Abstract
Objectives
We previously reported that adult patients with GH deficiency (GHD) due to a confirmed or likely pituitary defect, as compared to healthy controls individually matched for age, gender and BMI, have more slow-wave sleep (SWS) and higher delta activity (a marker of SWS intensity). Here we examined the impact of recombinant human GH (rhGH) therapy, compared to placebo, on objective sleep quality in a subset of patients from the same cohort.
Design
Single-blind randomized cross-over design study.
Methods
Fourteen patients with untreated GHD of confirmed or likely pituitary origin, aged 22–74 yr, participated in the study. Patients with associated hormonal deficiencies were on appropriate replacement therapy. Polygraphic sleep recordings, with bedtimes individually tailored to habitual sleep times, were performed after 4 months on rhGH or placebo.
Results
Valid data were obtained in 13 patients. At the end of rhGH treatment period, patients had a shorter sleep period time than at the end of the placebo period (479±11 vs 431±19 min respectively; p=0.005), primarily due to an earlier wake up time, and a decrease in the intensity of SWS (delta activity) (559±125 vs 794±219 μV2, respectively; p=0.048).
Conclusions
Four months of rhGH replacement therapy partly reversed sleep disturbances previously observed in untreated patients. The decrease in delta activity associated with rhGH treatment adds further evidence to the hypothesis that the excess of high intensity SWS observed in untreated pituitary GHD patients is likely to result from overactivity of the hypothalamic GHRH system due to the lack of negative feedback inhibition by GH.
Keywords: growth hormone, sleep, slow-wave activity, hormonal replacement
INTRODUCTION
A large body of evidence has demonstrated bidirectional interactions between somatotropic activity and sleep regulation 1. In particular, GHRH activity has been linked to the promotion of the deepest stages of non-rapid eye movement (NREM) sleep, i.e. slow-wave sleep (SWS) 2–5. The absence of negative feedback regulation of GHRH, as occurs in untreated GH deficiency of pituitary origin, may result in a stimulation of the depth or intensity of NREM sleep, which can be estimated as EEG spectral power in the frequency band characteristic of slow-waves (delta band: 0.75 – 4 Hz; also referred to as slow-wave activity [SWA] or delta activity).
Very few studies have objectively compared sleep quality in GHD patients and in appropriately matched controls and the results have been inconclusive 6–9. We recently reported that sleep is disturbed in patients with GHD due to pituitary damage compared to individually age-, gender- and BMI-matched controls. The disturbances involve excessive amounts of delta activity and an age-dependent increase in sleep fragmentation. Furthermore the patients reported poor subjective sleep quality and daytime sleepiness, as well as impaired quality of life (QoL) 10.
Treatment of adult GHD patients with recombinant human growth hormone (rhGH) is generally associated with a clear improvement in QoL 11–13. However, as far as sleep is concerned, the few studies that evaluated the effect of GH therapy did not detect any consistent change 9, 14–17. The purpose of the present study was therefore to assess the impact on sleep of 4 months of recombinant human GH (rhGH) treatment, as compared to placebo, in adult GHD patients, using a single-blind placebo-controlled randomized cross-over design. We hypothesized that rhGH replacement would restore the negative feedback regulation of central GHRH activity and therefore correct the excessive slow-wave activity that we had observed in patients with pituitary GHD in the absence of treatment.
SUBJECTS AND METHODS
Patients and experimental protocol
All 26 patients with GHD of confirmed or likely pituitary origin included in our study of sleep-wake regulation in untreated GH-deficient adults 10 were invited to participate in a single-blind randomized placebo-controlled cross-over design trial testing the impact of GH replacement therapy on sleep conducted at the Université Libre de Bruxelles (Belgium), the University of Pisa (Italy), the University of Liege (Belgium) and the University of Chicago (Illinois, USA). In each participating institution, the protocol was approved by the Institutional Review Board. Written informed consent was obtained from all participants.
Details of the inclusion criteria are given in our previous paper 10. In summary, GHD was diagnosed based on an i.v. insulin tolerance test performed within the last 5 yrs, with a maximum GH response less than 3 μg/liter. Patients with evidence of substance abuse, liver disease, renal insufficiency, heart failure, malignant disease, chronic infectious disease, neurological or psychiatric disease, clinically significant hyperprolactinemia, or diabetes requiring administration of insulin or sulfonylureas, were excluded from the study. Individuals employed as shift workers within the last 3 months and subjects having traveled across more than 2 time zones within 2 weeks prior to starting the study were not included. All subjects were off hypnotic drugs for at least 3 months. Additionally, patients were either rhGH-naïve or had been off treatment for at least 6 months prior to participation in the study. All patients with associated pituitary hormonal deficiencies received stable replacement therapy, as assessed by at least two clinical and biological evaluations performed at intervals of at least 3 months.
Of the 26 patients with pituitary GHD included in our baseline study, 6 patients declined participation. Thus, 20 adults with pituitary GHD were enrolled. The patients were studied in laboratories at the Universities of Chicago, Brussels (including 5 patients recruited at the University of Liège) and Pisa. The same procedures, equipment, instruments and recording techniques were used at each site.
Immediately after completing the baseline study of sleep-wake regulation 10, patients underwent a first intervention period during which either rhGH or placebo was administered daily for 4 months. The dose of rhGH (Genotropin, Pharmacia, Stockholm, Sweden) was sex- and age-specific, according to the Consensus guidelines for the diagnosis and treatment of adults with GH deficiency 18 (Table 1). Both Genotropin and placebo preparations were provided by Pharmacia. The dose of placebo was similarly titrated such that patients remained blind to the treatment condition. Patients were instructed to inject rhGH subcutaneously, approximately 30 minutes before bedtime. The participants returned for an outpatient visit at monthly intervals. Each visit included clinical examination, determination of IGF-I levels and assessment (whenever applicable) of the current replacement therapy for associated pituitary hormonal deficiencies. The rhGH dosage was titrated accordingly by one of the physician investigators (L.L.M., J.-J.L., R.E.W., J.M. or G.C.), who therefore did not remain blind to the nature of the injections. All other investigators and staff, including the sleep technologist who scored the recordings, were blind to the study condition.
Table 1.
Sex- and age-specific instructions for rhGH administration.
| Men ≤ 45 years | Men > 45 years | Women ≤ 45years | Women > 45 years | |
|---|---|---|---|---|
| Initial dose (mg/day) | 0.2 | 0.1 | 0.3 | 0.3 |
| Increments (mg/day) | 0.2 | 0.1 | 0.2 | 0.1 |
| Max. dose (mg/day) | 0.6 | 0.4 | 0.7 | 0.6 |
At the end of this first 4-month period, the participants had an outpatient admission that included clinical examination and routine laboratory blood tests. Following this outpatient admission, the patients underwent 6 days of ambulatory sleep monitoring by wrist actigraphy (Actiwatch, Philips Respironics, Bend, OR), a method providing accurate estimations of sleep onset and offset 19, 20. The mean habitual bedtimes from these recordings were used to individually design the bedtime schedule during a 2-day inpatient study, which occurred within one week after the end of ambulatory monitoring. The subjects were admitted to the laboratory between 17h00 and 19h00 on day 1, and remained in the laboratory until discharge in the morning of day 3. Regular hospital meals were served at 08h00, 12h30 and 19h00. Lights were turned off 5 min before scheduled bedtime and turned on 5 min after scheduled wake time. During bedtimes, sleep was polygraphically recorded (DigiTrace Care Services, Boston, MA).
Upon awakening on day 2, a blood sample was taken for measurement of plasma IGF-I. Thereafter, patients were maintained under normal indoor light (± 300 lux) until bedtime. During waking hours, they had sedentary activities (reading, watching TV and simple neurobehavioral tests) and were free to ambulate around the unit. Naps were not allowed.
Immediately after this inpatient study, the participants entered a 3-month wash-out period, followed by a second 4-month period during which either placebo or rhGH was administered. Objective sleep quality was re-evaluated at the end of the second 4-month intervention period using the same procedures as during the first intervention period.
Throughout the study, patients who did not strictly conform to the instructions, who experienced side-effects or had to modify the replacement therapy for associated pituitary hormonal deficiencies, were excluded from the study. At the end, valid sleep analyses in both placebo and rhGH conditions were obtained in 14 patients who completed the entire study. However, while reviewing the results of the spectral analysis of the sleep EEG, we noticed that one patient, a 29-year old man who suffered a transsection of the pituitary stalk (with associated hypoadrenalism, hypothyroidism and hypogonadism), had results at the end of the placebo period that were widely divergent from his own recordings obtained during the baseline study 10, i.e. also in the absence of active treatment. His delta activity levels were indeed approximately 7 times lower at the end of placebo treatment than at baseline. Delta activity is highly reproducible in a single individual 21–23, even in recordings separated by several months, with intra-subject correlation coefficients above 0.90. The most likely explanation for the large between-study difference observed in this patient is a technical artifact related to amplifier settings. The Grubbs test for detection of statistical outliers 24, 25 performed on differences of both delta power and theta power during NREM sleep between the two recordings indeed identified this patient as a significant outlier (p<0.01 for delta power; p<0.05 for theta power), and he was excluded from the analysis. Results are therefore presented for 13 patients.
Four of them had childhood-onset idiopathic GHD that persisted into adulthood (as assessed by the persistence of an abnormally low GH response to the i.v. insulin tolerance test). Nine patients had an adult-onset GHD: in 5 of them, the origin of GHD was had a primary pituitary defect without supra-pituitary involvement: surgical removal of a pituitary tumor without radiotherapy (n=4). None of them presented with diabetes insipidus. One patient was diagnosed with pituitary apoplexy in a non-functioning pituitary adenoma and had diabetes insipidus. In 2 patients with adult-onset GHD, the existence of primary pituitary lesions was confirmed but a supra-pituitary involvement could not be excluded (surgical removal of a craniopharyngioma, without any adherence to the hypothalamus or the optic chiasm with presence of diabetes insipidus (n=2)). Both pituitary and hypothalamic lesions were possible in one patient with histiocytosis and one patient with neurosarcoidosis. Individual patient descriptions are presented in Table 2.
Table 2.
Clinical characteristics of the patients None of the patients had visual field defects at presentation.
| Subj., Sex | Age (yrs) | BMI (kg/m2) | Diagnosis, Onset | Treatment (yrs before) | Adrenal | Additional hormonal
replacement therapy |
||
|---|---|---|---|---|---|---|---|---|
| Thyroid | Gonad | ADH | ||||||
| 1, M | 64 | 25.0 | NFPA, AO | Surgery (2) | CA 37.5 mg/d | |||
| 2, M | 60 | 23.2 | NFPA, AO | Surgery (7) | HC 25 mg/d | |||
| 3, M | 28 | 17.8 | Idiopathic, CO | n/a | T im 250mg/3wks | |||
| 4, F | 70 | 24.1 | Prolactinoma, AO | Surgery (14) | HC 35 mg/d | T4 125 μg/d | E2-PRG transd | |
| 5, M | 69 | 30.1 | NFPA, AO | Surgery (8) | HC 20 mg/d | T4 150 μg/d | T im 250 mg/mo | |
| 6, M | 22 | 21.4 | Idiopathic, CO | n/a | CA 37.5 mg/d | T4 150 μg/d | T im 250mg/3wks | |
| 7, M | 47 | 32.7 | Idiopathic, CO | n/a | CA 25 mg/d | T4 125 μg/d | T oral 120 mg/d | |
| 8, M | 36 | 44.0 | Neurosarcoidosis, AO hypothalamic infiltration | n/a | HC 20 mg/d | T4 200 μg/d | T im 250 mg/mo | DDVAP nasal 20 μg/d |
| 9, M | 47 | 26.7 | Idiopathic, CO | n/a | HC 20 mg/d | T4 100 μg/d | T im 250mg/2wks | |
| 10, M | 30 | 29.5 | Cranio, AO | Surgery (7) | CA 37.5 mg/d | T4 50 μg/d | T im 250 mg/2wks | DDVAP nasal 20 μg/d |
| 11, M | 27 | 24.0 | Cranio, AO | Surgery (7) | HC 25 mg/d, | T4 125 μg/d | T im 300 mg/3wks | DDVAP nasal 15 μg/d |
| 12, M | 51 | 28.4 | Histiocytosis, AO | RT (3) | HC 20 mg/d | T4 75 μg/d | T im 200 mg/2wks | |
| 13, M | 54 | 34.4 | NFPA+ pituitary apoplexy, AO | n/a | HC 20 mg/d | T4 75 μg/d | T transd 5 mg/d | DDVAP oral 100 μg/day |
NFPA non-functioning pituitary adenoma, Cranio craniopharyngioma, AO adult-onset, CO childhood-onset, RT radiotherapy, CA cortisone acetate, HC hydrocortisone, T4 L-thyroxine, T testosterone, E2 estradiol, PRG progesterone, DDAVP desmopressin, transd transdermal.
Sleep analysis
All polygraphic recordings were visually scored at 30-sec intervals in stages wake, I, II, III, IV and REM using standardized criteria 26 by the same experienced scorer who was blind to the subject’s condition. Sleep onset and morning awakening were defined as, respectively, the times of the first and last 30-sec intervals scored II, III, IV, or REM. The sleep period was defined as the time separating sleep onset from final morning awakening. Total sleep time was defined as the sleep period minus the total duration of wake after sleep onset (WASO). Sleep latency was defined as the time from lights off until sleep onset. Sleep efficiency was calculated as the total sleep time expressed as percentage of the time allocated to sleep (i.e. the interval between lights off and lights on). Periodic leg movements were identified only in one recording in one patient.
A spectral analysis on the central EEG lead was performed (PRANA, PhiTools, Strasbourg, France). Muscular, ocular and movement artifacts were eliminated prior to spectral analysis. Delta, theta, and alpha activities were calculated as the absolute spectral power in the frequency bands 0.75–4 Hz, 4.5–8 Hz, and 8.5–12 Hz, respectively. Mean power per 30-sec epoch was calculated for each band. Mean delta power in non-REM sleep quantifies the intensity or “depth” of non-REM sleep. Alpha activity is an independent marker of the synchronization of high-frequency cortical oscillations that typically appear during quiet wake with eyes closed; it originates in neuronal networks distinct from those responsible for the generation of delta activity. Theta activity reflects spectral power in an intermediate frequency that is often contaminated by delta activity. For illustrative purposes, the durations of NREM/REM cycles were also normalized to account for individual differences, as previously described 27.
All of the 56 nights recorded were scored; technical artifacts prevented spectral analysis for 4 of them. Comparisons between conditions used the second, rather than the first night of polysomnography, because patients were habituated to the experimental procedures and spent the preceding day in the same standardized and controlled environment, except in 4 cases due to highly artifactual recordings.
Quality of life (QoL) and subjective sleep quality
QoL was estimated by the global score of the QoL Assessment of Growth Hormone Deficiency in Adults questionnaire (QoL-AGHDA), which comprises 25 “yes or no” questions relative to specific complaints commonly reported by GHD patients 28. A higher score corresponds to lower QoL. Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index questionnaire (PSQI) 29. Additionally, the patients were asked to complete a Karolinska Sleep Log, a questionnaire used to assess subjective sleep duration and quality, for the 6 consecutive days preceding each inpatient session.
Statistical analysis
Comparisons between rhGH treatment and placebo were performed using the Wilcoxon signed rank test. All group values are expressed as means ± SEM. All statistical calculations were performed using JMP version 8.0.2 (S.A.S Institute Inc., Cary, NC, USA).
RESULTS
Six patients started the study with the placebo phase, and 7 started with the rhGH phase. The average dose of rhGH was 0.48 ± 0.03 mg/day; the “dose” of placebo averaged 0.50 ± 0.07 mg/day (p=1.000 vs GH). At the end of the rhGH period, plasma IGF-I levels averaged 231 ± 20 ng/ml, vs 97 ± 14 ng/ml at the end of the placebo period (p<0.001) (age-adjusted SD scores: −0.1 ± 0.3 SD, vs −2.1 ± 0.2 SD, respectively; p<0.001). Body mass index averaged 28.5 ± 2.1 kg/m2 at the end of the rhGH period vs 27.8 ± 2.2 kg/m2 at the end of the placebo period (p=0.266).
Objective sleep quality
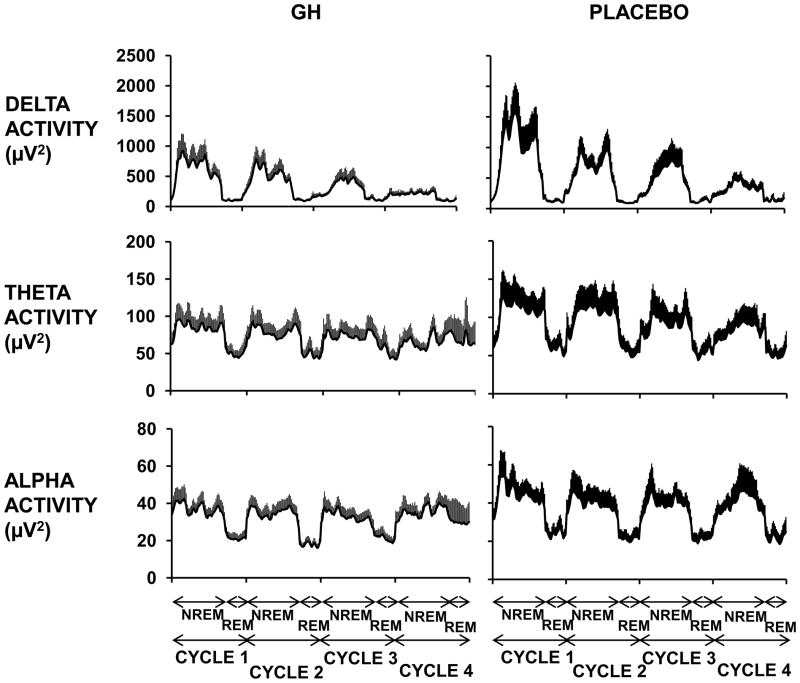
Table 3 summarizes the findings. The sleep period time (from sleep onset to final morning awakening) was reduced by an average of 48 min under rhGH as compared to placebo (p=0.005), primarily reflecting a trend to an earlier wake up time. The relative distribution of the different sleep stages was not affected by treatment. The mean duration of SWS (stages III+IV) was reduced by 27%, but this reduction did not reach statistical significance (p=0.110). However, mean delta activity during NREM sleep in the first 6 hours of sleep was decreased by 30% at the end of the rhGH treatment period, compared to the placebo period (p=0.048) (Table 3). In contrast, theta and alpha activity levels were not affected by treatment. The average profiles of EEG delta, theta and alpha power during the first four sleep cycles in the two conditions are presented in Fig. 1.
Table 3.
Mean (±SEM) values of sleep variables in 13 patients with pituitary GHD after 4 months of placebo or rhGH treatment.
| Placebo | GH | p level GH vs Placebo | |
|---|---|---|---|
| Time of lights out | 23h32±12 min | 23h44±18 min | 0.470 |
| Time of lights on | 07h54±19 min | 07h34±20 min | 0.092 |
| Time in bed (min) | 504±13 | 471±17 | 0.216 |
| Sleep period time (min) | 479±11 | 431±19 | 0.005 |
| Sleep latency (min) | 15±4 | 21±8 | 0.423 |
| Total sleep time (min) | 422±20 | 384±25 | 0.223 |
| Sleep efficiency (%) | 84±3 | 82±4 | 0.635 |
| WASO (min) | 57±16 | 47±12 | 0.191 |
| REM sleep (min) | 80±9 | 67±8 | 0.879 |
| REM sleep (%) | 19±2 | 17±2 | 0.588 |
| Stages I+II (min) | 261±13 | 248±15 | 0.376 |
| Stages I+II (%) | 63±4 | 66±4 | 0.542 |
| Stages III+IV (min) | 95±19 | 69±19 | 0.110 |
| Stages III+IV (%) | 18±4 | 17±4 | 0.455 |
| Delta activity (μV2)* | 794±219 | 559±125 | 0.048 |
| Theta activity (μV2)* | 102±25 | 82±17 | 0.376 |
| Alpha activity (μV2)* | 40±7 | 35±5 | 0.542 |
Spectral power is reported as the average absolute power during NREM sleep in the first 6 hours of sleep; WASO wake after sleep onset; REM sleep rapid eye movement sleep; NREM sleep non-rapid eye movement sleep.
Fig. 1.
Mean profiles (+SEM) of absolute EEG spectral power in the delta, theta and alpha ranges during the first four NREM-REM cycles in GHD patients under rhGH (left) and placebo (right).
Of note, 2 patients displayed low sleep duration (at least 1.5 h less sleep in the laboratory than assessed at home by wrist actigraphy) as well as low sleep quality (average number of awakenings 46; average duration of wake after sleep onset 2 h 40 min; average sleep efficiency 60%) in both study conditions. If those 2 patients were excluded from the analysis, the reduction in the sleep period time averaged 51 minutes (p=0.019), primarily reflecting earlier wake up time (p=0.027). The decrease in delta activity under rhGH treatment as compared to placebo averaged 32% (p=0.024). Results regarding sleep stages, theta and alpha activity levels remained similar in both conditions when these 2 patients were excluded.
Quality of life (QoL) and subjective sleep quality
The global score of the QoL-AGHDA scale was decreased under GH as compared to placebo (4.75 ± 0.57 vs 8.75 ± 0.89, p=0.023), indicating an improvement in QoL ratings. The PSQI averaged 4.1 ± 0.7 under rhGH vs 5.8 ± 1.1 under placebo, but this difference did not reach statistical significance (p=0.102). Consistent with the polysomnographic findings, at the end of the GH treatment period, patients reported an earlier wake up time (difference about 24 min, p=0.002), and a shorter sleep time (difference about 46 min, p=0.007) compared to the end of the placebo period.
DISCUSSION
The present study examined sleep duration, sleep architecture and EEG power in the delta, theta and alpha ranges in adult GHD patients of confirmed or likely pituitary origin, after 4 months of rhGH versus placebo administration. In light of the results of our previous study on adult patients with pituitary GHD 10, we hypothesized that the peculiar sleep disturbance observed in this cohort, i.e. an increase in NREM sleep intensity, would be reversed as the physiology of the somatotropic axis was restored by rhGH replacement. As compared to placebo, rhGH treatment in patients with pituitary GHD resulted in shorter sleep period time by almost one hour, and decreased intensity of NREM sleep as quantified by EEG delta activity. Additionally, in keeping with previous reports 11–13, patients experienced an improvement in their QoL under rhGH.
A unique aspect of our study is that, in each treatment condition, the time allotted for sleep during the inpatient study was based on habitual sleep time derived from one week of ambulatory wrist actigraphy immediately preceding the inpatient study. Following rhGH treatment, the patients had shorter habitual time in bed than under placebo, primarily because they got out of bed earlier. Accordingly, sleep period time was nearly one hour shorter under rhGH than under placebo. The shorter sleep period time could be explained by the lower levels of delta activity. Delta activity is a measure of sleep pressure and decreases exponentially throughout the night to reach minimal levels at the end of the night. This is thought to reflect the completion of the recovery process during sleep 30, 31. When delta activity is lower, the minimum is reached sooner and therefore the sleep episode is shorter. Vice versa, after a night of total sleep deprivation, a rebound of delta activity is typically observed during the following period of recovery sleep, which is typically longer than a regular night 32.
Sleep architecture (the proportions of the various sleep stages) was similar under both treatment conditions. The maintenance of a normal sleep architecture under rhGH treatment is in accordance with previous reports, as several authors observed no impact of replacement therapy administered for 6 months on sleep stages, compared to placebo 9 or to a baseline evaluation before treatment 14, 16, 17 (Table 4). The only exception is a very small study (n=5) that found a decrease in SWS duration in middle-aged patients with GHD of variable etiology after 6 months of rhGH withdrawal, compared to results obtained after 1 to 2 years of high dose rhGH replacement 15.
Table 4.
Review of studies evaluating the impact of hGH treatment on sleep in adult GHD patients
| Authors | n | Patients | Protocol | Results |
|---|---|---|---|---|
| Astrom et al., 1990 14 | 8 | Young adults with CO-isolated GHD | PSG before and after 6 months hGH
treatment No placebo control. No spectral analysis. |
↓ total sleep time ↑ REM sleep No change in SWS duration |
| Nolte et al., 2002 15 | 5 | Patients with AO-GHD due to pituitary disease | PSG after 1 yr rhGH treatment and after 6
months withdrawal No placebo control. No spectral analysis. |
rhGH: normal stages I, II,
SWS duration; ↓ sleep efficiency and REM
sleep rhGH withdrawal: ↓ SWS duration |
| Schneider et al., 2005 16 | 18 | CO-GHD patients | PSG before and after 6 months rhGH treatment.
No placebo control. Spectral analysis. |
No differences in sleep
parameters. Supraphysiologic IGF-1 levels in 10 patients. |
| Peker et al., 2006 17 | 19 | AO-GHD patients | PSG before and after 6 months rhGH
treatment No placebo control. No spectral analysis. |
No differences in sleep parameters |
| Ismailogullari et al., 2009 9 | 12 | Women with AO-GHD due to Sheehan’s syndrome | Parallel group design PSG before and after 6 months rhGH treatment, and vs 8 patients on placebo. No spectral analysis. |
No differences in sleep parameters |
| Morselli et al. (present study) | 13 | 9 adults with AO-GHD 4 adults with CO-GHD |
Randomized cross-over design PSG after 4 months treatment rhGH vs placebo Spectral analysis. |
rhGH vs
placebo: ↓ sleep period time (earlier wake up) ↓ SWS intensity (delta activity) |
CO childhood onset, AO adult onset, PSG polysomnography, REM rapid eye movement, SWS slow wave sleep.
Quantitative EEG analysis of our results revealed a decrease in delta activity after rhGH replacement. In our previous study comparing sleep in patients with GHD of pituitary origin versus controls individually matched for age, gender and BMI, we had observed excessive amounts of delta activity, associated with poor subjective sleep quality 10. We indicated that this combination of objective and subjective symptoms represents an unusual form of sleep disturbance as the most prevalent sleep disorders, such as obstructive sleep apnea, are characterized by the combination of reduced delta activity in association with increased daytime sleepiness 33, 34. While limited by the small sample size, the present findings nonetheless suggest that rhGH therapy corrects this peculiar sleep disturbance. Our results are consistent with the hypothesis of an enhanced GHRH drive in patients with GHD of pituitary origin, which could be reversed by rhGH treatment. Indeed, a large body of literature supports a role of GHRH in the modulation of sleep and in particular the generation of SWS/delta activity 2, 4, 5. The only previous study 16 that had performed quantitative EEG analysis before and after rhGH treatment in GHD patients did not find any change in delta activity after 6 months of treatment, as compared to baseline. No comparison was made with a placebo condition, and some patients experienced supraphysiologic IGF-I concentrations with mild side effects.
Two recent studies, conducted in patients previously treated by transsphenoidal surgery for pituitary tumours with or without suprasellar extension, have evidenced that hypothalamic involvement was associated with sleep fragmentation, resulting in shorter sleep duration, disturbed sleep architecture, and disturbed circadian movement rhythms, possibly via alterations of the suprachiasmatic nuclei 35, 36. Though a hypothalamic involvement could not be excluded in four of our patients, none had suffered from compression of the optic chiasm and only one had received radiotherapy. Moreover, sleep variables were similar in those patients and in the other 9 patients (data not shown). Thus, it seems unlikely that hypothalamic alterations were involved in sleep disturbances observed in our cohort.
Our study has several limitations. The small sample size is an obvious weakness of the study, which involved a demanding and labor-intensive protocol and challenging recruitment criteria. Second, our cohort consisted mostly of middle-aged and older men (8/13 patients over 40 years old). In men, the earliest sign of aging of sleep is a dramatic decrease in delta activity that is apparent already by 40 years of age 37, 38. Therefore, it is possible that the impact of rhGH treatment on sleep, and particularly SWS and delta activity, would have been more robust had we been able to recruit more young patients. A further limitation is that we did not record breathing parameters in these patients, and therefore could not control for the presence of sleep apnea. An increase in the prevalence and severity of sleep apnea could have contributed to the decrease in delta activity observed in our study. However, neither the distribution of sleep stages, nor the measures of sleep fragmentation (which would arguably increase in the case of worsening sleep apnea) were affected by treatment. The fact that the participants in the present study were only modestly overweight and did not gain a significant amount of weight during rhGH treatment also argues against a role of sleep apnea in the present findings. Finally, the only prospective study evaluating the incidence of sleep apnea in adult GHD patients treated with rhGH showed that treatment does not induce or aggravate sleep apnea 17.
CONCLUSION
In conclusion, although they need to be confirmed by larger studies, the present findings suggest that recombinant human GH treatment administered for 4 months may reverse some of the disturbances in the macro- and micro-architecture of sleep previously evidenced in adult patients with GHD. These findings add to a body of evidence indicating a central role of GHRH activity in modulating sleep quality.
Acknowledgments
FUNDING
This study was sponsored by an investigator-initiated grant from Pharmacia Corporation and received partial support from NIH grant MO1 RR-00055 to the General Clinical Research Center of the University of Chicago. During part of the study, Dr. A. Nedeltcheva was supported by the Northwestern University – University of Chicago Training Grant for Sleep Research (NIH grant T32 HL-07909). The analysis of sleep recordings was partly supported by grant PO1 AG-11412 from the National Institute on Aging.
Thanks are due to Claire van den Bril, M.S. (Université Libre de Bruxelles), Elisabeth Lallemant, M.S (CHR-Citadelle, Liège), and to the nursing staff of the General Clinical Research Center of the University of Chicago for excellent care of the subjects during inpatient studies. We are grateful to Dr. P. Penev for assistance with the screening of subjects recruited at the University of Chicago and to Dr. Barbara Lippe (formerly of Pharmacia Corporation) for helpful advice and suggestions during the development of the protocol.
Footnotes
DECLARATION OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
All authors in this manuscript have contributed substantially to the manuscript, either in design, data collection or analysis, and drafting of the article and have provided final approval before submission of the manuscript.
References
- 1.Van Cauter E, Plat L, Copinschi G. Interrelations between sleep and the somatotropic axis. Sleep. 1998;21:553–566. [PubMed] [Google Scholar]
- 2.Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F. Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology. 1992;56:566–573. doi: 10.1159/000126275. [DOI] [PubMed] [Google Scholar]
- 3.Kerkhofs M, Van Cauter E, Van Onderbergen A, Caufriez A, Thorner MO, Copinschi G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. American Journal of Physiology- Endocrinology and Metabolism. 1993;264:E594–E598. doi: 10.1152/ajpendo.1993.264.4.E594. [DOI] [PubMed] [Google Scholar]
- 4.Obal F, Jr, Krueger JM. GHRH and sleep . Sleep Medicine Reviews. 2004;8:367–377. doi: 10.1016/j.smrv.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Peterfi Z, McGinty D, Sarai E, Szymusiak R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology. 2010;298:R147–156. doi: 10.1152/ajpregu.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr W, Vogel G, Stahl M, Griffiths W, Seely J. Sleep patterns in growth hormone deficient children and age-matched controls: developmental considerations. Neuroendocrinology. 1977;24:347–352. doi: 10.1159/000122721. [DOI] [PubMed] [Google Scholar]
- 7.Wu RH, Thorpy MJ. Effect of growth hormone treatment on sleep EEGs in growth hormone-deficient children. Sleep. 1988;11:425–429. doi: 10.1093/sleep/11.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Aström C, Jochumsen PL. Decrease in delta sleep in growth hormone deficiency assessed by a new power spectrum analysis. Sleep. 1989;12:508–515. doi: 10.1093/sleep/12.6.508. [DOI] [PubMed] [Google Scholar]
- 9.Ismailogullari S, Tanriverdi F, Kelestimur F, Aksu M. Sleep architecture in Sheehan’s syndrome before and 6 months after growth hormone replacement therapy. Psychoneuroendocrinology. 2009;34:212–219. doi: 10.1016/j.psyneuen.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Copinschi G, Nedeltcheva A, Leproult R, Morselli LL, Spiegel K, Martino E, Legros JJ, Weiss RE, Mockel J, Van Cauter E. Sleep disturbances, daytime sleepiness, and quality of life in adults with growth hormone deficiency. Journal of Clinical Endocrinology & Metabolism. 2010;95:2195–2202. doi: 10.1210/jc.2009-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koltowska-Haggstrom M, Mattsson AF, Monson JP, Kind P, Badia X, Casanueva FF, Busschbach J, Koppeschaar HP, Johannsson G. Does long-term GH replacement therapy in hypopituitary adults with GH deficiency normalise quality of life? European Journal of Endocrinology. 2006;155:109–119. doi: 10.1530/eje.1.02176. [DOI] [PubMed] [Google Scholar]
- 12.Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocrine reviews. 2006;27:287–317. doi: 10.1210/er.2004-0022. [DOI] [PubMed] [Google Scholar]
- 13.Moock J, Albrecht C, Friedrich N, Volzke H, Nauck M, Koltowska-Haggstrom M, Kohlmann T, Wallaschofski H. Health-related quality of life and IGF-1 in GH-deficient adult patients on GH replacement therapy: analysis of the German KIMS data and the Study of Health in Pomerania. European Journal of Endocrinology. 2009;160:17–24. doi: 10.1530/EJE-08-0738. [DOI] [PubMed] [Google Scholar]
- 14.Aström C, Pedersen SA, Lindholm J. The influence of growth hormone on sleep in adults with growth hormone deficiency. Clinical Endocrinology (Oxf) 1990;33:495–500. doi: 10.1111/j.1365-2265.1990.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 15.Nolte W, Radisch C, Rodenbeck A, Wiltfang J, Hufner M. Polysomnographic findings in five adult patients with pituitary insufficiency before and after cessation of human growth hormone replacement therapy. Clin Endocrinol (Oxf) 2002;56:805–810. doi: 10.1046/j.1365-2265.2002.01531.x. [DOI] [PubMed] [Google Scholar]
- 16.Schneider HJ, Oertel H, Murck H, Pollmacher T, Stalla GK, Steiger A. Night sleep EEG and daytime sleep propensity in adult hypopituitary patients with growth hormone deficiency before and after six months of growth hormone replacement. Psychoneuroendocrinology. 2005;30:29–37. doi: 10.1016/j.psyneuen.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Peker Y, Svensson J, Hedner J, Grote L, Johannsson G. Sleep apnoea and quality of life in growth hormone (GH)-deficient adults before and after 6 months of GH replacement therapy. Clinical Endocrinology (Oxf) 2006;65:98–105. doi: 10.1111/j.1365-2265.2006.02555.x. [DOI] [PubMed] [Google Scholar]
- 18.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. European Journal of Endocrinology. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 19.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Perceptual & Motor Skills. 1997;85:207–216. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 20.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: II. A novel approach to scoring and interpreting sleep-wake activity. Perceptual & Motor Skills. 1997;85:219–226. doi: 10.2466/pms.1997.85.1.219. [DOI] [PubMed] [Google Scholar]
- 21.Tan X, Campbell IG, Palagini L, Feinberg I. High internight reliability of computer-measured NREM delta, sigma, and beta: biological implications. Biological psychiatry. 2000;48:1010–1019. doi: 10.1016/s0006-3223(00)00873-8. [DOI] [PubMed] [Google Scholar]
- 22.Tan X, Campbell IG, Feinberg I. Internight reliability and benchmark values for computer analyses of non-rapid eye movement (NREM) and REM EEG in normal young adult and elderly subjects. Clinical Neurophysiology. 2001;112:1540–1552. doi: 10.1016/s1388-2457(01)00570-3. [DOI] [PubMed] [Google Scholar]
- 23.Tasali E, Spiegel K, Latta F, Leproult R, Van Cauter E. Slow wave activity is a stable trait dependent characteristic in both young and older adults. Society for Research on Biological Rhythms Annual meeting; Amelia Island, FL. May 22–26, 2002; 2002. [Google Scholar]
- 24.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 25.Grubbs FE, Beck G. Extension of sample sizes and percentage points for significance tests of outlying observations. Technometrics. 1972;14:847–854. [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- 27.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochemical Pharmacology. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 28.McKenna SP, Doward LC, Alonso J, Kohlmann T, Niero M, Prieto L, Wiren L. The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Quality of Life Research. 1999;8:373–383. doi: 10.1023/a:1008987922774. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, III, Monk TH, Berman SB, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 31.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. American Journal of Physiology- Endocrinology and Metabolism. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 32.Dijk D, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1990;258:R650–R661. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 33.Heinzer R, Gaudreau H, Décary A, Sforza E, Petit D, Morisson F, Montplaisir J. Slow-Wave Activity in Sleep Apnea Patients Before and After Continuous Positive Airway Pressure Treatment. Chest. 2001;119:1807–1813. doi: 10.1378/chest.119.6.1807. [DOI] [PubMed] [Google Scholar]
- 34.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Medicine. 2001;2:477–491. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 35.Borgers AJ, Romeijn N, van Someren E, Fliers E, Alkemade A, Bisschop PH. Compression of the optic chiasm is associated with permanent shorter sleep duration in patients with pituitary insufficiency. Clinical endocrinology. 2011;75:347–353. doi: 10.1111/j.1365-2265.2011.04053.x. [DOI] [PubMed] [Google Scholar]
- 36.Biermasz N, Joustra S, Donga E, Pereira A, van Duinen N, van Dijk M, van der Klaauw A, Corssmit E, Lammers G, van Kralingen K. Patients previously treated for nonfunctioning pituitary macroadenomas have disturbed sleep characteristics, circadian movement rhythm, and subjective sleep quality. Journal of Clinical Endocrinology & Metabolism. 2011;96:1524–1532. doi: 10.1210/jc.2010-2742. [DOI] [PubMed] [Google Scholar]
- 37.Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently ? Journal of sleep research. 1997;6:211–215. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Journal of the American Medical Association. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]