25 ppb) (22) Furthermore, several of the EIB-negative athletes had markedly elevated eNO levels of greater than 70 ppb. The very poor correlation between eNO levels and objectively documented EIB suggests that eNO should not be used in lieu of bronchoprovocation testing in the diagnostic workup of exertional dyspnea.
Our study has limitations. Although we found a very low prevalence of EIB in teams that we tested, it is possible that other sports in our athletic program had a higher prevalence of EIB and our data are not representative of the entire athletic program as a whole. It is possible, as our data in this study suggest, that the prevalence of EIB in individual teams likely varies significantly year to year, which may explain in part why we did not see a high prevalence of EIB in this study, but did in past studies (5). Our testing methods, study personnel, and study location (laboratory based) were identical to our previous study. It is unlikely that seasonal influences (e.g., pollens/humidity/temperature) influenced the results of the current or previous study (5) as both studies were conducted over several months; hence, a confounding effect of one single variable seems implausible.
In addition, despite our goal of 100% participation of the athletes in the selected teams, we did not achieve that benchmark in all the teams. Accordingly, it is possible that the reported results may not reflect the true prevalence of EIB in the teams with <100% participation. It is possible that some athletes who did not participate avoided the study due to asthma symptoms, which would create a potential selection bias.
Additionally, although all four EIB-positive athletes were female, we were unable to further investigate any potential sex differences related to the presence of EIB in athletes, because our sample size of EIB-positive athletes was so small that it prevented statistically robust comparisons. Our data suggest screening all athletes for EIB may not be useful and there is a great need for further studies to determine if there are other factors (demographics, race, sex, comorbidities, etc.) that may indicate which athletes should be screened. In addition, future studies of college athletes should aim to include all athletes in teams being studied to avoid selection bias, although logistically and economically this may prove to be very difficult.
Conclusions
Our data argue that screening for EIB is not recommended given the surprisingly low prevalence of EIB in our population. In addition, the presence of symptoms is not predictive of EIB as virtually all of the athletes who complained of exercise-induced respiratory symptoms were EIB-negative. eNO quantification does not appear to be of diagnostic value in the workup of EIB. True prevalence of EIB may vary widely between groups of athletes over time, which would make screening an ineffective strategy to implement widely. Given the previously documented high prevalence of EIB in select athletes independent of symptoms, there remains a significant need for further studies to better determine which athletes should undergo objective testing for EIB.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Gotshall RW. Exercise-induced bronchoconstriction. Drugs. 2002;62 (12):1725–1739. doi: 10.2165/00003495-200262120-00003. [DOI] [PubMed] [Google Scholar]
- 2.Weiler JM, Bonini S, Coifman R, Craig T, Delgado L, Capão-Filipe M, Passali D, Randolph C, Storms W. American Academy of Allergy, Asthma & Immunology Work Group Report: Exercise-induced asthma. J Allergy Clin Immunol. 2007;119(6):1349–1358. doi: 10.1016/j.jaci.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128(6):3966–3974. doi: 10.1378/chest.128.6.3966. [DOI] [PubMed] [Google Scholar]
- 4.Rundell KW, Jenkinson DM. Exercise-induced bronchospasm in the elite athlete. Sports Med. 2002;32(9):583–600. doi: 10.2165/00007256-200232090-00004. [DOI] [PubMed] [Google Scholar]
- 5.Parsons JP, Kaeding C, Phillips G, Jarjoura D, Wadley G, Mastronarde JG. Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med Sci Sports Exerc. 2007;39(9):1487–1492. doi: 10.1249/mss.0b013e3180986e45. [DOI] [PubMed] [Google Scholar]
- 6.Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33(2):208–213. doi: 10.1097/00005768-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson J, McConnell A, Whyte G. Diagnosis of exercise-induced bronchoconstriction: eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise-induced bronchoconstriction. Br J Sports Med. 2011 Nov;45(14):1126–31. doi: 10.1136/bjsm.2010.072520. [DOI] [PubMed] [Google Scholar]
- 8.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 9.Silkoff PE, Erzurum SC, Lundberg JO, George SC, Marczin N, Hunt JF, Effros R, Horvath I American Thoracic Society; HOC Subcommittee of the Assembly on Allergy, Immunology, and Inflammation. ATS workshop proceedings: exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate. Proc Am Thorac Soc. 2006;3 (2):131–145. doi: 10.1513/pats.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SD, Argyros GJ, Magnussen H, Holzer K. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br J Sports Med. 2001;35(5):344–347. doi: 10.1136/bjsm.35.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiler JM, Ryan EJ., 3rd Asthma in United States Olympic athletes who participated in the 1998 olympic winter games. J Allergy Clin Immunol. 2000;106(2):267–271. doi: 10.1067/mai.2000.108605. [DOI] [PubMed] [Google Scholar]
- 12.Mannix ET, Roberts M, Fagin DP, Reid B, Farber MO. The prevalence of airways hyperresponsiveness in members of an exercise training facility. J Asthma. 2003;40(4):349–355. doi: 10.1081/jas-120018634. [DOI] [PubMed] [Google Scholar]
- 13.Mannix ET, Roberts MA, Dukes HJ, Magnes CJ, Farber MO. Airways hyperresponsiveness in high school athletes. J Asthma. 2004;41(5):567–574. doi: 10.1081/jas-120037658. [DOI] [PubMed] [Google Scholar]
- 14.Holzer K, Anderson SD, Douglass J. Exercise in elite summer athletes: challenges for diagnosis. J Allergy Clin Immunol. 2002;110(3):374–380. doi: 10.1067/mai.2002.127784. [DOI] [PubMed] [Google Scholar]
- 15.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122(2):225–235. doi: 10.1016/j.jaci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000;161(6):2086–2091. doi: 10.1164/ajrccm.161.6.9907025. [DOI] [PubMed] [Google Scholar]
- 17.Koh YI, Choi IS, Lim H. Atopy may be related to exercise-induced bronchospasm in asthma. Clin Exp Allergy. 2002;32(4):532–536. doi: 10.1046/j.0954-7894.2002.01353.x. [DOI] [PubMed] [Google Scholar]
- 18.Hull JH, Ansley L, Garrod R, Dickinson JW. Exercise-induced bronchoconstriction in athletes—should we screen? Med Sci Sports Exerc. 2007;39(12):2117–2124. doi: 10.1249/mss.0b013e3181578db2. [DOI] [PubMed] [Google Scholar]
- 19.Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122(2):238–246. doi: 10.1016/j.jaci.2008.06.014. quiz 47–8. [DOI] [PubMed] [Google Scholar]
- 20.Hallstrand TS, Curtis JR, Koepsell TD, Martin DP, Schoene RB, Sullivan SD, Yorioka GN, Aitken ML. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141(3):343–348. doi: 10.1067/mpd.2002.125729. [DOI] [PubMed] [Google Scholar]
- 21.Parsons JP, O’Brien JM, Lucarelli MR, Mastronarde JG. Differences in the evaluation and management of exercise-induced bronchospasm between family physicians and pulmonologists. J Asthma. 2006;43 (5):379–384. doi: 10.1080/02770900600709880. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, Zamel N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138 (3):682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 23.Terada A, Fujisawa T, Togashi K, Miyazaki T, Katsumata H, Atsuta J, Iguchi K, Kamiya H, Togari H. Exhaled nitric oxide decreases during exercise-induced bronchoconstriction in children with asthma. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1879–1884. doi: 10.1164/ajrccm.164.10.2009105. [DOI] [PubMed] [Google Scholar]
- 24.Scollo M, Zanconato S, Ongaro R, Zaramella C, Zacchello F, Baraldi E. Exhaled nitric oxide and exercise-induced bronchoconstriction in asthmatic children. Am J Respir Crit Care Med. 2000;161 (3 Pt 1):1047–1050. doi: 10.1164/ajrccm.161.3.9905043. [DOI] [PubMed] [Google Scholar]
- 25.ElHalawani SM, Ly NT, Mahon RT, Amundson DE. Exhaled nitric oxide as a predictor of exercise-induced bronchoconstriction. Chest. 2003;124(2):639–643. doi: 10.1378/chest.124.2.639. [DOI] [PubMed] [Google Scholar]