Abstract
Decades of research in molecular oncology have brought about promising new therapies that are designed to target specific molecules that promote tumor growth and survival. The epidermal growth factor receptor (EGFR) is one of the first identified important targets of these novel antitumor agents. Approximately half of cases of triple-negative breast cancer (TNBC) and inflammatory breast cancer (IBC) overexpress EGFR. Thus, EGFR inhibitors for treatment of breast cancer have been evaluated in several studies. However, results so far have been disappointing. One of the reasons for these unexpected results is the lack of biomarkers for predicting which patients are most likely to respond to EGFR inhibitors. Recent studies have shown that EGFR and its downstream pathway regulate epithelial-mesenchymal transition, migration, and tumor invasion and that high EGFR expression is an independent predictor of poor prognosis in IBC. Further, recent studies have shown that targeting EGFR enhances the chemosensitivity of TNBC cells by rewiring apoptotic signaling networks in TNBC. These studies indicate that EGFR-targeted therapy might have a promising role in TNBC and IBC. Further studies of the role of EGFR in TNBC and IBC are needed to better understand the best way to use EGFR-targeted therapy—e.g., as a chemosensitizer or to prevent metastases—to treat these aggressive diseases.
Keywords: EGFR, breast cancer, targeted therapy, triple-negative breast cancer, inflammatory breast cancer
Introduction
In oncology, the search for new molecular predictors of prognosis (prognostic factors) and response to therapy (predictive factors) is an area of intense investigation. Progress in this area will undoubtedly transform cancer drug therapy from use of non-targeted antitumor agents in unselected patients to use of targeted antitumor agents in patients selected on the basis of tumor molecular biology. Among the most notable cancer molecular targets identified to date are the members of the epidermal growth factor receptor (EGFR)/ErbB family: EGFR (also known as ErbB1 and HER1), HER2 (also known as HER2/neu and ErbB2), ErbB3 (also known as HER3), and ErbB4 (also known as HER4) [1]. HER2, which is overexpressed in 20% to 25% of breast cancers, is the most well established therapeutic target in breast cancer.
EGFR overexpression in breast cancer is associated with large tumor size, poor differentiation, and poor clinical outcomes [2, 3]. Though EGFR overexpression is observed in all subtypes of breast cancer, EGFR is more frequently overexpressed in triple-negative breast cancer (TNBC) and inflammatory breast cancer (IBC), which are especially aggressive [4–6]. Treatment of patients with these phenotypes has been challenging not only because of the aggressive behavior of these diseases but also because of the lack of established clinically relevant treatment targets. The role of EGFR in breast cancer has been scrutinized, and several therapies that target EGFR, including gefitinib, cetuximab, lapatinib, and others, have been developed. However, results of clinical studies of EGFR-targeted therapy in breast cancer have been disappointing.
Here, we review the latest studies of EGFR signaling and EGFR-targeted therapies in breast cancer, with a special focus on the relationship between EGFR and TNBC and IBC. We summarize the basic biological characteristics of EGFR and the latest findings from clinical trials of EGFR-targeted therapies for breast cancer.
EGFR in breast cancer
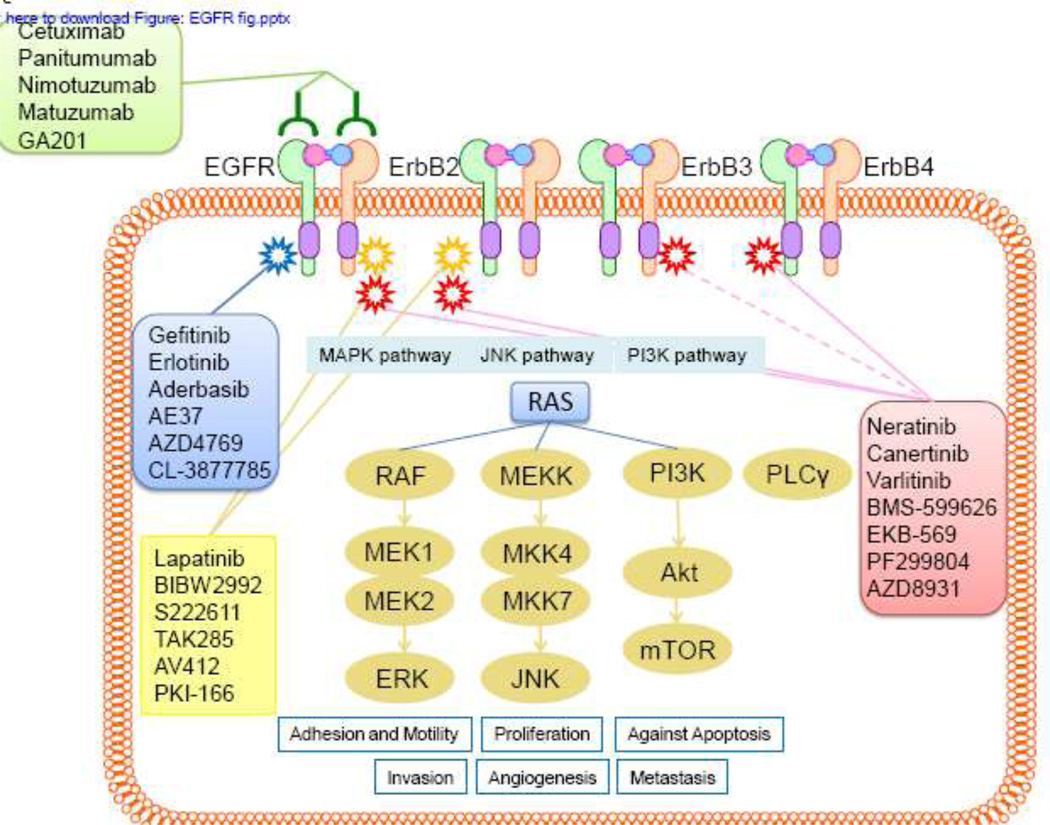
The human EGFR family comprises 4 closely related receptors that are transmembrane glycoproteins containing an extracellular ligand binding domain and an intracellular receptor tyrosine kinase domain. The major signaling pathways activated by EGFR receptors are mediated by PI3 kinase, Ras-Raf-MAPK, JNK, and PLCγ and result in a plethora of biological functions (Figure 1) [7, 8]. At the cellular level, the ligands not only induce cell proliferation but also alter adhesion and motility and protect against apoptosis; at the physiological level, the ligands promote invasion and angiogenesis [9]. Activation of members of the EGFR family promotes scattering and invasion of breast epithelial cells in 3-dimensional culture, which is associated with loss of cell polarization and other features of epithelial differentiation [10]. In vitroany of these effects may contribute to the malignant phenotype. Dysregulation of EGFR pathways by overexpression or constitutive activation can promote tumor processes including angiogenesis and metastasis and is associated with poor prognosis in many human malignancies [3, 11], [12]. In addition to cross-talk between members of the EGFR family, there is evidence for significant interactions between EGFR family members and other receptor tyrosine kinases, such as c-MET and IGF-1R, and it is possible that such alternative signaling pathways are linked to resistance to EGFR-targeted therapies [13].
Figure 1.
EGFR inhibitors and downstream signaling pathways. Activation of EGFR leads to homodimerization/heterodimerization and phosphorylation of specific tyrosine residues. The major signaling pathways activated by EGFR receptors are mediated by Ras-Raf-MAPK, JNK, PI3 kinase, and PLCγ and result in a plethora of biological functions [7, 8]. At the cellular level, the ligands not only induce cell proliferation but also alter adhesion and motility and protect against apoptosis; at the physiological level, the ligands promote invasion and angiogenesis.
It has been reported that the expression of both EGFR and HER2 is inversely correlated with estrogen receptor (ER) status, and EGFRHER2 heterodimers have been shown to increase the metastatic potential of breast cancer cell lines [14]. The rate of overexpression of EGFR is particularly high in TNBC, and the negative impact of EGFR overexpression is particularly pronounced in TNBC. Thus, EGFR has potential as a therapeutic target in TNBC, for which there are no specific targeted therapies at present.
One of the mechanisms of EGFR overexpression is amplification of the EGFR gene, which has been described in oligodendroglioma, [15] glioblastoma, lung cancer, [16] gastric cancer, and breast cancer [17]. EGFR gene amplification is infrequent in breast cancers overall: previous studies showed EGFR gene amplification in 0.8% to 14% of tumors [18, 19]. However, gene amplification has been shown in approximately 25% of cases of metaplastic breast cancer, a specific phenotype of TNBC [20–23].
Another mechanism of EGFR overexpression is through activating mutations of EGFR, which have been demonstrated in central nervous system tumors and lung cancer but is rare in breast cancer. Weber et al. found mutations of EGFR in 7 of 48 sporadic breast carcinomas and 11 of 24 hereditary breast carcinomas [23]. Surprisingly, mutations were found in both stromal and neoplastic epithelium. These authors also showed that EGFR mutations occurred at a significantly higher frequency in hereditary than in sporadic breast cancer (P=0.0079) and that the majority of missense mutations were in the tyrosine kinase domain of EGFR exon 20. These data are in agreement with the fact that the rate of TNBC is higher among patients with hereditary breast cancer than among patients with sporadic breast cancer [24]. Weber et al. suggested that future clinical trials employing molecular targeted therapy should evaluate EGFR mutations not only in neoplastic epithelia but also in the surrounding tumor stroma. This will establish the role of EGFR mutations in response to therapy and their value in predicting individual variation in response. In breast cancer, as has previously been done in lung cancer (with in-frame deletion of exon 19 and point mutations of exon 21) [25, 26], identification of EGFR mutations may be used to select patients most likely to respond to EGFR-targeted therapies.
In breast cancer, EGFR expression level or gene mutation status is increasingly being used to select patients for particular treatments. However, whether EGFR is truly a predictive biomarker remains to be proven.
Regulates epithelial-mesenchymal transition (EMT)
In several malignancies, EGFR alterations occur at an advanced stage of malignancy characterized by metastatic competence [27–29], and EGFR is thought to promote cancer cell migration and invasion. Recently, EGFR has been shown to promote epithelial-mesenchymal transition (EMT), a process by which cells undergo a morphologic switch from a polarized epithelial phenotype to a mesenchymal fibroblastoid phenotype, in a variety of epithelial cell lines. EMT has been identified as a key process of migration and tumor invasion [30, 31]. In breast cancer, there is some evidence that EMT is involved in development of the normal mammary gland, but EMT is likely to be most important in tumor progression [32, 33].
EMT is characterized by the loss of epithelial markers (E-cadherin and cytokeratins) and the presence of mesenchymal markers (vimentin and fibronectin). Reduction of the E-cadherin level has been associated with metastatic breast cancer, which indicates the importance of EMT in metastasis [34, 35]. EMT can be induced in vitro in several epithelial cell lines by growth factors such as EGFR, scatter factor/hepatocyte growth factor, fibroblast growth factors, and insulin-like growth factors 1 and 2 [32].
EMT ultimately results in a transcriptional reprogramming of the tumor cell and its transition to a mesenchymal phenotype, promoted by abnormal survival signals through plateletderived growth factor receptor, fibroblast growth factor receptor, cMET, transforming growth factor beta-receptor, insulin-like growth factor 1 receptor, ERKand AKT. These proteins and pathways can be targeted by molecular targeted therapies directed toward EGFR, insulin-like growth factor 1 receptor, mammalian target of rapamycin, vascular endothelial growth factor, and cKIT [36]. We have shown that erlotinib, an EGFR-tyrosine kinase inhibitor (TKI), inhibited cell motility and invasiveness and transformed IBC cells from a mesenchymal phenotype to an epithelial phenotype [37]. The fact that cells treated with erlotinib showed higher expression of E-cadherin and lower expression of vimentin suggested that the antimetastatic effect of erlotinib might be through inhibition of EMT [37]. Thus, EGFR is highly involved in EMT and might be a key target for inhibiting tumor metastasis.
Downstream of EGFR, the Ras-ERK pathway has been shown to also regulate EMT, tumor invasion, and metastasis. Activation of RSK by ERK is known to induce mesenchymal motility and invasion in cancer cells [38]. ERK has also been implicated in transforming growth factor-beta signaling: it was shown that transforming growth factor-beta1 induced EMT through activation of ERK1 [39]. However, recently, Ras-induced EMT that produced a dramatic morphological change in nontransformed human epithelial cell lines was shown to involve ERK2, not ERK1. The ERK2-induced EMT involved Fra1, a transcription factor that regulates expression of ZEB1/2, a marker associated with EMT [40].
EGFR in TNBC and basal-like breast cancer
At present, classification of breast cancers on the basis of common molecular features is indispensable for selecting the best treatment strategies. EGFR overexpression is found in at least 50% of cases of TNBC, which is a higher expression rate than the rates seen in other breast cancer subtypes [41]. Because of the high rate of overexpression of EGFR in TNBC, EGFR inhibitors are among the targeted agents being developed for treatment of TNBC.
TNBCs are negative for ER, progesterone receptor (PgR), and HER2 and are generally accepted as a clinical surrogate for basal-like breast cancer, one of the intrinsic subtypes based on microarray analysis [42]. EGFR and cytokeratin 5/6 are readily available positive markers for basal-like breast cancer that are applied to standard pathology specimens in clinics. Though TNBC is generally accepted as a clinical surrogate for basal-like breast cancer, it was recently hypothesized that TNBC is heterogeneous, and 50% to 85% of TNBC tumors were estimated to be true basal-like breast cancer [43, 44]. Recently, Lehmann et al. reported there are 6 subtypes of TNBC, and the EGF pathway is one of the top canonical pathways for Basal-like 2 and Mesenchymal-like subtypes [45].
Lee et al. recently showed that EGFR-targeted therapy may be used to enhance the initial sensitivity of TNBC cells to cytotoxic therapy [46], they identified new strategies to enhance the initial chemosensitivity of TNBC cells. They showed that enhanced cell death observed with the use of time-staggered erlotinib-doxorubicin combinations was directly mediated by sustained EGFR inhibition. After sustained EGFR inhibition, oncogene signatures such as RAS and MYC signatures were dramatically decreased in TNBC cells. The authors analyzed multiple types of quantitative data using advanced computation network modeling and found that the most effective strategy for killing aggressive TNBC cells was a time- and orderdependent combination of genotoxic agents with small molecule EGFR inhibitors, such as doxorubicin and erlotinib respectively. They also found that “the enhanced treatment efficacy resulted from dynamic network rewiring of an oncogenic signature maintained by active EGFR signaling to unmask an apoptotic process that involves activation of caspase-8.” They concluded that “phosphorylation of EGFR may constitute a useful biomarker of response to time-staggered inhibition in some tumor types that are EGFR driven, such as TNBC and lung cancer.”[46
EGFR in IBC
IBC, the most clinically aggressive subtype of breast cancer, is also associated with EGFR overexpression: 30% of IBCs express EGFR [47]. Several studies have documented a high frequency of negative ER and PgR status, up to 50%, and a high incidence of HER2 overexpression, up to 40%, in IBC tumors [47, 48]. Lack of expression of ER and PgR is clearly one of the reasons for the poor prognosis of IBC, but whether HER2 overexpression has a prognostic role in IBC has yet to be established. In contrast, EGFR overexpression is clearly correlated with poor prognosis in patients with IBC [49]. Patients with EGFR-positive IBC have a worse 5-year overall survival rate than patients with EGFR-negative tumors, and EGFR expression in IBC is associated with an increased risk of recurrence [49].
Because conventional chemotherapy regimens are not sufficient for the treatment of IBC, new therapies for IBC are needed. Among the potential candidates are therapies that target the EGFR pathway. An in vitro study showed that gefitinib, an EGFR-TKI, suppressed the growth of SUM149 cells, which overexpress EGFR and lack ER expression and are widely used as a model of aggressive IBC [50]. Another in vitro study showed that treatment with neutralizing antibody against amphiregulin, one of the ligands of EGFR, decreased EGFR activity and reduced cell proliferation in SUM149 cells [51]. These findings led to an ongoing study of panitumumab (an anti-EGFR antibody), albumin-bound paclitaxel (Abraxane), and carboplatin in IBC (NCT01036087). This study will elucidate the biological impact of anti-EGFR therapy on IBC tumors.
EGFR-targeted agents
To date, molecular targeted agents against EGFR have consisted of small molecule EGFR inhibitors (TKIs) (Table 1) and anti-EGFR monoclonal antibodies (MAbs) (Table 2).
Table 1.
EGFR family tyrosine kinase inhibitors (TKIs) under investigation for treatment of breast cancer
| TKI | Target | Class of action | Phase of study |
|---|---|---|---|
| Gefitinib | EGFR | Reversible TKI | Phase I, II |
| Erlotinib | EGFR | Reversible TKI | Phase I, II |
| Aderbasib | EGFR | Reversible TKI | Phase II |
| AE37 | EGFR | Reversible TKI | Phase II |
| AZD4769 | EGFR | TKI | Phase I, solid tumor |
| Lapuleucel-T | EGFR | Designed to stimulate cellular immune responses against HER2/neu |
Phase I |
| CL-3877785 | EGFR | Irreversible TKI | Preclinical |
| Lapatinib | EGFR, ErbB2 | Reversible TKI | In clinical use |
| BIBW2992 | EGFR, ErbB2 | Irreversible TKI | Phase II |
| S222611 | EGFR, ErbB2 | Reversible TKI | Phase I, solid tumor |
| TAK285 | EGFR, ErbB2 | TKI | Phase I, solid tumor |
| AV412 | EGFR, ErbB2 | Irreversible TKI | Phase I |
| PKI-166 | EGFR, ErbB2 | TKI | Phase I, solid tumor |
| Varlitinib (ARRY-334543) | EGFR, ErbB2, ErbB4 | Reversible TKI | Phase II |
| BMS-599626 | EGFR, ErbB2, ErbB4 | Reversible TKI | Phase I |
| EKB-569 | EGFR, ErbB2, ErbB4 | Irreversible TKI | Phase I |
| PF299804 | EGFR, ErbB2, ErbB4 | Irreversible TKI | Phase I, solid tumor |
| AZD8931 | EGFR, ErbB2, ErbB3 | Reversible TKI | Phase I, solid tumor |
| Vandetanib | EGFR, VEGF, RET | TKI | Phase I, II |
| CUDC101 | EGFR, ErbB2, HDAC | Irreversible TKI | Phase I, solid tumor, |
| phase Ib | |||
| Neratinib (HKI-272) | Pan-EGFR | Irreversible TKI | Phase I, II, III |
| Canertinib (CI-1033) | Pan-EGFR | Irreversible TKI | Phase I, II |
| BMS690514 | Pan-EGFR, VEGFR2 | Irreversible TKI | Phase l, solid tumor |
| XL647 | EGFR, ErbB2, EphB4, VEGF | Reversible TKI | Phase I |
| AEE788 | EGFR, ErbB2, VEGFR | Reversible TKI | Phase I |
| ARRY380 | ErbB2, AKT | Reversible TKI | Phase I |
EGFR, epidermal growth factor receptor; HDAC, histone deacetylase; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2.
Table 2.
Monoclonal antibodies (MAbs) against epidermal growth factor receptor under investigation for treatment of breast cancer
| MAb | Class of action | Phase of study |
|---|---|---|
| Cetuximab | Chimeric MAb | Phase I, II |
| Panitumumab | Humanized MAb | Phase II |
| GA 201 | MAb | Phase l, solid tumor |
| Nimotuzumab | Humanized MAb | Phase I |
| Matuzumab | Humanized MAb | Preclinical |
Tyrosine kinases are associated with the cytoplasmic domains of growth factor receptors and oncoproteins, and many tyrosine kinases have the potential to cause transformation if they are mutated or overexpressed. Tyrosine kinases therefore represent an excellent target for the development of cancer drugs [52, 53]. The small molecule inhibitors of EGFR are TKIs that bind to the ATP-binding site in the tyrosine kinase domain of EGFR [54]. TKIs act directly on EGFR but also affect the activities of other kinases in the cell; thus, there is some potential for unfavorable side effects. The small molecule inhibitors of EGFR can be classified as pure EGFR TKIs and dual EGFR and HER2 TKIs.
Whereas small molecule EGFR TKIs are not completely specific for EGFR tyrosine kinase receptors, MAbs are completely specific for the EGFR tyrosine kinase, which could be advantageous. MAbs have less capacity to reach normal intestinal epithelium, which appears to be an advantage for MAb-mediated EGFR blockade since diarrhea was a dose-limiting toxicity with the oral kinase inhibitor such as a Lapatinib but was not observed with the MAbs in general. Moreover, MAbs could work through other mechanisms, including activation of immune responses through mediating antibody-dependent cell-mediated cytotoxicity that is not seen with the use of small molecule EGFR inhibitors.
Clinical trials of EGFR inhibitors for breast cancer
EGFR inhibitors for treatment of breast cancer have been evaluated in several studies (Tables 3 and 4), but results so far have been disappointing.
Table 3.
Clinical trials of EGFR inhibitors as monotherapy
| Drug Studied | First Author, Year (Ref) |
No. of Patients |
Type of Study | Patient Population | Dosage | Response | Patient Outcome |
|---|---|---|---|---|---|---|---|
| Gefitinib | von Minckwitz 2005 (55) | 58 | Phase II | Taxane- and anthracycline-pretreated MBC | 500 mg/d | PR: 1.7% (1/58) SD: 0 PD: 89.7% (52/58) CB: 1.70% (1/58) |
Median time to progression, 61 days (95% CI: 54-82). EGFR evaluated, no correlation between EGFR expression and response. |
| Gefitinib | Baselga 2005 (57) | 31 | Pharmacodynamic, phase II | Advanced breast cancer | 500 mg/d | CR: 0 PR: 0 SD: 38.7% (12/31) PD: 61.3% (19/31) |
Good correlation between degree of inhibition of EGFR in skin and breast tumors. However, lack of EGFR dependence in tested population. |
| Erlotinib | Dickler 2009 (58) | 69 | Phase II | Unselected, previously treated MBC. | 150 mg/d | PR: 3% (2/69) SD: 11.6% (8/69) PD: 79.7% (55/69) |
Erlotinib showed minimal activity. |
| EKB-569 | Erlichman 2006 (70) | Cohort 1: 30 Cohort 2: 29 |
Phase I | Advanced solid tumors | Escalating doses: 25 mg, 50 mg, 75 mg, 125 mg, 175 mg, and 225 mg. Intermittent dosing or continuous dosing |
No major antitumor responses observed. Tolerable toxic effects and long half-life of this irreversible EGFR inhibitor warrant its further evaluation as a single agent and in combination with other drugs. | |
| Lapatinib | Burris 2005 (64) | 67 (30 patients with breast cancer) |
Phase I | ErbB1- and/or ErbB2-expressing advanced refractory solid tumors | 13 pts, 500 mg/d; 15 pts, 650 mg/d; 11 pts, 900 mg/d; 3 pts, 1000 mg/d; 12 pts, 1200 mg/d; 13 pts, 1600 mg/d | PR: 6% (4/67) (all breast cancer pts) SD: 36% (24/67) (10 breast cancer pts) |
Four patients with trastuzumab-resistant MBC, two of whom were classified as having inflammatory breast cancer, had partial responses. |
| Lapatinib | Johnston 2008 (65) | 45 Cohort A: 30 Cohort B: 15 |
Phase II | Anthracycline-refractory MIBC. Cohort A: HER2-overexpressing disease Cohort B: HER2-neg, EGFR-pos disease |
1500 mg/d | Cohort A: PR: 23% (7/30) SD: 23% (7/30) Cohort B: PR: 7% (1/15) |
Lapatinib well tolerated with clinical activity in heavily pretreated HER2-positive but not EGFR-pos/HER2-neg IBC. Co-expression of pHER2 and pHER3 in tumors seemed to predict for a favorable response. |
| Lapatinib | Burstein 2008 (71) | 229 Cohort A: 140 Cohort B: 89 |
Phase II | Previously treated MBC Cohort A: HER2-pos disease Cohort B: HER2-neg disease |
1500 mg/d | Cohort A: CB: 5.7% (CR, PR, or SD ≥ 24 weeks) Cohort B: CB: 0% Only one patients had SD ≥ 16 weeks |
Lapatinib had modest clinical activity in HER2-pos MBC that progressed on prior trastuzumab regimens. No apparent clinical activity was observed in chemotherapy-refractory, HER2-neg disease. |
| Canertinib | Calvo 2004 (72) | 24 (1 BC) | Phase I pharmacokinetic | Advanced solid malignancies | Escalating doses | Recommended dose on this schedule is 250 mg/d. No patient had objective evidence of a major response. | |
| Canertinib | Nemunaitis 2005 (73) | 32 (3 BC) | Phase I pharmacokinetic | Solid tumors refractory to standard therapy | 9 pts: 300 mg/d 3 pts: 350 mg/d 6 pts: 450 mg/d 8 pts: 500 mg/d 6 pts: 560 mg/d |
SD: 19% (6/32) | None of the 3 patients with breast cancer showed a response. |
| BIBW 2992 | Yap 2010 (74) | 53 (4 BC) |
Phase I | Advanced solid tumors | Escalating doses (10 to 50 mg/d) | PR: 15% (5/34) SD: 20% (7/34) |
PR: 4 pts with NSCLC, 1 with esophageal cancer. SD: 1 pt with mesothelioma, 1 with breast cancer, 2 with colorectal cancer, 1 each with cervical cancer, thyroid carcinoma, unknown tumor type. |
| BIBW 2992 | Schuler 2010 (66) | Cohort A: 29 Cohort B: 21 |
Phase II | Cohort A: HER2-neg, HR-pos MBC Cohort B: TNBC |
50 mg/d | Cohort A: PR: 0% SD: 0% Cohort B: SD: 14% (3/21) |
Side effects mainly affected the skin and gastrointestinal tract and were manageable. Clinical benefit was achieved in a fraction of pts with TNBC. |
| Cetuximab | Carey 2008 (59) | 102 | Phase II | TNBC with MBC, no prior platinum | Cetuximab alone (400 mg/m2 then 250 mg/m2 weekly) vs cetuximab + carboplatin | PR: 6% vs 18% SD: 4% vs 9% CB: 10% vs 27% (CB = PR or SD > 6 mo) |
Cetuximab alone was well tolerated and produced some responses. This arm was closed for insufficient activity. The cetuximab + carboplatin arm produced higher response and clinical benefit rates. |
CB, clinical benefit; EGFR, epidermal growth factor receptor; IBC, inflammatory breast cancer; MBC, metastatic breast cancer; neg, negative; MIBC, metastatic inflammatory breast cancer; pos, positive; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease; TNBC, triple-negative breast cancer. MIBC, metastatic inflammatory breast cancer
Table 4.
Clinical trial for EGFR inhibitor: Combination therapy
| Drug Studied, Trial Name |
First Author, Year (Ref) |
No. of Patients |
Type of Study |
Patient Population |
Combination Therapy |
Response | Patient Outcome |
|---|---|---|---|---|---|---|---|
| Gefitinib | Polychronis 2005 (75) | 56 | Phase II randomize d trial | Newly diagnosed postmenopausal, ER-pos, EGFR- pos breast cancer | Arm 1: gefitinib 250 mg/d + anastrozole 1 mg/d Arm 2: gefitinib 250 mg/d + placebo | Arm 1 vs arm 2: CR: 0% vs 0% PR: 44% (12/27) vs 48% (14/29) SD: 37% (10/27) vs 48% (14/29) PD: 0% vs 0% | Tumor size reduction on ultrasonography. Gefitinib either alone or in combination with anastrozole substantially reduced tumor size. |
| Gefitinib | Smith 2007 (76) | 206 | Phase II placebo- controlled randomized trial | Newly diagnosed stage I to IIIB HR-pos breast cancer | Arm 1: anastrozole + gefitinib Arm 2: anastrozole to anastrozole + gefitinib Arm3: anastrozole alone | Anastrozole vs Anastrozole + gefitinib: CR: 4% vs 7% PR: 57% vs 40% SD:33%vs 37% PD: 4% vs 5% CB:61%vs 48% | Study failed. |
| Gefitinib, | Mauriac 2008 (77) | 108 | Phase II randomized trial | MBC | Arm 1: anastrozole + gefitinib Arm 2: anastrozole + placebo | PD: 72% (26/36) | Thirty-six patients completed treatment. The estimated PFS rate at 1 year was 42% |
| Gefitinib | Cristofanilli 2008 (78) | 94 | Phase II randomized trial | Postmenopausal, newly diagnosed HR-pos MBC | Arm 1: anastrozole + gefitinib Arm 2: anastrozole + placebo | Arm 1 vs arm 2: CR: 2% (1/43) vs 2% (1/50) PR: 0% (0) vs 10% (10/50) SD: 47% (20/43)vs 22% (11/50) CB: 49% (21/43) vs 34% (17/50) | Anastrozole + gefitinib was well tolerated and associated with a marked advantage in PFS vs anastrozole + placebo. |
| Gefitinib, NSABP FB IR-002 | Dennison 2007 (79) | 33 | Phase II | MBC | Gefitinib 250 mg/d + docetaxel 75 mg/m2 | CR: 3% (1/33) PR: 36% (12/33) SD: 12% (4/33) PD: 48% (16/33) CB: 52% (17/33) | The median duration of CB was 10.9 months (95% CI, 6.0 to 17.6). |
| Gefitinib, 0024154 ECOG-1100 | Arteaga 2008 (80) | 35 | Phase II | HER2-pos MBC | Trastuzumab 2 mg/kg/week + gefitinib 250 mg/d or 500 mg/d | CR: 3% (1/32) PR: 6% (2/32) SD: 19% (6/32) PD: 28%(9/32) CB: 28% (9/32) | These results do not support the use of this combination in patients with HER2- pos breast cancer. |
| Erlotinib | Mayer 2006 (81) | 22 | Phase II | Postmenopausal HR-sensitive MBC | Letrozole 2.5 mg/d + erlotinib 150 mg/d | CR: 5% (1/20) PR: 20% (4/20) SD: 30% (6/20) PD: 25% (5/20) CB: 55% (11/20) | Median time to progression was 13 months. EGFR immunohistochemistry was negative in all cases. Two of 18 patients had HER2-pos disease. |
| Erlotinib | Twelves 2008 (82) | 24 | Phase II | MBC | Capecitabine + docetaxel + erlotinib in several dosages. | CR: 8% (2/24) PR: 50% (12/24) SD: 21% (5/24) PD: 8% (2/24) | Recommended for further study is erlotinib 100 mg/d continuously with capecitabine 825 mg/m2 bid and docetaxel 75 mg/m2 intravenously. The exposure to the three drugs is not diminished when they are given in combination. |
| Erlotinib | Venturini 2004 (83) | 9 | Phase II dose-finding study | MBC | Erlotinib 50 mg/d, 100mg/d, or 150 mg/d sequentially after capecitabine + vinorelbine for 6 cycles or 8 cycles | PR: 50% (3/6) SD: 33% (2/6) PD: 17% (1/6) | Established maximum tolerated dose. |
| Erlotinib | Kaur 2006 (84) | 31 | Phase II | MBC | Docetaxel 35 mg/m2 weekly + erlotinib 150 mg/d | PR: 55% (11/20) SD: 35% (7/20) PD: 10% (2/20) | Promising activity with favorable response compared to other studies. |
| Erlotinib | Dickler 2008 (85) | 38 | Phase II | MBC | Erlotinib 150 mg + bevacizumab 15 mg/kg intravenously every 3 weeks | PR: 2.7% (1/37) SD:51.3% (19/37) PD:51.3% (19/37) | EGFR expression was not predictive of response to therapy. |
| Erlotinib | Beeram 2005 (86) | 16 (14 patients with breast cancer) | Phase I | Advanced solid tumors | Erlotinib 50 mg/d, 100 mg/d, or 150 mg/d + trastuzumab 2 mg/kg/week + paclitaxel 80 mg/m2 | 1 CR, 2 PRs, 1 minor response observed in patients with breast cancer in whom prior trastuzumab therapy failed. | |
| Lapatinib | Johnston 2009 (87) | 1286 | Phase III randomized trial | Postmenopausal, HR-pos MBC | Arm A: letrozole 2.5 mg/d + lapatinib 1500 mg/d Arm B: letrozole 2.5 mg/d + placebo | HER2-pos patients, arm B: CR: 5% vs 4% PR: 23% vs 11% SD:20%vs 14% CB:48%vs 29% HER2-neg patients, arm Avs arm B: CR: 5% vs 4% 27% SD:26%vs CB:58%vs 56% | In HER2-pos patients (n=219), addition of lapatinib to letrozole significantly reduced the risk of disease progression (hazard ratio = 0.71; 95% CI, 0.53 to 0.96; P = 0.019). In HER2-neg patients (n=952), no improvement in PFS was observed with addition of lapatinib. |
| Lapatinib | Di Leo 2008 (88) | 579 | Phase III randomized trial | HER2-neg or HER2-untested MBC | Arm A: paclitaxel 175 mg/m2 every 3 weeks + lapatinib 1500 mg/d Arm B: paclitaxel 175 mg/m2 every 3 weeks + placebo | HER2-pos patients, arm Avs arm B: CR: 10% vs 3% PR: 53% vs 35% SD: 18%vs 30% PD: 14% vs 22% CB: 69.4% vs 40.5% P=0.011 HER2-neg patients, arm A vs arm B: CR: 3% vs 2% PR: 27% vs 21% SD: 34% vs 46% PD:25%vs 25% CB: 4.7% vs 31.9% P=0.806 | Neither patients with HER2-neg disease nor those with HER2- untested disease benefited from the addition of lapatinib to paclitaxel. |
| Canertinib | Garland 2006 (89) | 26 | Phase I | Advanced solid tumors | Canertinib 50–75 mg/d + docetaxel 75 mg/m2 | CR: 4.7% (1/21) PR: 4.7% (1/21) SD: 4.7% (1/21) PD: 85.7% (18/21) | Resulted in a recommended phase II dose of canertinib 50 mg/d + docetaxel 75 mg/m2. |
| Cetuximab | Modi 2006 (89) | 12 | Phase I dose-escalation study | EGFR-pos MBC | Cetuximab + paclitaxel | SD: 17% (2/12) PD: 67% (8/12) | Because of prohibitive dermatologic toxic effects and disappointing preliminary efficacy, the combination was not considered promising in this population. |
| Cetuximab | Baselga2010 (61) | 173 | Phase II | TNBC or MBC | Arm 1: cetuximab 400 mg/m2 initial dose then 250 mg/m2 weekly + cisplatin 75 mg/m2 Arm 2: cisplatin alone (after 6 cycles could switch to cetuximab + cisplatin) | Overall response rate, arm 1 vs arm 2: 20.0% vs 10.3% | Adding cetuximab to cisplatin nearly doubled the overall response rate. Cetuximab + cisplatin was associated with a significant 32.5% reduction in the risk of disease progression compared with cisplatin alone (hazard ratio = 0.675; P =0.032). |
| Cetuximab, USOR04-070 | O'Shaughnessy 2010(60) | 154 | Phase II randomized trial | MBC | Arm 1: irinotecan + carboplatin Arm 2: irinotecan + carboplatin + cetuximab | Cetuximab did not improve antitumor activity, but subset analysis showed that the addition of cetuximab increased the overall response rate associated with irinotecan + cisplatin in TNBC (49% vs 38%). | |
| Cetuximab | Rivera 2011 | 43 | Retrospective | Advanced TNBC | Cisplatin 50 mg/m2 or carboplatin + cetuximab 400 mg/m2 initial dose then 250 mg/m2 weekly | Objective response rate: 45.9% | Median treatment duration was 2.3 months. The objective response rate was 45.9% among 37 evaluable patients. Median time to progression was 2.3 months (95% CI = 1.9 to 4.0). |
| Panitumumab | Nabholtz2011 (62) | 58 | Pilot phase II | Newly diagnosed TNBC | FEC100 (fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2) every 3 weeks for 4 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 4 cycles + panitumumab 9 mg/kg every 3 weeks for 8 cycles | Pathologic CR: 17 patients Overall clinical response rate: 80% | Preliminary results suggest that panitumumab in combination with FEC100 followed by docetaxel appears efficacious with acceptable toxicity for neoadjuvant therapy for operable TNBC. |
bid, twice a day; CB, clinical benefit; CR, complete response; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ESMO, European Society for Medical Oncology; HR, hormone receptor; neg, negative; neg, negative; NSABP, National Surgical Adjuvant Breast and Bowel Project; PD, progressive disease; PFS, progression-free survival; pos, positive; PR, partial response; SD, stable disease; TNBC, triple-negative breast cancer; USOR, US Oncology Research.
Gefitinib monotherapy did not significantly improve response rates in most studies [55–57]. In a phase II trial of erlotinib monotherapy in patients with metastatic breast cancer (n=69), no patients had a complete response, and only 2 had a partial response [58]. These rather disappointing results may relate to the patient selection criteria: these trials did not select patients on the basis of EGFR expression; rather, they included unselected breast cancer patients who had often been heavily pretreated. Subgroup analysis of results of previous clinical trials may offer clues to optimal patient selection for EGFR-targeted therapy.
Two phase II clinical trials evaluated the efficacy of cetuximab alone or in combination with platinum-based chemotherapy and found that addition of cetuximab to carboplatin did not improve outcome [59, 60]. However in one of these trials [59], cetuximab alone was associated with a 6% response rate, suggesting that this drug could have some efficacy in selected patients. In that trial, the authors also investigated EGFR pathway activation using gene expression profiling with Agilent DNA microarrays. The findings suggested that cetuximab blocked expression of the EGFR pathway in only a minority of patients, indicating that most patients had alternate mechanisms of pathway activation [59].
Another phase II trial in patients with advanced breast cancer showed that cetuximab improved the overall response rate only in patients with TNBC: in this subgroup, overall response rates were 38% for patients treated with irinotecan and carboplatin and 49% for patients treated with irinotecan, carboplatin, and cetuximab [60]. EGFR positivity was not an eligibility criterion for this trial, but retrospective assessment showed that EGFR positivity was significantly associated with the TNBC subtype (P<0.0001).
European researchers were the first to prove that anti-EGFR therapy can provide substantial clinical benefit for patients with TNBC [61]. These researchers conducted a phase II randomized trial of cisplatin versus cisplatin plus cetuximab in 173 heavily pretreated patients with TNBC. The overall response rate was twice as high with the cisplatin-cetuximab combination as it was with cisplatin alone (20% vs. 10.3%). Also, the median progression-free survival time was more than twice as long with cetuximab as it was with cisplatin alone (3.7 months vs. 1.5 months).
Panitumumab, an antibody targeting EGFR, has also been investigated for its efficacy in patients with TNBC. In a phase II trial of panitumumab in combination with 5-fluorouracil, epidoxorubicin, and cyclophosphamide followed by docetaxel for neoadjuvant therapy, the overall response rate was 80% [62]. A trial of panitumumab in combination with carboplatin for patients with metastatic TNBC is ongoing (NCT00894504).
Taken together, the findings to date on EGFR-targeted agents suggest that further investigation of these agents in patients with TNBC is warranted [63].
Clinical trials of dual EGFR and HER2 inhibitors for breast cancer
In a phase I clinical trial, lapatinib monotherapy showed clinical activity in patients with trastuzumab-refractory breast cancer. Four of the 59 evaluable patients with metastatic breast cancer positive for both EGFR and HER2, including 2 with IBC, had a partial response [64]. In a phase II trial of lapatinib monotherapy for heavily pretreated patients with IBC, the response rate was 50% among the 30 patients with HER2-positive tumors but only 7% among the 15 patients with HER2-negative, EGFR-positive tumors [65]. The investigators also evaluated ErbB3 status and found that co-expression of phosphorylated HER2 and phosphorylated ErbB3 was associated with a better response rate. In a phase II trial, BIBW 2992, a novel oral, irreversible EGFR and HER2 inhibitor, had a rate of clinical benefit of 14% in 21 patients with metastatic TNBC [66]. At present, response to dual EGFR and HER2 inhibitors seems to depend on HER2 expression rather than EGFR expression. Further studies of dual inhibitors are required.
Conclusions
Previous trials showed that many patients with EGFR-expressing tumors did not respond to EGFR-targeted therapy, which suggests that EGFR expression alone does not indicate tumor cell dependence on the EGFR pathway. There is now evidence for significant interactions of EGFR with other receptor tyrosine kinases, such as c-MET and IGF-1R, and it is possible that such alternative signaling pathways are linked to resistance to targeted therapies [36]. Thus, we have to consider combining EGFR-targeted therapy with drugs targeting these alternate signaling pathways to improve efficacy. Further, EGFR activation drives migration and invasion through EMT and alters chemosensitivity by rewiring the apoptotic signaling network. Therefore, EGFR-targeted therapy may not produce cancer shrinkage due to suppression of cell proliferation; rather, EGFR-targeted therapy may produce a therapeutic effect by inhibiting metastasis or sensitizing cancer cells to the effects of cytotoxic therapy.
Recent studies confirm that EGFR is a potentially important target in breast cancer, especially TNBC, basal-like breast cancer, and IBC. EGFR-targeted therapy has finally shown some promise in terms of improving outcomes in breast cancer patients, but molecular prognostic and predictive factors need to be identified to optimize selection of patients for EGFR-targeted therapies. Mechanistic, hypothesis-oriented clinical trials are needed rather than trials based on the assumption that EGFR-targeted therapy will be effective against EGFR-overexpressing breast cancer. Further, recent data suggest that expecting EGFR-targeted therapy to produce tumor shrinkage may be unrealistic. EGFR-targeted therapy may be most effective as a chemosensitizer or therapy designed to prevent metastases.
Acknowledgments
Financial support: This research was supported by NIH grant R01 CA123318 (NT Ueno), MD Anderson’s Cancer Center Support Grant, CA016672, the Morgan Welch Inflammatory Breast Cancer Research Program and Clinic, a State of Texas Rare and Aggressive Breast Cancer Research Program grant (NT Ueno), and Susan G. Komen Postdoctoral Fellowship KG091192 (D Zhang).
Footnotes
Conflicts of interest: The authors have no conflicts to declare.
References
- 1.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 2.Sainsbury JR, Farndon JR, Needham GK, Malcolm AJ, Harris AL. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987;1:1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- 3.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 4.Burness ML, Grushko TA, Olopade OI. Epidermal growth factor receptor in triplenegative and basal-like breast cancer: promising clinical target or only a marker? Cancer J. 2010;16:23–32. doi: 10.1097/PPO.0b013e3181d24fc1. [DOI] [PubMed] [Google Scholar]
- 5.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 6.Guerin M, Gabillot M, Mathieu MC, Travagli JP, Spielmann M, Andrieu N, et al. Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer. 1989;43:201–208. doi: 10.1002/ijc.2910430205. [DOI] [PubMed] [Google Scholar]
- 7.Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984;307:521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- 8.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005–2008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eccles SA. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol. 2011;55:685–696. doi: 10.1387/ijdb.113396se. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 12.Martinazzi M, Crivelli F, Zampatti C, Martinazzi S. Relationships between epidermal growth factor receptor (EGF-R) and other predictors of prognosis in breast carcinomas. An immunohistochemical study. Pathologica. 1993;85:637–644. [PubMed] [Google Scholar]
- 13.Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:485–498. doi: 10.1007/s10911-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 14.Menard S, Balsari A, Casalini P, Tagliabue E, Campiglio M, Bufalino R, et al. HER-2-positive breast carcinomas as a particular subset with peculiar clinical behaviors. Clin Cancer Res. 2002;8:520–525. [PubMed] [Google Scholar]
- 15.Fallon KB, Palmer CA, Roth KA, Nabors LB, Wang W, Carpenter M, et al. Prognostic value of 1p, 19q, 9p, 10q, and EGFR-FISH analyses in recurrent oligodendrogliomas. J Neuropathol Exp Neurol. 2004;63:314–322. doi: 10.1093/jnen/63.4.314. [DOI] [PubMed] [Google Scholar]
- 16.Giaccone G. Epidermal growth factor receptor inhibitors in the treatment of non-small-cell lung cancer. J Clin Oncol. 2005;23:3235–3242. doi: 10.1200/JCO.2005.08.409. [DOI] [PubMed] [Google Scholar]
- 17.Al-Kuraya K, Schraml P, Torhorst J, Tapia C, Zaharieva B, Novotny H, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64:8534–8540. doi: 10.1158/0008-5472.CAN-04-1945. [DOI] [PubMed] [Google Scholar]
- 18.Ro J, North SM, Gallick GE, Hortobagyi GN, Gutterman JU, Blick M. Amplified and overexpressed epidermal growth factor receptor gene in uncultured primary human breast carcinoma. Cancer Res. 1988;48:161–164. [PubMed] [Google Scholar]
- 19.Spyratos F, Delarue JC, Andrieu C, Lidereau R, Champeme MH, Hacene K, et al. Epidermal growth factor receptors and prognosis in primary breast cancer. Breast Cancer Res Treat. 1990;17:83–89. doi: 10.1007/BF01806288. [DOI] [PubMed] [Google Scholar]
- 20.Yatabe Y, Kosaka T, Takahashi T, Mitsudomi T. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol. 2005;29:633–639. doi: 10.1097/01.pas.0000157935.28066.35. [DOI] [PubMed] [Google Scholar]
- 21.Reis-Filho JS, Pinheiro C, Lambros MB, Milanezi F, Carvalho S, Savage K, et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol. 2006;209:445–453. doi: 10.1002/path.2004. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, Ladanyi M, et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005;18:1027–1033. doi: 10.1038/modpathol.3800438. [DOI] [PubMed] [Google Scholar]
- 23.Weber F, Fukino K, Sawada T, Williams N, Sweet K, Brena RM, et al. Variability in organ-specific EGFR mutational spectra in tumour epithelium and stroma may be the biological basis for differential responses to tyrosine kinase inhibitors. Br J Cancer. 2005;92:1922–1926. doi: 10.1038/sj.bjc.6602557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall- cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 26.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 27.Sainsbury JR, Nicholson S, Angus B, Farndon JR, Malcolm AJ, Harris AL. Epidermal growth factor receptor status of histological sub-types of breast cancer. Br J Cancer. 1988;58:458–460. doi: 10.1038/bjc.1988.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa S, Ueda M, Ando N, Shimizu N, Abe O. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer. 1989;63:2169–2173. doi: 10.1002/1097-0142(19890601)63:11<2169::aid-cncr2820631117>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, et al. Invasive ductal carcinoma of the breast with the "triple-negative" phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 30.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 33.Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. discussion 5. [DOI] [PubMed] [Google Scholar]
- 34.Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol. 2009;5:1129–1143. doi: 10.2217/fon.09.94. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–541. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, et al. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res. 2009;15:6639–6648. doi: 10.1158/1078-0432.CCR-09-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doehn U, Hauge C, Frank SR, Jensen CJ, Duda K, Nielsen JV, et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol Cell. 2009;35:511–522. doi: 10.1016/j.molcel.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santamaria PG, Nebreda AR. Deconstructing ERK signaling in tumorigenesis. Mol Cell. 2010;38:3–5. doi: 10.1016/j.molcel.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 42.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 43.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 44.Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 45.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011:121. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, Macbeath G, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zell JA, Tsang WY, Taylor TH, Mehta RS, Anton-Culver H. Prognostic impact of human epidermal growth factor-like receptor 2 and hormone receptor status in inflammatory breast cancer (IBC): analysis of 2,014 IBC patient cases from the California Cancer Registry. Breast Cancer Res. 2009;11:R9. doi: 10.1186/bcr2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabioglu N, Gong Y, Islam R, Broglio KR, Sneige N, Sahin A, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18:1021–1029. doi: 10.1093/annonc/mdm060. [DOI] [PubMed] [Google Scholar]
- 50.Mueller KL, Yang ZQ, Haddad R, Ethier SP, Boerner JL. EGFR/Met association regulates EGFR TKI resistance in breast cancer. J Mol Signal. 2010;5:8. doi: 10.1186/1750-2187-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baillo A, Giroux C, Ethier SP. Knock-down of amphiregulin inhibits cellular invasion in inflammatory breast cancer. J Cell Physiol. 2011;226:2691–2701. doi: 10.1002/jcp.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 53.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 55.von Minckwitz G, Jonat W, Fasching P, du Bois A, Kleeberg U, Luck HJ, et al. A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005;89:165–172. doi: 10.1007/s10549-004-1720-2. [DOI] [PubMed] [Google Scholar]
- 56.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 57.Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 58.Dickler MN, Cobleigh MA, Miller KD, Klein PM, Winer EP. Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2009;115:115–121. doi: 10.1007/s10549-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 59.Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012 doi: 10.1200/JCO.2010.34.5579. published online on June 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khambata-Ford S, O'Shaughnessy J, Brickman D, et al. Candidate predictive biomarkers of cetuximab benefit in triple-negative breast cancer. J Clin Oncol. 2010;28(suppl) abstr 1056. [Google Scholar]
- 61.Baselga J, Steemmer S, Pego A, et al. Cetuximab+cisplatin in estrogen receptor-negative, progesterone receptor-negative, HER2-negative (triple-negative) metastatic breast cancer: results of the randomized phase II BALI-1 trial. Cancer Res. 2010;70(24) Suppl SABCS10-PD01-01. [Google Scholar]
- 62.Nabholtz J, Weber B, Mouret-Reynier M, et al. Panitumumab in combination with FEC100 (5-fluorouracil, epidoxorubicin, cyclophosphamide) followed by docetaxel (T) in patients with operable, triple negative breast cancer (TNBC): preliminary results of a multicenter neoadjuvant pilot phase II study. J Clin Oncol. 2011;29(suppl) abstr e11574. [Google Scholar]
- 63.Corkery B, Crown J, Clynes M, O'Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20:862–867. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 64.Burris HA, 3rd, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O'Neil B, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 65.Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 66.Schuler MH, Uttenreuther-Fischer MM, Piccart-Gebhart MJ, et al. BIBW 2992, a novel irreversible EGFR/HER1 and HER2 tyrosine kinase inhibitor, for the treatment of patients with HER2-negative metastatic breast cancer after failure of no more than two prior chemotherapies. J Clin Oncol. 2010;28(suppl) abstr 1065. [Google Scholar]
- 67.Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 68.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 69.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 70.Erlichman C, Hidalgo M, Boni JP, et al. Phase I study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2006;24:2252–2260. doi: 10.1200/JCO.2005.01.8960. [DOI] [PubMed] [Google Scholar]
- 71.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 72.Calvo E, Tolcher AW, Hammond LA, et al. Administration of CI-1033, an irreversible pan-erbB tyrosine kinase inhibitor, is feasible on a 7-day on, 7-day off schedule: a phase I pharmacokinetic and food effect study. Clin Cancer Res. 2004;10:7112–7120. doi: 10.1158/1078-0432.CCR-04-1187. [DOI] [PubMed] [Google Scholar]
- 73.Nemunaitis J, Eiseman I, Cunningham C, et al. Phase 1 clinical and pharmacokinetics evaluation of oral CI-1033 in patients with refractory cancer. Clin Cancer Res. 2005;11:3846–3853. doi: 10.1158/1078-0432.CCR-04-1950. [DOI] [PubMed] [Google Scholar]
- 74.Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 75.Polychronis A, Sinnett HD, Hadjiminas D, et al. Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. Lancet Oncol. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- 76.Smith IE, Walsh G, Skene A, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25:3816–3822. doi: 10.1200/JCO.2006.09.6578. [DOI] [PubMed] [Google Scholar]
- 77.Mauriac L, Cameron D, Dirix L, et al. Results of randomized phase II trial combining Iressa (gefitinib) and Arimidex in women with advanced breast cancer (ABC) EORTC protocol 10021. 2008:S6133. [Google Scholar]
- 78.Cristofanilli M, Valero V, Mangalik A, et al. A phase II multicenter, double-blind, randomized trial to compare anastrozole plus gefitinib with anastrozole plus placebo in postmenopausal women with hormone receptor-positive (HR+) metastatic breast cancer (MBC) J Clin Oncol. 2008;26 (May 20 suppl): abstr 1012. [Google Scholar]
- 79.Dennison SK, Jacobs SA, Wilson JW, et al. A phase II clinical trial of ZD1839 (Iressa) in combination with docetaxel as first-line treatment in patients with advanced breast cancer. Invest New Drugs. 2007;25:545–551. doi: 10.1007/s10637-007-9055-6. [DOI] [PubMed] [Google Scholar]
- 80.Arteaga CL, O'Neill A, Moulder SL, et al. A phase I–II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2)-overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14:6277–6283. doi: 10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayer I, Granja N, Shyr Y, et al. A phase II trial of letrozole plus erlotinib in post menopausal women with hormone-sensitive metastatic breast cancer (MBC): preliminary results of toxicities and correlative studies. 2006:S4052. [Google Scholar]
- 82.Twelves C, Trigo JM, Jones R, et al. Erlotinib in combination with capecitabine and docetaxel in patients with metastatic breast cancer: a dose-escalation study. Eur J Cancer. 2008;44:419–426. doi: 10.1016/j.ejca.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Venturini M, Catzeddu T, Del L, et al. Erlotinib given sequentially to capecitabine and vinorelbine as first-second line chemotherapy in metastatic breast cancer patients. A dose finding study. J Clin Oncol. 2004;22(suppl) abstr 834. [Google Scholar]
- 84.Kaur H, Silverman P, Singh D, et al. Toxicity and outcome data in a phase II study of weekly docetaxel in combination with erlotinib in recurrent and/or metastatic breast cancer (MBC) J Clin Oncol. 2006;24 (June 20 suppl): abstr 10623. [Google Scholar]
- 85.Dickler MN, Rugo HS, Eberle CA, et al. A phase II trial of erlotinib in combination with bevacizumab in patients with metastatic breast cancer. Clin Cancer Res. 2008;14:7878–7883. doi: 10.1158/1078-0432.CCR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beeram M, De Bono JS, Pamaik A, et al. Phase I and pharmacokinetics (PK) of combined erbB1 and erbB2 blockade with OSI-774 (Erlotinib; E) and trastuzumab (T) in combination with weekly paclitaxel (P) in patients (pts) with advanced solid tumors. 2005:S2034. [Google Scholar]
- 87.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 88.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garland LL, Hidalgo M, Mendelson DS, et al. A phase I clinical and pharmacokinetic study of oral CI-1033 in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2006;12:4274–4282. doi: 10.1158/1078-0432.CCR-05-2507. [DOI] [PubMed] [Google Scholar]
- 90.Modi S, D'Andrea G, Norton L, et al. A phase I study of cetuximab/paclitaxel in patients with advanced-stage breast cancer. Clin Breast Cancer. 2006;7:270–277. doi: 10.3816/CBC.2006.n.040. [DOI] [PubMed] [Google Scholar]
- 91.Rivera P, Filleron T, Gladieff L, et al. Efficacy of cetuximab plus platinum agent in advanced, triple-negative breast carcinoma: Results of a retrospective analysis. J Clin Oncol. 2011;29(suppl) abstr e11581. [Google Scholar]