Abstract
Background
Functional interactions between mu- and delta- opioid receptors (MOPr and DOPr, respectively) are implicated in morphine tolerance and dependence. The contribution of DOPr to the conditioned rewarding effects of morphine and the enhanced conditioned response that occurs following repeated morphine administration is unknown. This issue was addressed using the conditioned place preference procedure (CPP).
Methods
Rats received home cage injections of saline or morphine (5.0 mg/kg/day × 5 days) prior to conditioning. For sensitization studies, DOPr antagonists (DOPr1/2: naltrindole, DOPr2: naltriben, DOPr1: 7-benzylidenenaltrexone) were administered prior to morphine injections. Conditioning sessions (2 morphine; 2 saline) commenced 3 days later. To assess the influence of acute DOPr blockade on the conditioning of morphine reward in naïve animals, 3 morphine and 3 saline conditioning sessions were employed. Antagonists were administered prior to morphine conditioning sessions.
Results
Morphine was ineffective as a conditioning stimulus after 2 conditioning sessions in naïve rats. However, doses ≥ 3.0 mg/kg produced significant CPP in morphine pre-exposed rats confirming that sensitization develops to the conditioned rewarding effects of morphine. In animals that received morphine pre-exposure with naltrindole or naltriben, but not 7-benzylidenenaltrexone, sensitization was prevented. No attenuation of morphine CPP was observed in animals that received DOPr antagonists acutely, prior to conditioning sessions.
Conclusion
These data indicate a critical role of DOPr systems in mediating sensitization to the conditioned rewarding effects of morphine. The efficacy of naltrindole and naltriben in preventing the enhanced response to morphine suggest the specific involvement of DOPr2 in the sensitization process.
Keywords: morphine, sensitization, conditioned place preference, delta opioid receptor, naltrindole, naltriben
Introduction
Exposure to stimuli associated with opiate administration elicits drug-craving and increased risk of relapse (1, 2). Accordingly, one focus of addiction research is the identification of the neural substrates mediating the conditioned reinforcing effects of drugs and pharmacological treatments that can prevent their development and/or expression.
The conditioned place preference (CPP) paradigm has been used extensively to study the contextual/cued associations that develop to drugs of abuse in experimental animals (3, 4). CPP studies have demonstrated that the rewarding effects of opiates become conditioned to stimuli associated with their administration. Several laboratories have shown that the conditioned rewarding effects of mu-opioid receptor (MOPr) agonists are enhanced in animals with a prior history of opiate exposure (5–8). Morphine doses that fail to produce CPP in naïve rats produce robust CPP in animals that received MOPr agonists repeatedly prior to the commencement of conditioning. The efficacy of morphine is also increased.
The opioid receptor types mediating the sensitized response to MOPr agonists are unclear. Repeated administration of the non-selective opioid receptor antagonist, naloxone (0.5 mg/kg) with morphine or fentanyl blocked the enhancement of CPP confirming opioid receptor mediation. However, a lower naloxone dose (0.1 mg/kg) that antagonizes the effects of MOPr-, but not delta-(DOPr) or kappa-opioid receptor (KOPr) agonists was ineffective (7). KOPr antagonism enhances sensitization to MOPr agonists (9). Therefore, attenuation of sensitization can not be attributed to KOPr blockade. These findings suggest that a DOPr-dependent mechanism may underlie sensitization to the conditioned rewarding effects of MOPr agonists.
Functional interactions of MOPr and DOPr have been described. Antisense oligonucleotides to DOPr attenuate morphine dependence (10, 11). Similarly, pharmacological antagonism or genetic deletion of DOPr reduces dependence and analgesic tolerance (12). Repeated MOPr agonist administration increases DOPr cell surface expression in brain and spinal cord (13, 14). MOPr and DOPr heterodimerization has been reported (15, 16). Evidence that the DOPr2 subtype may correspond to a MOPr-DOPr complex has been obtained (17, 18). Together these data indicate that chronic morphine exposure alters DOPr function and that aberrant DOPr activity may contribute to the behavioral dysregulation produced by repeated MOPr activation.
An involvement of DOPr in morphine tolerance and dependence has been demonstrated (10, 11, 12). The role of DOPr in sensitization to the rewarding effects of morphine is unknown. An unbiased CPP procedure was used to address this issue. Morphine conditioning was assessed in naïve rats and those that received a sensitizing morphine treatment alone or with a DOPr antagonist prior to conditioning. Because pharmacological data indicate DOPr subtypes (DOPr1; DOPr2), the DOPr 1/2 antagonist, naltrindole (19), the DOPr2 antagonist, naltriben, (20) and the DOPr1 antagonist, 7-benzylidenenaltrexone (BNTX; 21) were evaluated.
Materials and Methods
Animals
Male Sprague-Dawley rats (300–350 g; Charles River) were housed in a temperature controlled colony room and maintained on a 12 h:12 h light:dark cycle with food and water available ad-libitum. Facilities were accredited by AALAC and experiments were approved by the NIDA Institutional Care and Use Committee.
Drug Treatments
For sensitization studies, rats received home-cage injections of saline or morphine (5.0 mg/kg/day × 5 days; s.c). Fifteen min prior to injections they received an s.c injection of saline, naltindole, naltriben or BNTX. Conditioning commenced 3 days later. Experiments were conducted in squads of twenty animals. Separate squads of animals were run for each antagonist treatment. Each squad consisted of control and experimental animals. Controls animals received vehicle prior to repeated morphine injections. Experimental animals received vehicle or one of the assigned antagonist doses prior to morphine injection. Morphine-evoked conditioning was then assessed. The influence of prior, repeated administration of antagonist, alone, on morphine conditioning was assessed in additional squads of animals. Conditioning commenced 3 days later.
To assess the effects of antagonist treatment on the establishment of morphine CPP, additional rats received injections of antagonist or vehicle 15 min prior to morphine conditioning sessions.
CPP Procedure
Conditioning was conducted in Plexiglas boxes (7, 22) under dim illumination (8.5–10 lux). Under these conditions, rats exhibit no preference for either of the place cues. Two conditionings sessions (50 min)/day were conducted with at least 6 hrs separating each. Rats were injected with saline and confined to one compartment. Following morphine administration (3.0; 5.0 mg/kg; s.c) they were confined to the other compartment. For sensitization studies, four conditioning sessions (2 morphine; 2 saline) were conducted since 2 drug pairings is insufficient for the development of CPP in animals that are opiate naïve prior to the commencement of conditioning (7, 22). To assess the effects of acute DOPr antagonism on the establishment of CPP, six conditioning sessions (3 morphine; 3 saline) were conducted since three drug pairings produces robust CPP in opiate naive animals (7, 22; 23). Treatment compartment and the presentation order of morphine and saline were counterbalanced. Conditioning was assessed 1 day after the last conditioning session. Uninjected rats were allowed access to both compartments for 900 sec. Time spent in each was assessed by analysis of the video-recorded test session. Data analysis was conducted by a blinded observer. The rat’s location was determined by the position of the front paws.
Statistical Analysis
Conditioning scores represent time spent in the morphine-paired place minus that spent in the saline-paired place (mean ± SEM). The Wilcoxon test was used to determine whether individual doses produced significant CPP. A two-factor (pretreatment × morphine dose) ANOVA or when appropriate, a single-factor ANOVA or the Student t-test was used to determine the effects of treatments upon conditioning. Post-hoc analysis was conducted using the Dunnet’s test.
Drugs
Drugs, generously provided by NIDA Drug Supply (Bethesda, MD, USA), were prepared in saline.
Results
Rats conditioned with saline exhibited no preference for either of the place cues. The mean time spent in the black and white compartments, respectively, was: 330 ± 17 vs. 350 ± 22 sec (4 sessions) and 385 ± 51 vs. 329 ± 38 sec (6 sessions). There was no difference between groups in time spent in the two compartments (4 sessions: t=0.8, df=7; p ≥ 0.5; 6 sessions: t=0.6, df=7; p ≥ 0.6) confirming that the conditioning procedure was unbiased.
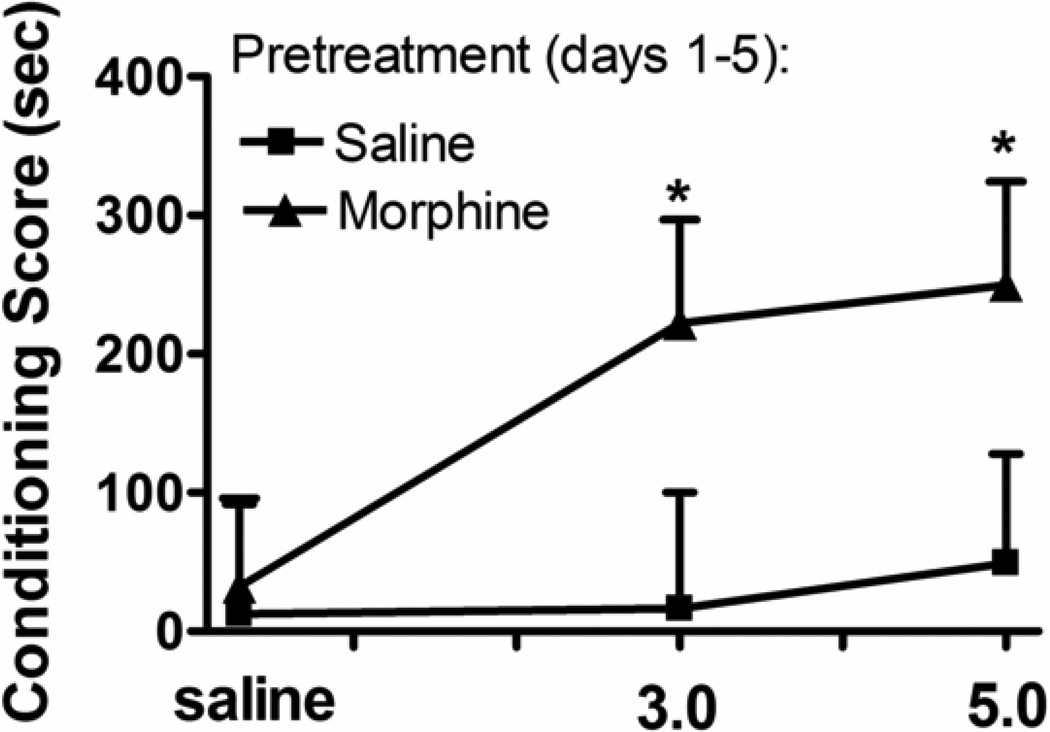
As previously reported (7, 8, 22), morphine failed to induce CPP in control animals after 2 drug conditioning sessions (Fig 1). In animals previously exposed to morphine, doses of 3.0 and 5.0 mg/kg produced significant CPP. ANOVA revealed a significant effect of pretreatment [F(1,51) = 4.6; p = 0.04] but no dose [F (2,51) = 1.3; p ≥ 0.3] or interaction [F (2,46) = 0.8; p≥ 0.5] effects. The CPP observed in animals with a prior morphine history is evidential of the development of sensitization.
Fig. 1.
Influence of prior context-independent morphine administration on morphine-evoked place conditioning. Rats received home-cage injections of saline or morphine (5.0 mg/kg/day, s.c × 5 days). Place conditioning sessions (2 morphine; 2 saline) commenced three days later. Conditioning scores represent the time (sec) spent in the morphine-paired environment minus that spent in the saline-paired environment. Each data point represents the mean ± SEM of 7–10 rats. * denotes significant CPP (Wilcoxon test).
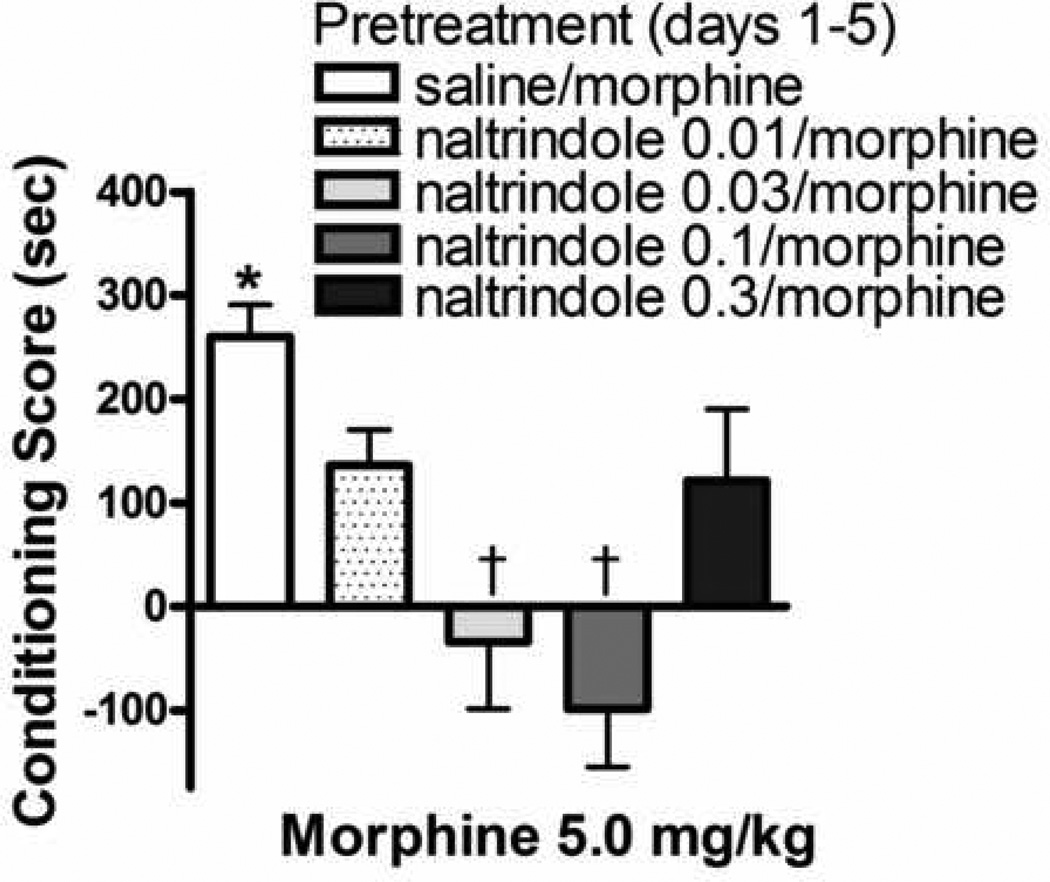
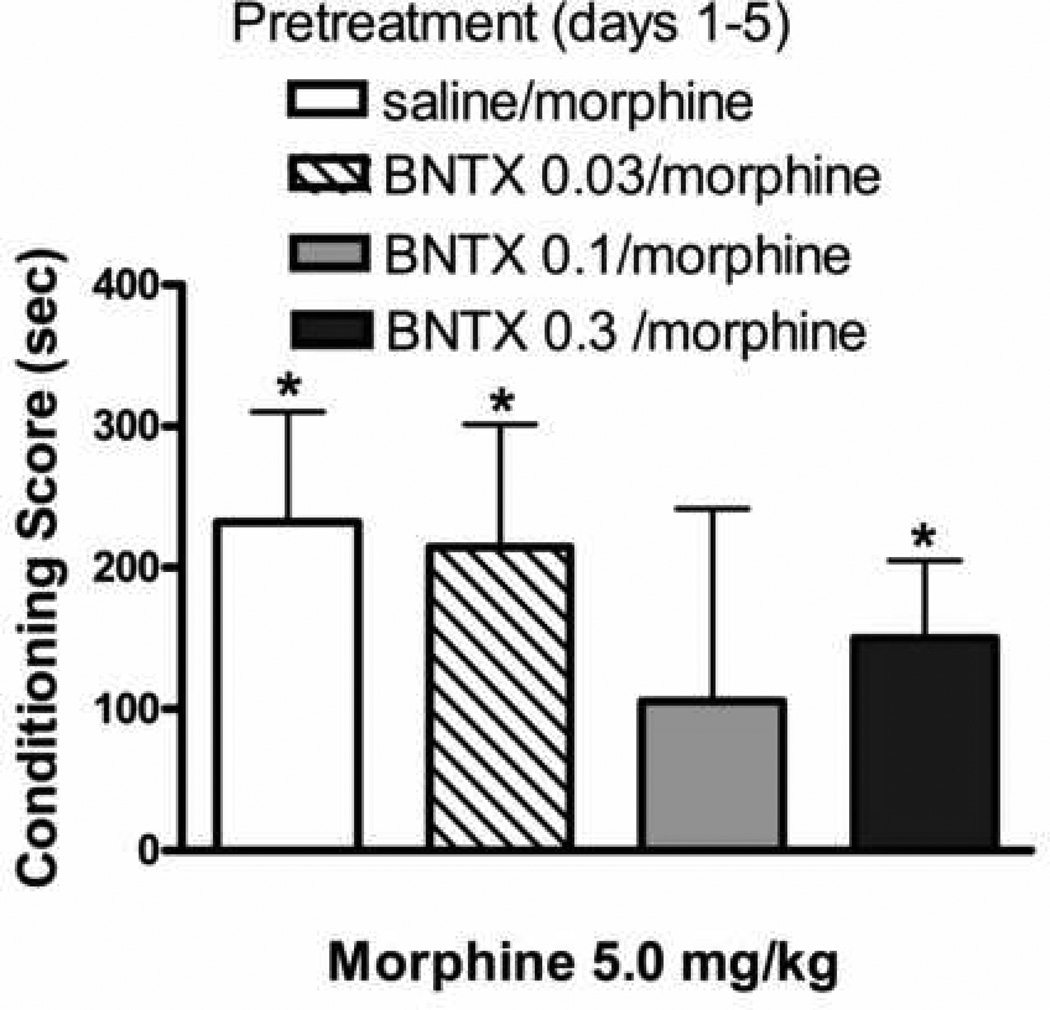
Fig. 2 shows conditioning produced by morphine (5.0 mg/kg) in rats that had received the repeated morphine pretreatment regimen with the DOPr1/2 antagonist, naltrindole. The naltrindole doses employed were ineffective in producing a conditioned response in naïve animals (24). ANOVA revealed a significant effect of naltrindole [F(4,38)=6.2; p≤ 0.001]. Morphine produced CPP in animals that had received once daily injections of morphine with saline but was ineffective in animals that received the morphine pretreatment with 0.03 or 0.1 mg/kg naltrindole.
Fig. 2.
Influence of prior, repeated administration of morphine or naltrindole + morphine on sensitization to the conditioned rewarding effects of morphine. Rats received once daily injections of saline or the DOPr1/2 antagonist, naltrindole (0.03–1.0 mg/kg; s.c) 15 min prior to morphine (5.0 mg/kg, s.c) injections on days 1–5. Conditioning sessions (2 morphine; 2 saline) commenced three days later. Each data point represents the mean ± SEM of 7–9 rats. * denotes significant CPP (Wilcoxon test). † indicates significant difference relative to animals that received the 5 day morphine treatment regimen in combination with saline.
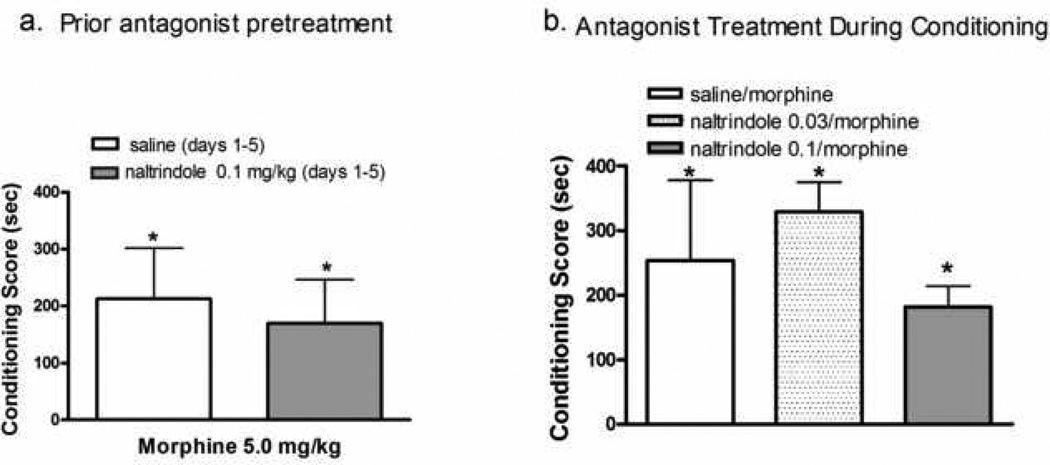
To determine whether prior repeated administration of naltrindole, alone, prevents acquisition of morphine CPP in naïve animals, additional rats received home cage injections of naltrindole (0; 0.1 mg/kg/day × 5 days). Conditioning sessions (3 morphine: 3 saline) commenced 3 days later. As previously reported (22, 23), morphine produced significant CPP in naïve animals when the number of environmental/drug pairings was increased (Fig 3a). CPP was also observed in animals that received naltrindole, repeatedly, prior to the commencement of conditioning. There was no difference between groups in this effect (t=0.4; df =1,14; p ≥ 0.5). Similarly, naltrindole administration prior to each morphine conditioning session did not affect morphine CPP [Fig. 3b: F(2,25)=1.3; p≥0.3].
Fig. 3.
Naltrindole is ineffective in attenuating morphine CPP in naïve animals. (a) Rats received once daily injections of naltrindole (0.1 mg/kg, s.c) for five days. Conditioning sessions (3 morphine; 3 saline) commenced 3 days later. (b) Rats received an injection of naltrindole (0.03; 0.1 mg/kg, s.c) 15 min prior to morphine conditioning sessions. A total of six conditioning sessions (3 morphine; 3 saline) were conducted. Data represent the mean SEM of n=8–11 rats. * indicates significant CPP (Wilcoxon test).
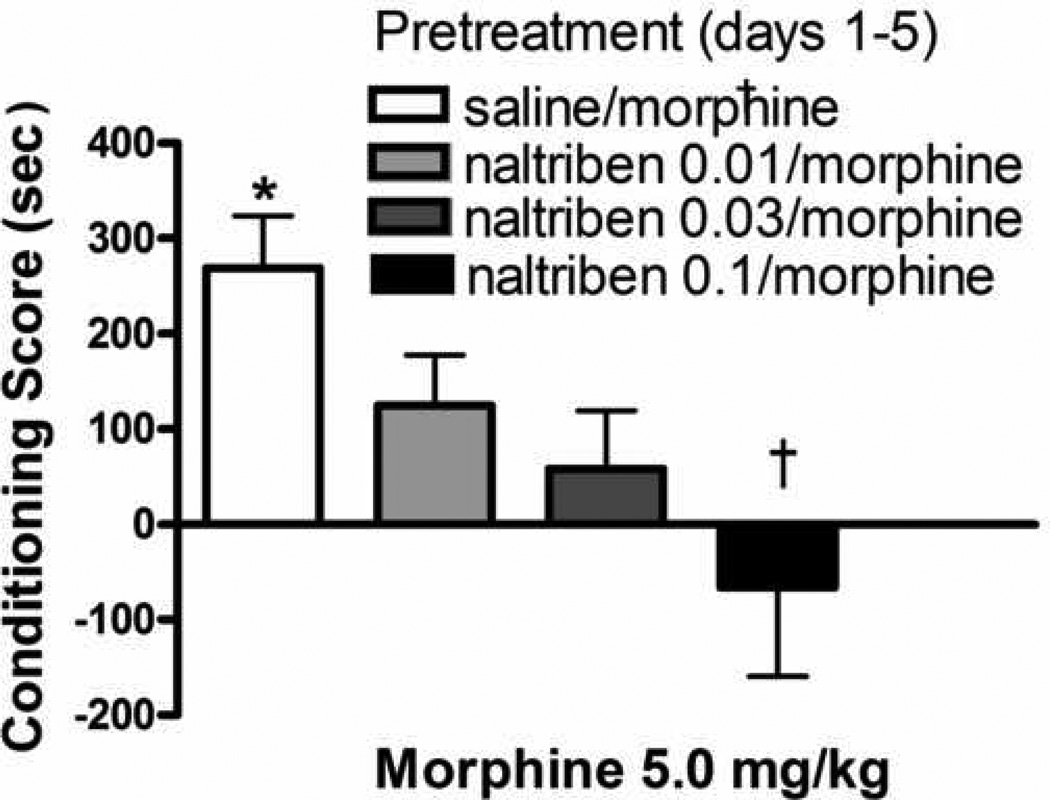
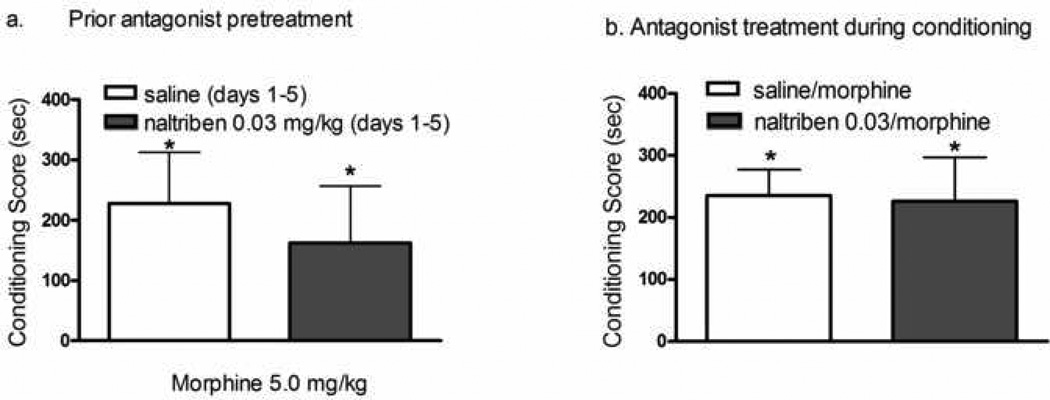
Fig. 4 shows morphine conditioning (2 sessions) in animals that received the five day morphine pretreatment with the DOPr2 antagonist, naltriben. In animals that had received naltriben with morphine for five days, the conditioned response to morphine response was attenuated [F(3,43)=4.7; p≤0.007]. Thus, morphine failed to produce significant CPP in animals that had received 0.01–0.3 mg/kg naltriben with morphine. Furthermore, following the 0.1 mg/kg naltriben + morphine treatment, morphine CPP was significantly reduced relative to vehicle + morphine treated animals. Repeated naltriben administration (0.03 mg/kg/day × 5 days), alone, prior to the commencement of conditioning sessions (3 morphine; 3 saline) did not alter morphine CPP (Fig 5a). The magnitude of CPP did not differ between the control and naltriben pretreatment groups (t=0.5; df=1,17; p ≥ 0.6). Similarly, naltrindole administration prior to each of morphine conditioning sessions did not attenuate morphine CPP (Fig. 5b: t=1.2; df=1,18; p≥ 0.3).
Fig. 4.
Influence of prior, repeated administration of naltriben + morphine on sensitization to the conditioned rewarding effects of morphine. Rats received once daily injections of saline or the DOPr2 antagonist, naltriben (0.01–0.1 mg/kg; s.c) 15 min prior to injections of morphine (5.0 mg/kg, s.c) on days 1–5. Conditioning sessions (2 morphine; 2 saline) commenced three days later. Each data point represents the mean ± SEM of 10–13 rats. * indicate significant CPP (Wilcoxon test). † indicates significant difference relative to animals that received the 5 day morphine treatment regimen with saline.
Figure 5.
Naltriben is ineffective in attenuating morphine-induced CPP in naïve animals. (a) Rats received once daily injections of naltriben (0.03 mg/kg, s.c) for five days. Conditioning sessions (3 morphine; 3 saline) commenced 3 days later. (b) Rats received an injection of naltriben (0.03 mg/kg, s.c) 15 min prior to morphine conditioning sessions. A total of six conditioning sessions (3 morphine; 3 saline) were conducted. Each column represents the mean SEM of n=9–10 rats. * indicate significant place conditioning (Wilcoxon test).
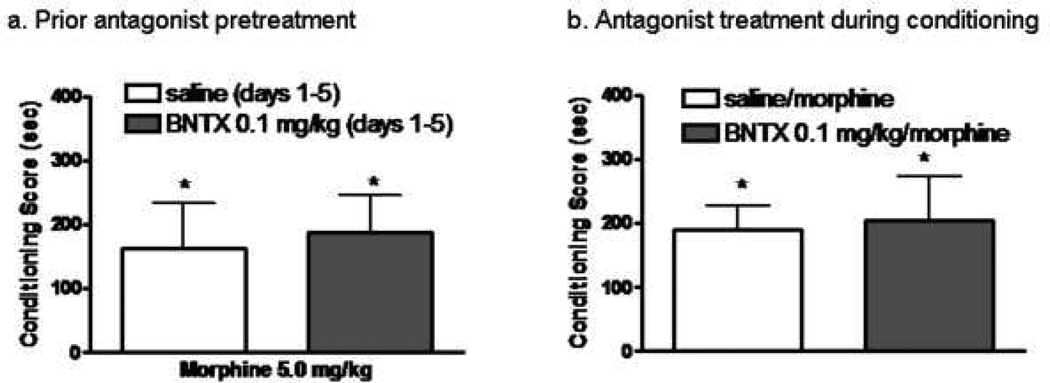
The DOPr1 antagonist, BNTX (0.03–0.3mg/kg), was ineffective in attenuating the enhancement of morphine conditioning produced by prior morphine administration (Fig 6a) [F(3,36)=0.3; p≥ 0.8]. Prior repeated administration of BNTZ alone (Fig 6b) or its administration prior to morphine conditioning sessions were also without effect (6c).
Figure 6.
Influence of prior, repeated administration of BNTX + morphine on sensitization to the conditioned rewarding effects of morphine. Rats received once daily injections of saline or the DOPr1 antagonist, BNTX (0.03–0.3 mg/kg; s.c) 15 min prior to injections of morphine (5.0 mg/kg, s.c) on days 1–5. Conditioning sessions (2 morphine; 2 saline) commenced three days later. Each data point represents the mean ± SEM of 10 rats. * indicates significant place conditioning (Wilcoxon Test).
DISCUSSION
These studies confirm that the conditioned rewarding effects of morphine are enhanced in animals with a prior history of morphine administration. In animals that received morphine repeatedly with a DOPr1/2 or a selective DOPr2 antagonist, no enhancement of the conditioned response was seen. Selective DOPR1 antagonism was without effect.
We previously showed that six conditioning sessions (3 morphine; 3 saline) is necessary for the conditioning of morphine reward in rats that are opiate naïve prior to place conditioning. In animals with a prior history of MOPr agonist administration, the potency and efficacy of morphine in producing CPP is increased (7, 22). In the present study, morphine doses which were ineffective as conditioning stimuli after 2 environmental pairings produced robust CPP in animals that had received context independent injections of morphine prior to the commencement of conditioning. These findings confirm that sensitization develops to the conditioned rewarding effects of morphine. Previous time course studies revealed that a period of abstinence is necessary for expression of the enhanced conditioned response (7). Thus, it can not be attributed to an increase in the total dose of morphine administered. Rather, neuroadaptations occurring in response to repeated morphine administration underlie the development of sensitization.
In animals that received naltrindole with the morphine pretreatment regimen, the enhancement of CPP was prevented. The effective doses of naltrindole are DOPr selective (25, 26) suggesting that the abolition of sensitization results from specific DOPr blockade. The abolition of sensitization can not be attributed to malaise or any conditioned effects of the antagonist since the naltrindole doses employed lack aversive effects (7) and naltrindole administered alone for 5 days prior to conditioning was without effect.
Naltrindole produced biphasic effects. Although an explanation for the decreased potency of higher doses is lacking, naltrindole binds with low affinity to MOPr and KOPr (27) and exerts DOPr agonist effects at high doses (28). Non-opioid receptor mediated effects of high naltrindole concentrations have been reported (29). Therefore, the diminished response to higher doses likely reflects loss of receptor selectivity.
DOPr subtypes have been proposed on the basis of differential antagonism of agonist induced antinociception, binding assays and tolerance studies (30). Recent data suggest that the cloned DOPr corresponds to DOPr2 whereas hetero-oligomerization of DOPr and KOPr gives rise to DOPr1 (31–33). BNTX is considered a selective DOPr1 antagonist whereas naltriben is DOPr2 selective (20, 21).
BNTX failed to modify the development of sensitization. The dose range employed was that which antagonized the behavioral effects of DOPr1 agonists (34, 35). Therefore, the lack of effect of BNTX is consistent with the hypothesis that sensitization to the conditioned rewarding effects of morphine occurs via a DOPr1-independent mechanism. The DOPr2 antagonist, naltriben, produced a dose-related attenuation of sensitization. The differential effects of naltrindole and naltriben as compared to BNTX suggest a critical role of DOPr2 in mediating the sensitized conditioned response to morphine that develops as a consequence of repeated morphine administration.
Given the influence of naltrindole and naltriben on sensitization, we examined whether these antagonists can attenuate the establishment of morphine CPP in opiate naïve animals. Morphine produced significant CPP in naïve animals when the number of environmental-drug pairings was increased from 2 to 3. CPP was also observed in rats that received antagonists repeatedly, prior to conditioning commencement. Thus, in the absence of concurrent MOPr activation, repeated DOPr blockade does not attenuate the conditioned rewarding effects of morphine.
CPP was observed when naltrindole or naltriben were administered acutely, prior to each of the three morphine conditioning sessions. These data suggest that although DOPr2 receptor activation is necessary for sensitization, it does not contribute to the conditioning of morphine reward in previously opiate naïve rats. Consistent with these findings, the DOPr antagonist, DN-20, does not attenuate CPP produced by a MOPr agonist in ICR mice (37). Similarly the locomotor activating and dopamine releasing effects produced by acute morphine administration are unaltered in DOPr knock out mice (36). Interestingly, DOPr antagonists were previously shown to attenuate morphine-induced CPP in ddY mice (38) and pharmacological inactivation or DOPr gene deletion attenuates conditioning in C57bl/6 mice (39). These data suggest that the role of DOPr in mediating the acute conditioned rewarding effects of MOPr agonists differ depending on the species and strain tested and highlight the need for caution in extrapolation of data to the human condition.
The mechanism by which DOPr2 blockade prevents the development of sensitization is unknown. Repeated MOPr activation increases DOPr function and cell surface expression (13, 14, 40). Whether repeated morphine administration produces similar effects in brain regions important for the motivational effects of opiates is unknown. However, DOPR recruitment by repeated morphine may be one mechanism by which DOPr2 antagonism attenuates sensitization.
MOPr and DOPr heterodimerization has been reported (15, 41, 42) in heterologous systems. Using bivalent ligands composed of a MOPr agonist pharmacophore linked to a DOPr antagonist pharmacophore through variable-length spacers, Daniels et al (43) obtained evidence that a physical interaction between DOPr and MOPr modulates MOPr-mediated tolerance and dependence. In contrast to monovalent MOPr agonists, bivalent ligands composed of a MOPr agonist and a DOPr antagonist did not produce CPP in ICR mice (37). Such findings indicate that heteromeric MOPr and DOPr contribute to the rewarding effects of MOPr agonists in this strain.
In conclusion, these data provide the first demonstration that DOPr2 activation is required for the development of sensitization to the conditioned rewarding effects of morphine. DOPr antagonists prevent the development of tolerance to the antinociceptive effects of MOPr agonists (10–12, 44). Given the postulated roles of sensitization and conditioning in addiction, these findings suggest that drugs which function as MOPr agonists/ DOPr2 antagonists may be effective analgesics with reduced tolerance and addiction liability. Studies, however, are needed to determine whether DOPR/MOPr interactions result from activation of opioid receptors located on separate neurons or at the intracellular level, via receptor cross-talk, and whether these receptor interactions are observed in human subjects.
Figure 7.
BNTX does not affect morphine-induced CPP in naïve animals. (a) Rats received once daily injections of BNTX(0.1 mg/kg, s.c) for five days. Conditioning sessions (3 morphine; 3 saline) commenced 3 days later. (b) Rats received an injection of BNTX (0.01 mg/kg, s.c) 15 min prior to morphine conditioning sessions. A total of six conditioning sessions (3 morphine; 3 saline) were conducted. Each column represents the mean SEM of n=9–10 rats. * indicate significant place conditioning (Wilcoxon test).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann NY Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Developing treatments that address classical conditioning. NIDA Res Monogr. 1993;135:71–91. [PubMed] [Google Scholar]
- 3.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 4.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 5.Gaiardi M, Bartoletti M, Bacchi A,Gubellini C, Costa M, Babbini M. Role of repeated exposure to morphine in determining its affective properties: place and taste conditioning studies in rats. Psychopharmacology (Berl) 1991;103:183–186. doi: 10.1007/BF02244201. [DOI] [PubMed] [Google Scholar]
- 6.Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- 7.Shippenberg TS, Heidbreder C, Lefevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur J Pharmacol. 1996;299:33–39. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- 8.Simpson GR, Riley AL. Morphine pre-exposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Spanagel R, Shippenberg TS. Modulation of morphine-induced sensitization by endogenous kappa opioid systems in the rat. Neurosci Lett. 1993;153:232–236. doi: 10.1016/0304-3940(93)90329-j. [DOI] [PubMed] [Google Scholar]
- 10.Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Blazquez P, Garcia-Espana A, Garzon J. Antisense oligodeoxynucleotides to opioid mu and delta receptors reduced morphine dependence in mice: role of delta-2 opioid receptors. J Pharmacol Exp Ther. 1997;280:1423–1431. [PubMed] [Google Scholar]
- 12.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 13.Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- 17.Porreca F, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI. Modulation of mu-mediated antinociception in the mouse involves opioid delta-2 receptors. J Pharmacol Exp Ther. 1992;263:147–152. [PubMed] [Google Scholar]
- 18.Xu H, Partilla JS, de Costa BR, Rice KC, Rothman RB. Differential binding of opioid peptides and other drugs to two subtypes of opioid delta ncx binding sites in mouse brain: further evidence for delta receptor heterogeneity. Peptides. 1993;14:893–907. doi: 10.1016/0196-9781(93)90064-n. [DOI] [PubMed] [Google Scholar]
- 19.Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- 20.Sofuoglu M, Portoghese PS, Takemori AE. Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: evidence for delta opioid receptor subtypes. J Pharmacol Exp Ther. 1991;257:676–680. [PubMed] [Google Scholar]
- 21.Portoghese PS, Sultana M, Nagase H, Takemori AE. A highly selective delta 1-opioid receptor antagonist: 7-benzylidenenaltrexone. Eur J Pharmacol. 1992;218:195–196. doi: 10.1016/0014-2999(92)90167-3. [DOI] [PubMed] [Google Scholar]
- 22.Shippenberg TS, LeFevour A, Thompson AC. Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol. 1998;345:27–34. doi: 10.1016/s0014-2999(97)01614-2. [DOI] [PubMed] [Google Scholar]
- 23.Mucha RF and Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- 24.Shippenberg TS, Heidbreder C. The delta-opioid receptor antagonist naltrindole prevents sensitization to the conditioned rewarding effects of cocaine. Eur J Pharmacol. 1995;280:55–61. doi: 10.1016/0014-2999(95)00185-n. [DOI] [PubMed] [Google Scholar]
- 25.Crook TJ, Kitchen I, Hill RG. Effects of the delta-opioid receptor antagonist naltrindole on antinociceptive responses to selective delta-agonists in post-weanling rats. Br J Pharmacol. 1992;107:573–576. doi: 10.1111/j.1476-5381.1992.tb12785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baamonde A, Dauge V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournie-Zaluski MC, Roques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- 27.Takemori AE, Sultana M, Nagase H, Portoghese PS. Agonist and antagonist activities of ligands derived from naltrexone and oxymorphone. Life Sci. 1992;50:1491–1495. doi: 10.1016/0024-3205(92)90138-f. [DOI] [PubMed] [Google Scholar]
- 28.Stapelfeld A, Hammond DL, Rafferty MF. Antinociception after intracerebroventricular administration of naltrindole in the mouse. Eur J Pharmacol. 1992;214:273–276. doi: 10.1016/0014-2999(92)90129-r. [DOI] [PubMed] [Google Scholar]
- 29.Gaveriaux-Ruff C, Filliol D, Simonin F, Matthes HW, Kieffer BL. Immunosuppression by δ-opioid antagonist naltrindole: δ- and triple μ, δ, κ-opioid receptor knockout mice reveal a nonopioid activity. J Pharmacol Exp Therap. 2001;298:1193–1198. [PubMed] [Google Scholar]
- 30.Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Opioid receptor types and subtypes: the delta receptor as a model. Ann Rev Pharmacol Toxicol. 1996;36:379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- 31.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 32.Portoghese PS, Lunzer MM. Identity of the putative δ-1 opioid receptor as a δ-k heteromer in the mouse spinal cord. Eur J Pharmacol. 2003;467:233–234. doi: 10.1016/s0014-2999(03)01599-1. [DOI] [PubMed] [Google Scholar]
- 33.Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68:1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- 34.Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Tsuji M, Mori T, Ikeda H, Misawa M, Nagase H. Involvement of dopamine-dependent and -independent mechanisms in the rewarding effects mediated by delta opioid receptor subtypes in mice. Brain Res. 1997;744:327–334. doi: 10.1016/S0006-8993(96)01119-5. [DOI] [PubMed] [Google Scholar]
- 36.Chefer VI, Kieffer BL, Shippenberg TS. Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice. Eur J Neurosci. 2003;18:1915–1922. doi: 10.1046/j.1460-9568.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- 37.Leonard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Yoshike M, Mizoguchi H, Kamei J, Misawa M, Nagase H. Blockade of delta opioid receptors prevents morphine-induced place preference in mice. Jpn J Pharmacol. 1994;66:131–137. doi: 10.1254/jjp.66.131. [DOI] [PubMed] [Google Scholar]
- 39.Chefer VI, Shippenberg TS. Augmentation of Morphine-Induced Sensitization but Reduction in Morphine Tolerance and Reward in Delta-Opioid Receptor Knockout Mice. Neuropsychopharmacol. 2008 doi: 10.1038/npp.2008.128. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- 41.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 42.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of μ and opioid receptors: a role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelhamid E.E, Sultana M, Portoghese PS, Takemori A E. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]