Abstract
We have fractionated crude porcine heparin to obtain highly active as well as relatively inactive species of molecular weight ≈7000 with specific anticoagulant activities of 360 and 12 units/mg, respectively. Nitrous acid degradation of both of these polymers yielded a tetrasaccharide fraction, 1β, that contained equimolar amounts of iduronic and glucuronic acids, possessed an internal N-acetylated glucosamine, and carried anhydromannitol at the reducing end position. The 1β tetrasaccharide derived from the highly active heparin, 1βa, was recovered in a yield of 1.1 mol/7000 daltons. Our analyses indicate that at least 95% of the 1βa is a single structure that consists of the following unique monosaccharide sequence: L-iduronic acid → N-acetylated D-glucosamine-6-sulfate → D-glucuronic acid → N-sulfate D-glucosamine-6-sulfate. The 1β tetrasaccharide fraction from relatively inactive mucopolysaccharide, 1βi, was recovered in a yield of 0.3 mol/7000 daltons and was a mixture of several components. Only 8.5% of the 1βi tetrasaccharide fraction exhibited the same uronic acid placement and sulfate group position found in 1βa. Thus, 2.6% of relatively inactive mucopolysaccharide molecules contain the unique tetrasaccharide sequence found within each molecule of highly active heparin. Given the correlation between abundance of this unique 1βa tetrasaccharide sequence and biologic potency, we suggest that this structure represents the critical site responsible for anticoagulant activity.
Keywords: mucopolysaccharide, anticoagulant function
Full text
PDF
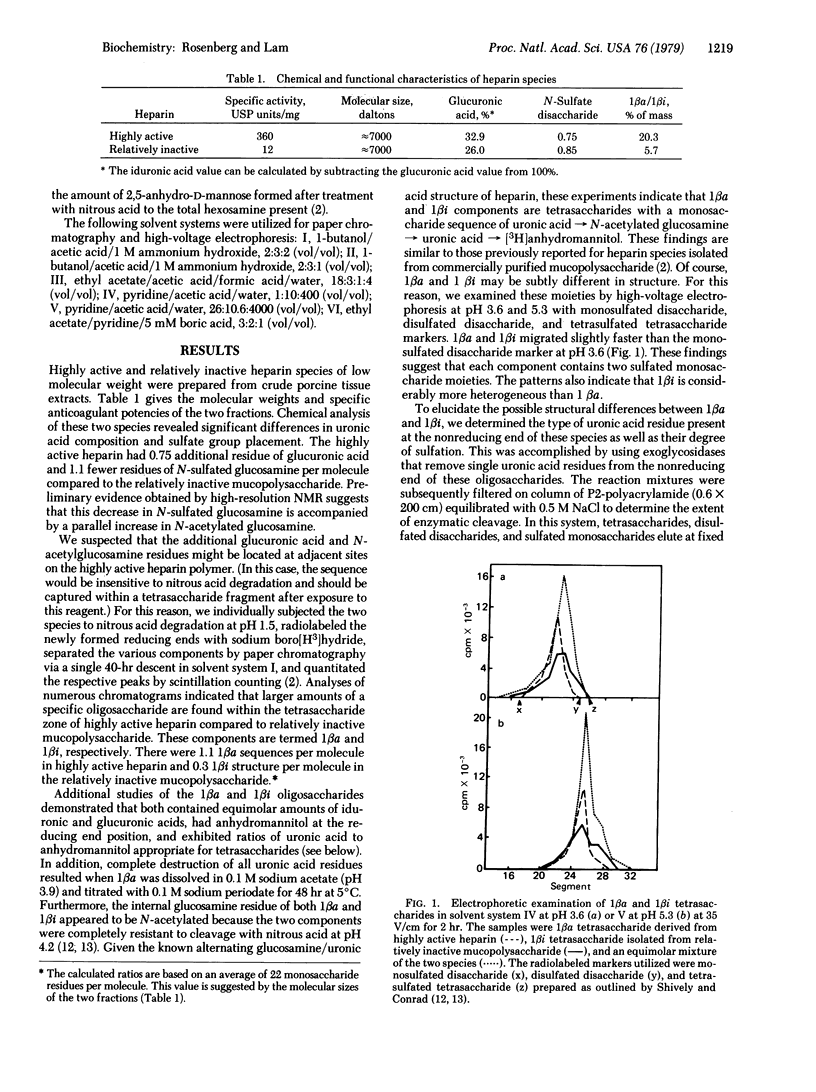

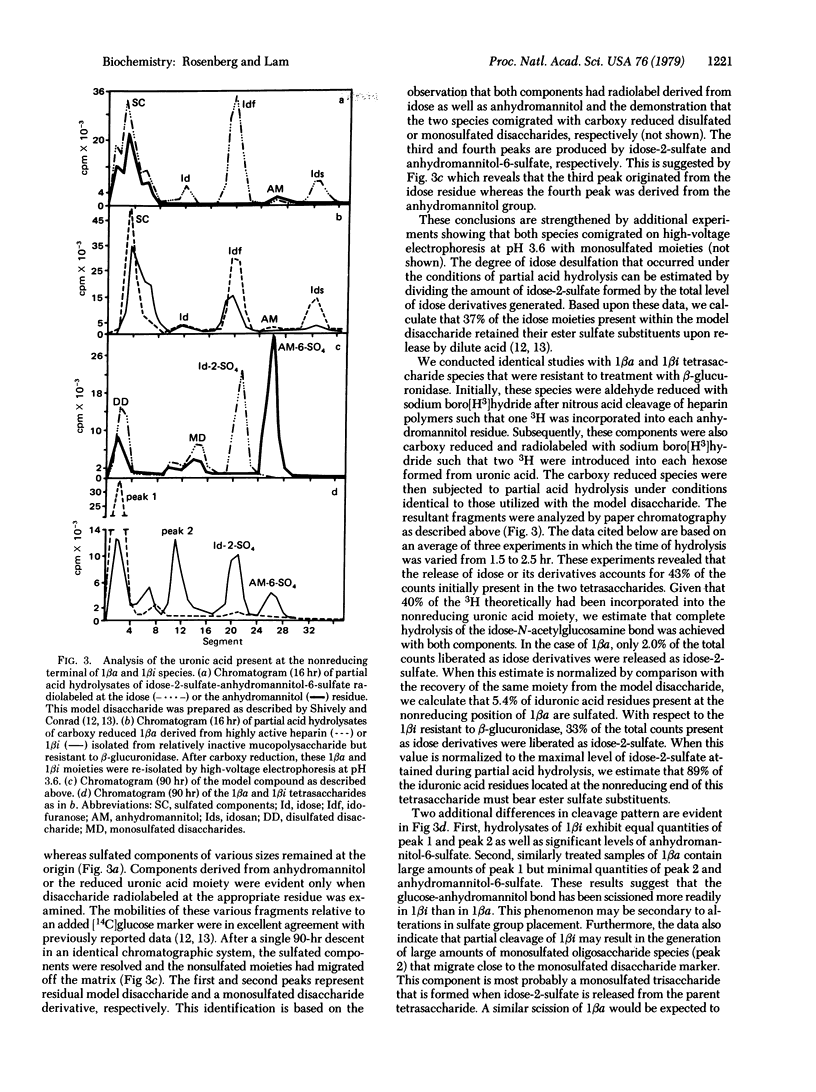

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Balazs E. A., Berntsen K. O., Karossa J., Swann D. A. An automated method for the determination of hexosamines. Anal Biochem. 1965 Sep;12(3):559–564. doi: 10.1016/0003-2697(65)90222-8. [DOI] [PubMed] [Google Scholar]
- Brot F. E., Bell C. E., Jr, Sly W. S. Purification and properties of beta-glucuronidase from human placenta. Biochemistry. 1978 Feb 7;17(3):385–391. doi: 10.1021/bi00596a001. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Huckerby T. N., Nieduszynski I. A. alpha-L-iduronate ring conformations in heparin and heparin derivatives. 13-C Nuclear-magnetic-resonance analysis and titration data for variously desulphated and periodate-oxidized heparins. Biochem J. 1978 Oct 1;175(1):299–309. doi: 10.1042/bj1750299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Lim T. W., Leder I. G., Bach G., Neufeld E. F. An assay for iduronate sulfatase (Hunter corrective factor). Carbohydr Res. 1974 Oct;37(1):103–109. doi: 10.1016/s0008-6215(00)87067-6. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp B. V., Cole R. D. Purification and characterization of bovine liver beta-glucuronidase. Arch Biochem Biophys. 1966 Sep 26;116(1):193–206. doi: 10.1016/0003-9861(66)90027-0. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]



