Abstract
Purpose.
We previously showed that pre-exposure of the cornea to Toll-like receptor (TLR)5 ligand flagellin induces strong protective innate defense against microbial pathogens and hypothesized that flagellin modulates gene expression at the transcriptional levels. Thus, we sought to determine the role of one transcription factor, interferon regulatory factor (IRF1), and its target gene CXCL10 therein.
Methods.
Superarray was used to identify transcription factors differentially expressed in Pseudomonas aeruginosa-challenged human corneal epithelial cells (CECs) with or without flagellin pretreatment. The expression of CXCL10, IRF1, LI-8(CXCL2), and IFNγ was determined by PCR, immunohistochemistry, Western/dot blotting, and/or ELISA. IRF1 knockout mice, CXCL10 and IFNγ neutralization, and NK cell depletion were used to define in vivo regulation and function of CXCL10. The severity of P. aeruginosa was assessed using clinical scoring, slit-lamp microscopy, bacterial counting, polymorphonuclear leukocytes (PMN) infiltration, and macrophage inflammatory protein 2/Chemokine (C-X-C motif) ligand 2 (MIP-2/CXCL2) expression.
Results.
Flagellin pretreatment drastically affected P. aeruginosa-induced IRF1 expression in human CECs. However, flagellin pretreatment augmented the P. aeruginosa–induced expression of Irf1 and its target gene Cxcl10 in B6 mouse corneas. Irf1 deficiency reduced infection-triggered CXCL10 expression, increased keratitis severity, and attenuated flagellin-elicited protection compared to values in wild-type (WT) controls. CXCL10 neutralization in the cornea of WT mice displayed pathogenesis similar to that of IRF1−/− mice. IFNγ receptor neutralization and NK cell depletion prevented flagellin-augmented IRF1 and CXCL10 expression and increased the susceptibility to P. aeruginosa infection in mouse corneas.
Conclusions.
IRF1 plays a role in the corneal innate immune response by regulating CXCL10 expression. IFNγ-producing NK cells augment the epithelial expression of IRF1 and CXCL10 and thus contribute to the innate defense of the cornea against P. aeruginosa infection.
Keywords: cell reprogramming, inflammation, toll-like receptors, IRF1, CXCL10
This study defines the role of IRF1 in mediating expression of CXCL10 in the cornea in response to bacterial infection. CXCL10 expression induced by P. aeruginosa and augmented by flagellin is regulated by IFNγ-IRF1-CXCL10 axis in a NK-cell dependent manner and is required for mucosal innate immunity.
Introduction
The innate immune system is critical for host defense against infectious challenges.1–3 The protective ability of innate defense is largely dependent on germ-line–encoded pattern-recognition receptors (PRRs).4 Prominent among PRRs are the Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs) to initiate innate immune responses including the production/secretion of inflammatory mediators, antimicrobial effectors, and signals inducing adaptive immune responses.5 The ocular surface, like other mucosal surfaces including the respiratory,6 gastrointestinal,2 and urogenital tracts,7 is covered by epithelial cells (ECs) that form a physiological barrier. These ECs also possess the ability to sense and promote the host immune response.1,8,9 Although the innate response is important for host defense against invading pathogens, unregulated inflammation could be detrimental to the host tissue. Several negative regulators, including Tollip,10 IRAK-M,11 SOCS1,12 and ST2,13,14 have been shown to play a role in the resolution of inflammation and in restoring homeostasis. At the cellular level, pre-exposure of mucosal surfaces or cultured cells to TLR ligands has resulted in suppression of inflammatory cytokine release yet enhanced tissue resistance to infection and other adverse environmental challenges.15,16 Inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation has been demonstrated to be responsible for silencing the expression of proinflammatory cytokines. However, the underlying mechanisms for the expression of antimicrobial effectors, mostly antimicrobial peptides such as human BD2 (hBD2) and LL-37, and other protective genes remain elusive.17 Hence, it is reasonable to postulate that in addition to NF-κB, other transcription factors, the effectors controlling gene expression, may also be involved in TLR ligand-mediated modification of gene expression, also termed “reprogramming.”17,18 Identification of these factors is of great interest, as they may mediate the expression of a subgroup of genes and serve as a more specific target for controlling TLR-triggered inflammation and/or accelerating the resolution of inflammation. Thus identification may lead to the development of more advanced therapeutic molecules to treat bacterial keratitis and other infectious diseases.
Most TLR ligands are known to desensitize the inflammatory response to the same (homotolerance) and/or different (cross-tolerance) TLR ligands, generating certain protective effects against infection or deleterious inflammation.15,17 The best studied is lipopolysaccharide (LPS)-induced endotoxin tolerance or hyporesponsiveness.16,17 Among the 10 identified human TLRs, TLR5 is unique as it recognizes a bacterial protein flagellin and requires only MyD88 for signal transduction.19 Using flagellin as a stimulus, we found that prestimulation of the cornea provided robust protection against microbial keratitis, including forms caused by flagellin-bearing Pseudomonas aeruginosa or unrelated pathogens such as fungi.20–24 Flagellin has also been reported to induce protection against lethal radiation and chemicals in mice and monkeys,25,26 to restore antibiotic-impaired innate immune defenses,27 and to protect mice from acute Clostridium difficile colitis.28 Moreover, the flagellin-TLR5 axis, but not the Ipaf pathway,29,30 exhibits several distinctive properties: (1) more potent stimulation of mucosal ECs compared to immune cells such as dendritic cells and macrophages,31 (2) unique expression of anti-inflammatory genes (such as IL-1Ra, but not IL-1β),26,32 and (3) preservation of epithelial barrier function.27,28 While flagellin or its derivatives have great potential as a prophylactic and/or therapeutic reagent, its clinical application faces many obstacles due to its potential ability to alter the cell's response to other adverse challenges, its untested usability in repeated applications on the airway and cornea or through systematic injections, and the generation of antibodies in the host that neutralize its effects in vivo.33,34 Hence, harnessing the flagellin-induced protective power requires much more insight into the underlying mechanisms of cell reprogramming. One of the pressing questions is how, while hyporesponsiveness can be attributed to impairment of the classic NF-κB activation, the expression of antimicrobial effectors such as LL-37 and hBD3 (which are generally known to require NF-κB at least for initial activation35,36) remains unchanged or even enhanced in flagellin-pretreated cells. As TLR-mediated expression of proinflammatory and cytoprotective genes is mostly controlled at the transcription level, it is of much interest to identify the transcription factors involved in TLR-induced reprogramming and mucosal surface protection.
In this study, we used a real-time PCR array to identify transcription factors with differential expression profiles in human corneal epithelial cells (HCECs) challenged with bacteria with or without flagellin pretreatment. We identified interferon regulatory factor (IRF)1 as one such transcription factor and demonstrated that its upregulation is important for the expression of CXCL10 in corneal epithelial cells (CECs). Our results suggest that the IFNγ-IRF1-CXCL10 axis plays a role in flagellin-induced corneal epithelial response to and protection against P. aeruginosa infection in the cornea by accelerating the recruitment of NK cells into the cornea.
Materials and Methods
Reagents and Antibodies
Anti-human IRF1 and anti-BD2 antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA). Anti-β-actin and anti-mouse CXCL10 antibodies were purchased from Sigma-Aldrich (St. Louis, MO) and Peprotech (Rocky Hill, NJ), respectively. Anti-NK1.1 was purchased from eBiosciences (San Diego, CA). Anti-IFNγR2 was purchased from R&D Systems (Minneapolis, MN). Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Bio-Rad (Hercules, CA). Fluorescein isothiocyanate–conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Defined keratinocyte serum-free medium (DK-SFM) was purchased from Invitrogen (Carlsbad, CA). Keratinocyte basal medium (KBM) was purchased from BioWhittaker (Walkersville, MD).
Animals
Wild-type (WT) C57BL6 (B6) mice (8 weeks of age; 20–24 g weight) and IRF1−/− mouse breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME). IRF1−/− mice were bred in-house, and their pups were subjected to genotyping before use. All investigations conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the National Institutes of Health guidelines, and the guidelines of the Animal Investigation Committee of Wayne State University.
Infection Protocol
Mice were anesthetized with ketamine/xylazine and placed beneath a stereoscopic microscope for needle scratching with three 1-mm incisions using a 26-gauge needle. Purified flagellin (500 ng in 5 μL PBS) or PBS (the control) was topically applied on the needle-injured corneas. At 24 hours after pretreatment, the corneas were scarified again and inoculated with 5 μL bacterial suspension containing 1 × 105 CFU of strain PAO1 and 1 × 104 CFU of P. aeruginosa strain ATCC 19660 (American Type Culture Collection, Manassas, VA). Eyes were examined daily to monitor the disease progression with a dissection microscope equipped with a digital camera.
Clinical Examination
For assessment of clinical scores, mice were color coded and examined in a blinded fashion by two independent observers at 1, 2, and 3 days postinfection (dpi) to visually grade the severity of disease after P. aeruginosa infection. The ocular disease was graded, and clinical scores were assigned using the following previously described scale37: 0, clear; 1, slight opacity partially or fully covering the pupil; 2, slight opacity fully covering the anterior segment; 3, dense opacity partially or fully covering the pupil; and 4, dense opacity covering the anterior segment and corneal perforation. At 3 dpi, all infected corneas were photographed with a slit-lamp microscope to illustrate the disease response.
Bacterial Strains and Flagellin
Two strains of P. aeruginosa, PAO1 and ATCC 19660, were used in the study. ATCC 19660, a cytotoxic strain, can infect the B6 mouse corneas at 104 CFU/cornea and cause severe keratitis and corneal perforation at approximately day 5. To illustrate the profound protection induced by flagellin we have been using ATCC 19660. However, our interest is in the early innate immune response in the cornea, particularly the epithelium, which has been shown to play a key role in innate immunity and in shaping adaptive immunity as well. ATCC 19660 usually causes EC death in vitro and epithelial sheet loss in vivo. To that end, we had to use PAO1 with 10-fold increase in CFU (105) to challenge B6 mouse corneas and assess host responses at 6 hours by isolating ECs for PCR analysis or immunohistochemistry. Flagellin was prepared from PAO1 using a previously described method.38 For direct bacterial challenge of HCECs, P. aeruginosa was grown in tryptic soy broth (Sigma-Aldrich) at 37°C until absorbance at 600 nm reached optical density value 0.5. The bacterial culture was centrifuged at 6000g for 10 minutes. Bacteria were washed in PBS and resuspended in KBM (PAO1) or PBS (PAO1 or ATCC 19660) and then used to challenge HCECs at a cell-to-bacteria ratio of 1:50 or to infect mouse corneas at 105 for PAO1 and 104 CFU for ATCC 19660, respectively.
Human Corneal Epithelial Cell Culture
Primary HCECs were isolated from human donor corneas obtained from the Midwest Eye Bank (Ann Arbor, MI) using a previously described method.24 HCECs were used between passages 3 and 5 in experiments. Prior to bacterial challenge (1:50 multiplicity of infection [MOI]), HCECs were cultured in KBM overnight to starve cells from growth factors and then pretreated with 50 ng/mL flagellin or PBS for 24 hours.
RNA Isolation, RT-PCR, and Real-Time PCR
Total RNA was isolated from mice corneal epithelia using RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer instructions. One microgram of total RNA was reverse-transcribed with a first-strand synthesis system for RT-PCR (SuperScript; Invitrogen). Complementary DNA was amplified by PCR using primers for mouse Ifnγ, Irf1, Cxcl10, Cxcl2, Bd3, and glyceraldehyde-3-phosphate dehydrogenase (Gapdh; Table). The PCR products and internal control Gapdh were subjected to electrophoresis on 1.5% agarose gels containing ethidium bromide. Stained gels were captured using a digital camera, and band intensity was quantified using 1D Image Analysis Software (EDAS 290 system; Eastman Kodak, Rochester, NY). Real-time PCR was conducted using the Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA), based on expression of Gapdh, on the StepOnePlus Real-Time PCR System (Applied Biosystems).
Table.
Mouse Primer Sequences Used for PCR
|
Gene |
Primer |
Sequence (5′→3′) |
Product Size, bp |
| Ifnγ | Forward | ACGGCACAGTCATTGAAAGC | 198 |
| Reverse | TGCTGATGGCCTGATTGTCTT | ||
| Irf1 | Forward | CAGAGGAAAGAGAGAAAGTCC | 208 |
| Reverse | CACACGGTGACAGTGCTGG | ||
| Cxcl10 | Forward | ATGAACCCAAGTGCTGCCGTC | 297 |
| Reverse | TTAAGGAGCCCTTTTAGACCTTT | ||
| Cxcl2 | Forward | AGTTTGCCTTGACCCTGAAGCC | 466 |
| Reverse | TGGGTGGGATGTAGCTAGTTCC | ||
| Bd3 | Forward | GCTTCAGTCATGAGGATCCATTACCTTC | 318 |
| Reverse | GCTAGGGAGCACTTGTTTGCATTTAATC | ||
| Gapdh | Forward | ACCACAGTCCATGCCATCAC | 452 |
| Reverse | TCCACCACCCTGTTGCTGTA |
Transcription Factor PCR Array
Total RNA was isolated using the following protocol. First-strand DNA was created using RT2 First Strand kit along with the protocol and cycling times as recommended by the manufacturer (SABiosciences, Frederick, MD). Two-stage real-time reverse transcriptase PCR of TLR-related transcription factors was performed on the RT2 Profiler PCR Array (SABiosciences, catalogue no. PAHS-018). The PCR was performed on an ABI 7000 (Applied Biosystems). The cycle threshold (CT) was determined for each sample and normalized to the average CT of GAPDH. Comparative CT method was used to calculate relative gene expression. Data are represented as fold change relative to control. All solutions, including the SYBR Green reverse transcriptase PCR mix, were purchased from SABiosciences. The data were analyzed using online analysis software provided by the manufacturer. Briefly, the PCR Array Pathway number is entered, followed by uploading the Microsoft Excel (Redmond, WA) file containing the PCR data. Housekeeping genes on the PCR array were used for data normalization, whereas HCECs without any treatment were used as the reference for infected HCECs with PAO1 and for flagellin pretreated and infected with the same strain. The “Fold Change” (increase as positive and decrease as negative) and “p-value” (only values <0.05 were chosen) were used by the software for subsequent graphical presentation.
Western Blot Analysis
Human CECs challenged with bacteria were subjected to Western blot analysis using a previously described method.24
ELISA Measurement of Cytokines
Secretion IL-8 from HCECs was determined by ELISA. Human CECs were plated at 1 × 106 cells/well in six-well plates. After growth factor starvation, the cells were either left untreated or pretreated with flagellin followed by challenge with live bacteria (∼MOI 50). At the end of the incubation period, the media were harvested for measurement of cytokines, and ELISA was performed according to the manufacturer's instructions (R&D Systems). To measure mouse CXCL2 and CXCL10, corneal extracts were prepared by homogenization in 200 μL phosphate-buffered saline (PBS) with a glass micro tissue grinder, followed by centrifugation at 14,000g for 10 minutes. Protein concentration of the corneal lysate was determined by Micro BCA protein assay kit (Pierce, Rockford, IL), and equal amounts of protein were used to perform ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Slot-Blot Determination of hBD2
Accumulation of hBD2 in the culture media was detected by slot blot.39,40 Briefly, 150 μL supernatant was applied to a nitrocellulose membrane (0.2 μm; Bio-Rad) by vacuum using a slot-blot apparatus (Bio-Rad). The membrane was fixed by incubating with 10% formalin for 1 hour at room temperature, followed by blocking in Tris-buffered saline (TBS) containing 5% nonfat powdered milk for 1 hour at room temperature. The membrane was then incubated overnight at 4°C with rabbit anti-hBD2 antibody diluted 1:500 in TBS containing 5% nonfat powdered milk, and 0.05% Tween-20. After washing, the membrane was incubated for 1 hour at room temperature with goat anti-rabbit IgG conjugated to HRP diluted 1:2000 with 5% nonfat powdered milk. Immunoreactivity was visualized by chemiluminescence (Supersignal reagents; Pierce) using the Kodak Image Station 4000R Pro.
Polymorphonuclear Leukocytes (PMN) Infiltration Assay
Measurement of myeloperoxidase (MPO) activity was employed to determine PMN infiltration in the cornea as previously described.23 The corneas were excised from enucleated eyes at 5 dpi and homogenized in 1 mL hexadecyltrimethylammonium bromide (HTAB) buffer (0.5% HTAB in 50 mM phosphate buffer, pH 6.0). The samples were then subjected to four freeze–thaw cycles, followed by centrifugation at 16,000g for 20 minutes. Each supernatant was mixed with 50 mM phosphate buffer (pH 6.0) containing 16.7 mg/mL O,O-dianisidine hydrochloride and 0.0005% hydrogen peroxide at a 1:30 ratio to obtain a total volume of 3 mL. The change in absorbance at 460 nm was monitored continuously for 5 minutes. The results were expressed in units of MPO activity/cornea.
Bacterial CFU Determination in the Cornea
Corneas (n = 5–7 per group) from P. aeruginosa (PA)-infected mice were collected, and the numbers of viable bacteria were determined by plate count method. Individual corneas were homogenized in sterile PBS, and aliquots (100 μL) of serial dilutions were plated onto Pseudomonas isolation agar (BD Biosciences, San Jose, CA) plates in triplicate. The plates were incubated overnight at 37°C, and bacterial colonies were counted. The results are expressed as the mean number of CFU/cornea.
Immunohistochemistry
Mouse eyes were enucleated and embedded in Tissue-Tek (Miles, Inc., Elkhart, IN) optimal cutting temperature (OCT) compound and frozen in liquid nitrogen. Sections (10 μm thick) were cut and mounted to polylysine-coated glass slides. After a 10-minute fixation in 4% paraformaldehyde, slides were blocked with 10 mmol/L sodium phosphate buffer containing 2% BSA for 1 hour at room temperature. Sections were then incubated with primary antibody for CXCL10, 5 μg/mL. For fluorescence microscopy, the slides were incubated with anti-rabbit IgG conjugated with fluorescein isothiocyanate (1:100; Jackson ImmunoResearch Laboratories) and then mounted with Vectorshield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) mounting medium (Vector Laboratories, Burlingame, CA). Controls were similarly treated, but the primary antibody was replaced with rabbit IgG. The images were taken using Nikon Eclipse 90i (Brighton, MI).
Statistical Analysis
An unpaired, two-tailed Student's t-test was used to determine statistical significance for data from bacterial count, cytokine ELISA, MPO assay, and real-time PCR. Mean differences were considered significant at a P value of <0.05. Experiments were repeated at least twice to ensure reproducibility.
Results
Flagellin Pretreatment Suppresses P. aeruginosa–Induced IRF1 Expression in Cultured HCECs
We previously showed that exposure of mucosal surfaces to the TLR5 ligand flagellin induced innate immune protection against microbial infection, regardless whether or not the invading pathogens expressed flagellin.21 Flagellin pretreatment alters cell response to infection by suppressing the expression of proinflammatory cytokines and augmenting the expression of innate defense genes such as β-defensins and LL-37 while the activation of NF-κB is dampened. To determine other transcription factors that might be involved in flagellin-induced cell reprogramming and protection against microbial infection, we used the transcription factor RT2 Profiler PCR Array (SABiosciences) to compare the expression profiles of transcription factors in HCECs in response to P. aeruginosa PAO1 (MOI, 50 bacteria per cell), with or without 50 ng/mL flagellin pretreatment, for 24 hours (Fig. 1A). Compared to the control, PAO1 challenge of HCECs resulted in greatly elevated expression of Irf1 (29.1-fold) and Atf3 (42-fold). Strikingly, while flagellin pretreatment further enhanced the expression of Atf3 (61.5-fold), Irf1 was downregulated by −2.93-fold compared to control, giving an 85.2 (29.1 × 2.93)-fold decrease for IRF1 mRNA in flagellin-pretreated HCECs over that of PAO1 challenge alone (Fig. 1A).
Figure 1.

Reprogramming of transcription factor expression caused by flagellin pretreatment in primary HCECs. Normal or flagellin-pretreated (50 ng/mL, 24 hours) cells were challenged with live PAO1 (1:50 MOI) for 4 hours, and transcription factor RNA expression alterations were quantified by comparing RNA fold changes of PAO1-infected normal cells versus PAO1-infected flagellin-pretreated cells using the real-time RT2 Profiler PCR Array (A). For confirmation of PCR array results, cells were cultured and challenged under the same conditions and lysed and subjected to Western blotting analyses with IRF1 and β-actin antibodies (D). Cell culture media were collected 4 hours after bacterial challenge for the analyses of IL-8 by ELISA (B) and hBD2 using slot blot (C). P values were generated using unpaired Student's t-test (**P < 0.01). The figure is a representative of four independent experiments.
To further confirm the expression pattern of IRF1 at the protein level, Western blotting of HCEC lysates, ELISA for IL-8, and dot blot for hBD2 detection in culture media were performed (Figs. 1B–D). As we have demonstrated previously,24 flagellin pretreatment (50 ng/mL for 24 hours) by itself had minimal effects on the expression of proinflammatory cytokine IL-8 and antimicrobial peptide hBD2. PAO1 strongly induced their production in naïve cells, whereas in flagellin-pretreated cells, the expression of IL-8 but not hBD2 was dampened at 6 hours post-PAO1 challenge. Consistent with the PCR array data, PAO1 challenge induced an abundant expression of IRF1, and flagellin pretreatment dampened PAO1-induced IRF1 expression (Fig. 1D). Thus, IRF1 is differentially expressed in HCECs in response to bacterial challenge in the presence or absence of flagellin pretreatment, suggesting IRF1 as a good candidate to study flagellin-induced cell reprogramming. Given its distinction as an important regulator of inflammation and immunity,40–43 we focused on IRF1 in subsequent experiments.
IRF1 Deficiency Dramatically Reduced Pseudomonas-Induced CXCL10 Expression in B6 Mouse Corneal Epithelium
Having shown the dramatic effects of flagellin pretreatment on P. aeruginosa PAO1-induced IRF1 expression in HCECs, we next investigated if flagellin pretreatment can also dampen IRF1 expression in vivo in mouse CECs in response to PAO1 (Fig. 2). Surprisingly, in contrast to our observations in vitro cultured HCECs, we observed augmented expression of IRF1 by flagellin pretreatment in PAO1-infected corneas (Fig. 2). To assess the consequences of altered expression of Irf1, we evaluated the expression of the Irf1 target gene Cxcl1044 in comparison with two known flagellin reprogrammed genes, Cxcl2 (Mip-2, a homolog of human IL-8) and mBD3 (a human homolog of inducible hBD2), in WT and Irf1 knockout mice. The expression patterns of Cxcl10 in these mice were consistent with IRF1 expression except for a faint band that could be detected in IRF1−/− mice by RT-PCR, suggesting additional pathways to induce Cxcl10 expression in response to PAO1. Flagellin-augmented Cxcl10 expression in PAO1-exposed WT but not Irf1−/− mouse CECs was verified with real-time PCR showing 32- and 47-fold increases in Cxcl10 mRNA levels in infected WT CECs without or with flagellin pretreatment, respectively; flagellin-augmented Cxcl10 protein expression is mentioned below. There was no significant increase of Cxcl10 in PAO1-infected corneas of Irf1−/− mice. As a control, the expression pattern of mBD3 was the same in WT and Irf1−/− mice (Fig. 2B).
Figure 2.
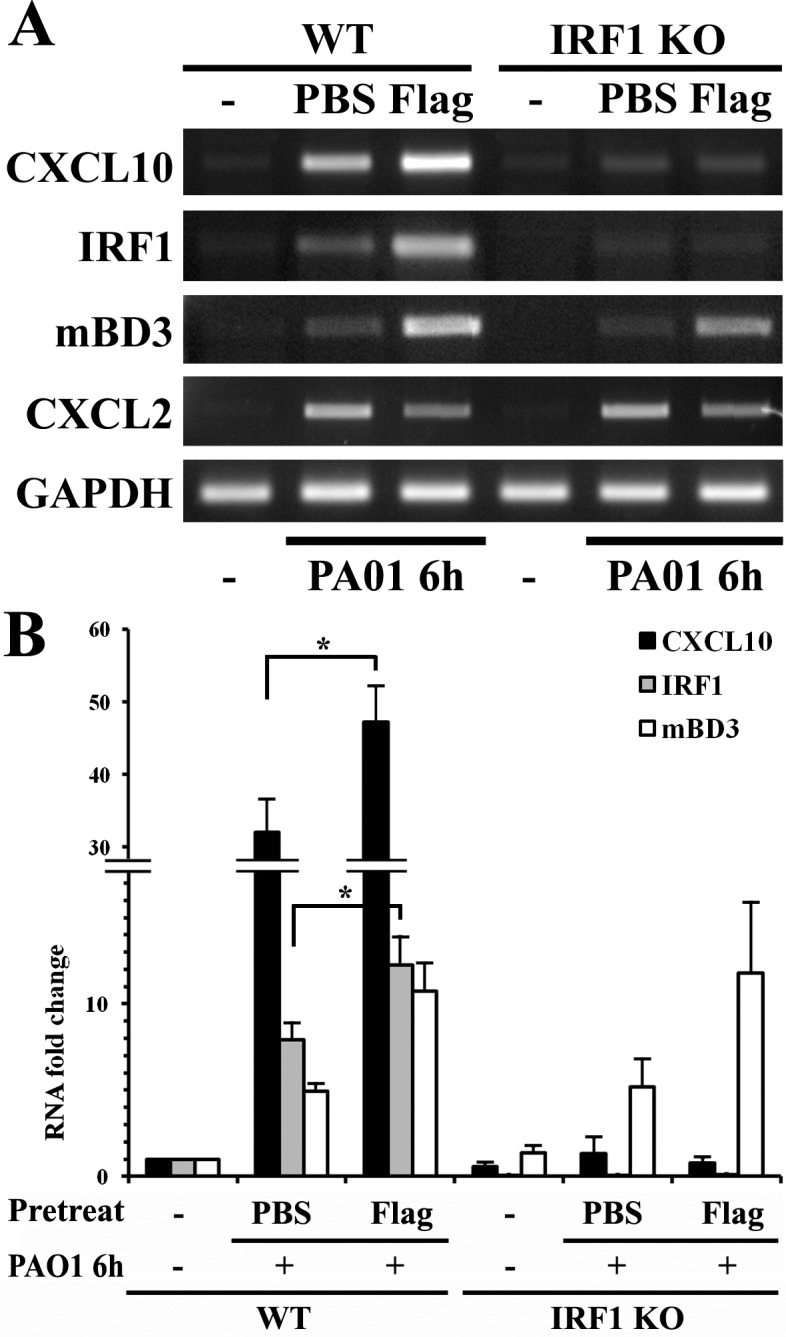
Detection of Cxcl10 expression in corneal epithelia of B6 WT and Irf1−/− mice. Wild-type and Irf1−/− B6 mice were topically pretreated with 500 ng flagellin in 5 μL PBS, a dosage we showed to induce protection in B6 mice, or 5 μL PBS for 24 hours, and then infected with PAO1 at 105 CFU for 6 hours. The epithelial cells were collected and RNA was isolated for semiquantitative RT-PCR (A) and quantitative real-time PCR (B) analysis of Cxcl10, Irf1, and mBd3. Gapdh was used as the internal control. P values were generated using unpaired Student's t-test (*P < 0.05). The figure is a representative of three independent experiments.
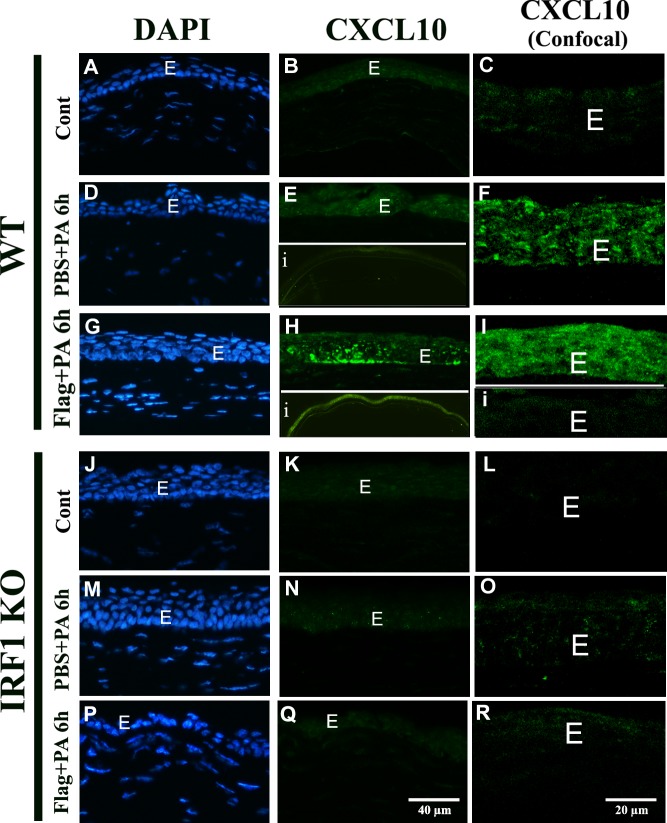
We next investigated CXCL10 expression and distribution in the corneas in response to flagellin pretreatment and PAO1 infection using immunohistochemistry with epifluorescence microscopy to show whole cornea and confocal microscopy in order to focus on epithelia (Fig. 3). Consistent with mRNA levels, no expression of CXCL10 was detected in uninfected corneas of WT and Irf1−/− mice (Figs. 3A–C, 3J–L). PAO1 infection of needle-scratched corneas at 6 hours induced CXCL10 expression in WT (Figs. 3D–F) but not in IRF1−/− (Figs. 3M–O) mice. Flagellin pretreatment greatly increased CXCL10 immunoreactivity in corneal epithelia of WT mice (Figs. 3G–I with inset i as the isotype control). The elevated CXCL10 expression can be seen in the entire cornea (inset, Fig. 3H). No CXCL10 staining was observed in epithelium of IRF1−/− mice (Figs. 3P, 3R). It should be noted that the isolated mouse CEC lysates contain intraepithelial dendritic cells,44 but the strong epithelial staining of CXCL10 suggests that most, if not all, CXCL10 mRNA detected in Figure 2 were from mouse CECs. Hence, IRF1 is required for PAO1-induced and flagellin-augmented CXCL10 expression in mouse CECs.
Figure 3.
Distribution of CXCL10 expression in corneal epithelia of WT and Irf1−/− mice. WT and Irf1−/− B6 mice were topically pretreated with 500 ng flagellin in 5 μL PBS, a dosage we showed to induce protection in B6 mice, or 5 μL PBS for 24 hours, and then infected with PAO1 at 105 CFU for 6 hours. Mouse eyes were enucleated in OCT, sectioned, stained with mouse anti-CXCL10 antibody, and examined with fluorescence microscopy middle panels with DAPI used to stain the nuclei (A, B, D, G, J, K, M, N, P, Q) and ×4 lens to view the entire mouse corneas (insert [i] in [E, H) or confocal microscopy (C, E, F, H, I, L, O, R). Scale bars are given in the figures.
Flagellin-Induced Corneal Protection Against Pseudomonas Is Diminished in Irf1−/− Mice
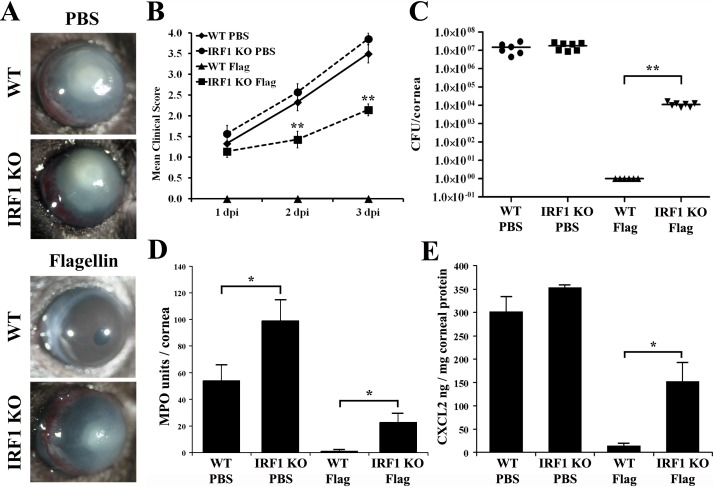
To examine the role of IRF1 in P. aeruginosa keratitis, we employed a well-accepted P. aeruginosa keratitis scratch model using a cytotoxic strain (ATCC 19660) that causes severe corneal keratitis and perforation, allowing better assessment of the protective effects of flagellin. As we showed previously,23 104 CFU ATCC 19660/cornea inoculated onto epithelial scratched corneas caused severe inflammation in WT B6 mice with high bacterial burden, and pretreatment with 500 ng flagellin for 24 hours resulted in total protection with no sign of inflammation and no viable bacteria at 3 dpi (Fig. 4). The corneas of Irf1−/− mice at 3 dpi exhibited signs of perforation, which usually occurs in WT B6 mice at 5 dpi or later22,23 (Figs. 4A, 4B), albeit bacterial burden was comparable to that in B6 mice (Fig. 4C). Flagellin pretreatment ameliorated the sensitivity phenotype in the Irf1−/− mice and protected the cornea from perforation (Fig. 4A). Compared to the WT, the protection was much reduced and incomplete as Irf1−/− mice displayed significant, albeit much lower, corneal clinical score and bacterial burden compared to the controls at 3 dpi (Figs. 4A–C). The severe keratitis observed in Irf1−/− mice appeared to be correlated to PMN infiltration while expression of CXCL2 was correlated to bacterial burden in these mice (Figs. 4D, 4E). As the severity continued to increase (Fig. 4B) during the first 3 days, the flagellin-induced protective mechanisms in the absence of Irf1−/− may not be sufficient to halt P. aeruginosa infection but rather may delay the disease progress. Thus, Irf1−/− mice are susceptible to P. aeruginosa infection with diminished flagellin-induced protection.
Figure 4.
IRF1 deficiency increased the severity of Pseudomonas keratitis at 3 dpi and attenuated flagellin-induced protection in B6 mice. WT or IRF1−/− B6 mice were topically pretreated with 500 ng flagellin or PBS for 24 hours, and then infected with ATCC 19660 at 104 CFU for 3 days. Keratitis progression was photographed (A), and clinical scores were assigned at 1, 2, and 3 dpi (B). The whole cornea was then collected for viable bacterial count (C), MPO activity assay (D), and CXCL2 ELISA (E). P values were generated using unpaired Student's t-test (*P < 0.05; **P < 0.01). The figure is a representative of three independent experiments.
Neutralization of CXCL10 Activity Impedes the Protective Properties of Flagellin Pretreatment Against P. aeruginosa Infection
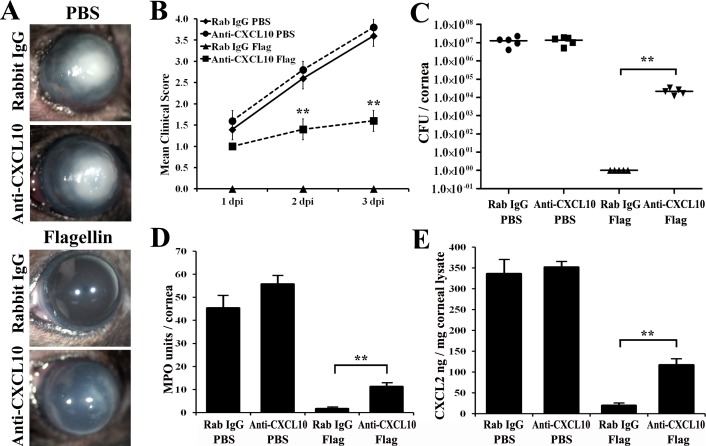
How might IRF1 influence corneal innate immunity and flagellin-induced protection? As Cxcl10 was a target gene of Irf1 and upregulated more apparently in protected corneas, we reasoned that it could play a role as an effector of IRF1 in bacterial keratitis. To test this hypothesis, we injected into the mouse subconjunctival space an anti-CXCL10 neutralizing antibody or isotype rabbit IgG 4 hours prior to flagellin/PBS pretreatment, followed by P. aeruginosa infection for 3 days (Figs. 5A, 5B). At 3 dpi, we assayed the corneas for viable bacterial count, MPO activity, and CXCL2 expression (Figs. 5C–E). CXCL10 neutralization did not significantly increase bacterial burden, PMN infiltration, and CXCL2 expression in infected corneas at 3 dpi in PBS pretreated WT B6 mice. However, in flagellin-pretreated corneas, CXCL10 neutralization resulted in visible infection and inflammation (Figs. 5A, 5B), significant increases in bacterial count (Fig. 5C), MPO activity (Fig. 5D), and CXCL2 expression (Fig. 5E) compared to anti-rabbit IgG-injected controls. However, compared to the control, PBS-pretreated corneas, flagellin-induced protection remained apparent. These results support the role of CXCL10 in mediating flagellin-induced corneal protection in the mouse model of P. aeruginosa keratitis.
Figure 5.
Neutralization of CXCL10 activity in the cornea attenuated flagellin-induced protection in B6 mice at 3 dpi. WT mice were injected with neutralizing anti-CXCL10 antibody (3 μg in 5 μL) or isotype rabbit IgG 24 hours prior to topical PBS or flagellin pretreatment, and then infected with ATCC 19660 at 104 CFU. Keratitis progression was photographed (A), and clinical scores were assigned at 1, 2, and 3 dpi (B). The corneas were then excised and homogenized for viable bacterial count (C), MPO activity assay (D), and CXCL2 ELISA (E). P values were generated using unpaired Student's t-test (**P < 0.01). The figure is a representative of three independent experiments.
Flagellin-Augmented CXCL10 Expression and Protection Involve Ifnγ and NK Cells in the Cornea
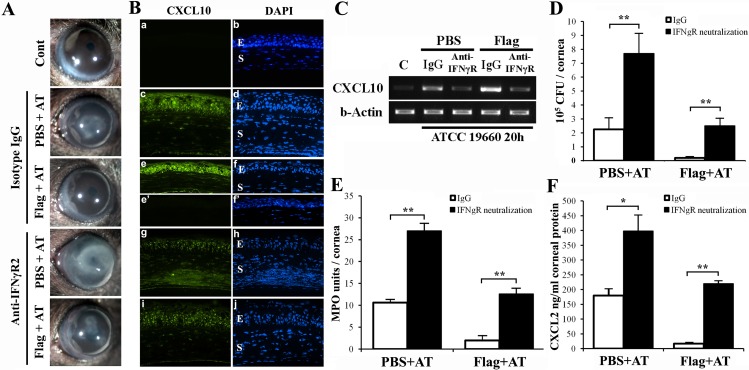
There was a major disparity between in vitro and in vivo results regarding IRF1 expression in flagellin-pretreated CECs in response to P. aeruginosa: Flagellin pretreatment dampened Irf1 expression in cultured HCECs, whereas in vivo infection-induced Irf1 expression was further augmented. This disparity could be due to human versus mouse CECs and/or to the pathogen used, PAO1 versus ATCC 19660. We would argue that the disparity is primarily due to the response of other cells such as infiltrated innate immune cells that are lacking in the cell culture setting. We hypothesized that Ifnγ, the strongest inducer of Irf1 and Cxcl10, secreted from infiltrating NK cells,45 is the missing link in cultured HCECs. To test this hypothesis, we first blocked Ifnγ function by injecting anti-IFNγR2 neutralizing antibodies (2.5 μg/cornea) subconjunctivally 4 hours prior to flagellin pretreatment, followed by ATCC 19660 inoculation. As shown in Figure 6, IFNγR2 neutralization increased the severity of keratitis and reduced flagellin-induced protection (Fig. 6A). Immunohistological staining and RT-PCR of IFNγR2-neutralized and infected corneas revealed that while isotype IgG had no effects on CXCL10 expression (compared to what is shown in Fig. 3), IFNγR2 neutralization attenuated infection-induced and flagellin-augmented expression of CXCL10 (Figs. 6B, 6C) in epithelia. Moreover, IFNγR2 neutralization resulted in significantly reduced bacterial clearance (Fig. 6D), increased neutrophil infiltration (Fig. 6E), and elevated CXCL2 (Fig. 6F) expression compared to isotype IgG-injected controls. Note that at this stage of infection (20 hpi), the parameters used to measure the severity of keratitis shown in Figures 6 and 7 were at much lower scales than shown in Figures 4 and 5 (3 dpi).
Figure 6.
Neutralization of IFNγR2 activity in the cornea attenuated epithelial CXCL10 production and flagellin-induced protection in B6 mice. WT mice were injected with IFNγ receptor neutralizing anti-IFNγR2 antibody (3 μg in 5 μL) or isotype rabbit IgG 24 hours prior to topical PBS or flagellin pretreatment, and then infected with ATCC 19660 at 104 CFU. At 20 hours postinfection the corneas were photographed (A) and then harvested for immunohistochemistry (B), RT-PCR (C) analysis of CXCL10 expression, bacterial count (D), MPO activity assay (E), and CXCL2 ELISA (F). P values were generated using unpaired Student's t-test (*P < 0.05; **P < 0.01). The figure is a representative of six corneas (n = 3 mice) per condition.
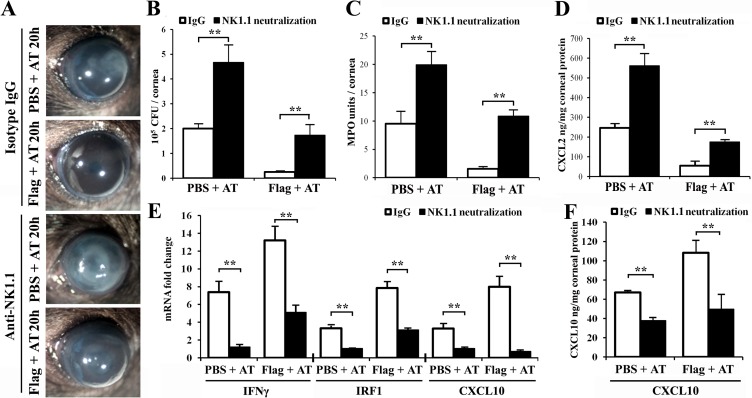
Figure 7.
Neutralization of NK cells in the cornea blocked IFNγ, IRF1, and CXCL10 expression and compromised flagellin-induced corneal protection in B6 mice. WT mice were injected with NK cell neutralizing anti-NK1.1 antibody (3 μg in 5 μL) or isotype rabbit IgG 24 hours prior to topical PBS or flagellin pretreatment, and then infected with ATCC 19660 at 104 CFU. At 20 hours postinfection the corneas were photographed (A) and then harvested for bacterial count (B); MPO activity assay (C); CXCL2 (D) and CXCL10 (F) ELISA; and real-time PCR analysis of IFNγ, IRF1, and CXCL10 expression (E). P values were generated using unpaired Student's t-test (**P < 0.01). The figure is a representative of six corneas (n = 3 mice) per condition.
Using the same approach, we depleted NK cells with antibody against NK1.1 (Fig. 7). Similarly, the isotype-matched antibody had no detectable effects on P. aeruginosa keratitis and on flagellin-induced protection. Depletion of NK cells resulted in an increase in the severity of PA keratitis and impaired flagellin-induced protection, including increased opacity (Fig. 7A), bacterial burden (Fig. 7B), PMN infiltration (Fig. 7C), and CXCL2 expression (Fig. 7D). As expected, the infection-induced and flagellin-augmented expressions of IFNγ, IRF1, and CXCL10 were greatly suppressed by NK cell depletion as detected by real-time PCR (Fig. 7E) and by ELISA for CXCL10 (Fig. 7F). These results support the importance of IFNγ and NK cells in the enhanced expression of CXCL10 and corneal innate immunity against P. aeruginosa.
Discussion
Our study of screening for transcription factors differentially expressed in PAO1-infected primary HCECs revealed that IRF1 expression was increased in response to bacterial challenge but that pre-exposure of cells to a low dosage of flagellin for 24 hours prior to PAO1 challenge caused a dramatic decrease in IRF1 expression. While our in vitro study of cultured human primary CECs revealed suppressive effects of flagellin on IRF1 expression, our in vivo study using B6 mice indicates an enhanced expression of IRF1 as well as its target gene CXCL10 in flagellin-pretreated CECs exposed to PAO1 for 6 hours. The expression of CXCL10 was primarily in epithelia. To understand the role of IRF1 in infectious keratitis, we used the mouse model of P. aeruginosa keratitis with ATCC 19660 as pathogen. Our results indicated that IRF1 deficiency increased cornea susceptibility to P. aeruginosa infection and abolished flagellin-induced protection against this common keratitis-causing pathogen in B6 mice. To assess if CXCL10 is an effector gene of IRF1, we neutralized CXCL10 and found indeed that CXCL10 is required for optimal flagellin-induced protection. To understand how the IRF1-CXCL10 axis is regulated, we blocked IFNγ signaling by neutralizing its receptor and found that the infection-induced and flagellin-augmented epithelial expression of CXCL10 was dampened in anti-IFNγR2- but not the control antibody-treated corneas. IFNγR2 neutralization also increased the severity of P. aeruginosa keratitis and significantly reduced flagellin-induced protection. Moreover, as NK cells are known to be a major source of IFNγ in the cornea,46,47 depletion of NK cells had effects similar to those of IFNγR2 neutralization on the severity of P. aeruginosa keratitis and on flagellin-induced protection. The upregulated expressions of IFNγ, IRF1, and CXCL10 in NK-depleted corneas were significantly reduced. Taken together, our data showed that infection-induced and flagellin-augmented expression of CXCL10 in CECs is IRF1 dependent and plays a major role in the elimination of inoculated P. aeruginosa—and that NK cells, similar to neutrophils,20,22 are critical for flagellin-induced protection and innate defense against P. aeruginosa in the cornea.
Irf1 is a member of the IRF family of transcription factors consisting of nine members, Irf1 through 9.48–50 Irfs were first characterized as IFN-inducible transcription factors. Recent studies revealed their involvement in the regulation of gene expression in responses triggered by TLRs, including upregulation of IRF3 and 7 by TLR3 and 4 through the Toll-receptor–associated activator of interferon-mediated pathway, and of Irf5, 7, and 1 by intracellular TLRs (7, 8, and 9), the latter only in myeloid dendritic cells.50,51 Our study is the first to show elevated expression of Irf1 in response to Gram-negative bacterial challenge in nonprofessional immune cells. In the mouse model of P. aeruginosa infection, we showed that P. aeruginosa infection resulted in the upregulation of Irf1 and that this upregulation, in contrast to in vitro data, was further augmented by flagellin pretreatment. This upregulation is important for controlling P. aeruginosa keratitis. While it is mostly studied in viral infection, Irf1 has been shown to be a master regulator of mycobacteria-induced immunopathology, involved in the development of centrally necrotizing granulomatous lesions in the lung.52,53 Interestingly, our results showed that IRF1-mediated gene expression plays a protective role in P. aeruginosa keratitis. Irf1 deficiency resulted in much more severe keratitis, and corneas of Irf1−/− mice were perforated within 3 days; in contrast, in WT B6 mice, the pathology will need 5 days to develop. It is also interesting that while PMN infiltration is significantly higher than in the WT mice, the bacterial burden and the levels of CXCL2 are similar between Irf1−/− and WT B6 mice, suggesting that excessive infiltration of PMNs is a major pathogenic factor for cornea tissue destruction. Importantly, although it is significantly compromised in Irf1−/− mice, flagellin-induced protection remains strong in Irf1−/− mice, as the severity of keratitis—evidenced by increased corneal opacity, bacterial burden, PMN infiltration, and the expression of Cxcl10 in flagellin-pretreated Irf1−/− mice—was significantly milder than that of the control, PBS-pretreated corneas. Our study further revealed that the epithelial-expressed Cxcl10 is a major effector of Irf1-mediated protection in the corneas.
Cxcl10 is a member of the interferon-inducible tripeptide motif Glu-Leu-Arg-negative (ELR−) CXC chemokines.54 It, along with CXCL9 and CXCL11, signals through a G-protein-coupled receptor, CXCR3, and functions primarily in the recruitment of activated CD4+ and CD8+ T cells, NK cells, and plasmacytoid dendritic cells to sites of infection and inflammation.55,56 In addition to their roles in leukocyte recruitment, CXCL9, CXCL10, and CXCL11 exert direct antimicrobial effects that are comparable to the effects mediated by cationic antimicrobial peptides, including defensins.57 Our study showed that flagellin pretreatment greatly augmented infection-induced upregulation of Cxcl10 in CECs in an Irf1-dependent manner.
We recently observed CXCL10 possessing bactericidal and fungicidal activities in vitro (Dong C, et al. IOVS 2013;54:ARVO E-Abstract 2064). Hence, the epithelium-expressed CXCL10 may play a key role in bacterial clearance at the early stage of infection. In our epithelial scratch model, pathogens were inoculated on epithelial injury sites with intact or minimally injured basement membrane. It has been reported that the basement membrane possesses physical barrier effects, resulting in the trapping of pathogens in epithelium for several hours,58,59 allowing the elevated CXCL10, along with other antimicrobial peptides such as β-defensins60 and LL-37 (or cathelicidin-related antimicrobial peptide, CRAMP),22,61 to kill these invading pathogens before they reach the stromal layer. Indeed, neutralizing CXCL10 resulted in much increased bacterial clearance, PMN infiltration, and CXCL2 expression and mild keratitis in flagellin-pretreated corneas, but exhibited no detectable effects on naïve mice; this suggests either that the efficiency of anti-CXCL10 antibody neutralization is low or that other effectors, antimicrobial peptides and/or infiltrated innate immune cells, also contribute significantly in flagellin-induced protection. Moreover, the fact that phenotypes of Irf1−/− and flagellin-treated Irf1−/− mice seem to be more severe than those of the anti-CXCL10-treated mice can also be explained by either low efficiency of CXCL10 neutralization or involvement of other effectors. Hence, future studies using CXCL10 and/or CXCR3 (receptor of CXCL10) knockout mice to define the role of CXCL10 as a chemokine and/or antimicrobial peptides (AMP) are warranted. Nevertheless, based on the results presented, we conclude that flagellin-induced expression of CXCL10 contributes to innate defense activated by topical flagellin and that epithelium-expressed CXCL10 is involved in pathogen clearance in the corneas.
Surprisingly, in contrast to in vitro results, flagellin pretreatment in B6 mice further enhanced the expression of Irf1 and its target gene Cxcl10. We suspected that this disparity is due to the molecular and/or cellular component(s) present in the mouse cornea and/or tears in vivo but not in cultured HCECs. One such factor is IFNγ, which is known not to be expressed in ECs. Our recent study of genome-wide cDNA array revealed a large number of interferon-induced genes in the ECs of flagellin-pretreated and PAO1-infected B6 mouse corneas.62 Using IFNγ receptor neutralizing antibody, we showed that inactivation of Ifnγ signaling increased the severity of P. aeruginosa keratitis in the control corneas and significantly reduced flagellin-induced protection to a level similar to that in nontreated corneas. As expected, infection-induced and flagellin-augmented expressions of IRF1 and CXCL10 were dampened in IFNγ receptor-neutralized corneas. Then, what are the cellular sources of IFNγ in infected corneas? We speculated that IFN-γ secreted from NK cells, which are absent in the in vitro system, enables flagellin-augmented IRF1 and CXCL10 expression in mouse cornea. Although NK cells and CD8+ T cells are both known to produce a large amount of IFNγ,63–65 we focused on NK cells at an early stage of infection (as early as 6 hpi, or 30 hours after flagellin pretreatment when augmented expression of IRF1 and CXCL10 was detected; Figs. 2, 3) in the cornea because CD8+ T cells were observed only at 3 dpi or thereafter in response to P. aeruginosa infection in the cornea.66 In a parallel study, we demonstrated that epithelial-expressed CXCL10 may recruit CXCR3-positive NK cells, which are present underneath CXCL10-expressing epithelia in flagellin-pretreated corneas without infection (Xiaowei L, Nan G, Chen D, et al., manuscript in preparation). Hence, we used neutralizing antibody to deplete NK cells by subconjunctival injection and assess its effects on P. aeruginosa and on the expression of IFNγ, IRF1, and CXCL10, an axis of IFNγ signaling pathway. While depletion of NK cells had effects on the severity of P. aeruginosa keratitis similar to those of IFNγ receptor neutralization, NK depletion indeed significantly affected the expression of all three genes. Intriguingly, the most affected is CXCL10, the effector of the axis, suggesting that NK1.1-positive cells may release additional factor(s) required to sustain elevated expression of CXCL10 in flagellin-pretreated and P. aeruginosa-infected corneas. The suppression of CXCL10 expression in flagellin-pretreated and P. aeruginosa-infected corneas by IFNγ neutralization and NK cell depletion may provide an explanation for diminished protection from microbial keratitis in these corneas. In vitro, IRF1 is induced by NF-κB, which was blunted by prolonged exposure to flagellin. We suggest that the lack of IFNγ in cell culture resulted in IRF1 being reprogrammed in vitro, but not in vivo.
Taken together, our data suggest that Irf1 is an important responsive transcription factor in corneal innate immunity and in flagellin-induced protection mediated by CXCL10 expression. We propose that flagellin pretreatment elicits CXCL10 expression in the corneal epithelia, resulting in the recruitment of a small yet significant number of CXCR3-positive NK cells to the stroma, along with PMNs.22,44 Challenge of flagellin-treated corneas with pathogens resulted in rapid activation of NK cells to produce IFNγ, which in turn augments the expression CXCL10 in ECs, resulting in the eradication of invading pathogens by the coordinated action of massively produced AMPs including CXCL10, β-defensins, and cathelicidin peptides and infiltrated NK cells and PMNs.22,44 Early elimination of pathogens results in greatly reduced inflammatory response and profound protection observed in flagellin-pretreated corneas, and potentially in the lung.67 Hence, Irf1 and its target gene Cxcl10 play an important role in corneal innate defense and more apparently in flagellin-induced mucosal protection against P. aeruginosa infection.
Acknowledgments
The authors thank all the members of the Yu and Mi laboratories for assistance and comments on the work.
Supported by National Institutes of Health/National Eye Institute Grants R01EY10869, EY17960 (F-SXY), p30 EY04078 (National Eye Institute Core Grant to Wayne State University), and HL097564 (TJS); and Research to Prevent Blindness (to Kresge Eye Institute).
Disclosure: G.S. Yoon, None; C. Dong, None; N. Gao, None; A. Kumar, None; T.J. Standiford, None; F.-S.X. Yu, None
References
- 1. Ueta M, Kinoshita S. Innate immunity of the ocular surface. Brain Res Bull. 2010; 81: 219–228 [DOI] [PubMed] [Google Scholar]
- 2. Santaolalla R, Fukata M, Abreu MT. Innate immunity in the small intestine. Curr Opin Gastroenterol. 2011; 27: 125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaible B, Schaffer K, Taylor CT. Hypoxia, innate immunity and infection in the lung. Respir Physiol Neurobiol. 2010; 174: 235–243 [DOI] [PubMed] [Google Scholar]
- 4. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34: 637–650 [DOI] [PubMed] [Google Scholar]
- 5. Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011; 34: 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004; 23: 327–333 [DOI] [PubMed] [Google Scholar]
- 7. Song J, Abraham SN. Innate and adaptive immune responses in the urinary tract. Eur J Clin Invest. 2008; 38 (suppl 2): 21–28 [DOI] [PubMed] [Google Scholar]
- 8. Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006; 6: 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu FS, Hazlett LD. Toll-like receptors and the eye. Invest Ophthalmol Vis Sci. 2006; 47: 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004; 126: 1054–1070 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002; 110: 191–202 [DOI] [PubMed] [Google Scholar]
- 12. Gingras S, Parganas E, de Pauw A, Ihle JN, Murray PJ. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of toll-like receptor signaling. J Biol Chem. 2004; 279: 54702–54707 [DOI] [PubMed] [Google Scholar]
- 13. Liew FY, Liu H, Xu D. A novel negative regulator for IL-1 receptor and Toll-like receptor 4. Immunol Lett. 2005; 96: 27–31 [DOI] [PubMed] [Google Scholar]
- 14. Feterowski C, Novotny A, Kaiser-Moore S, et al. Attenuated pathogenesis of polymicrobial peritonitis in mice after TLR2 agonist pre-treatment involves ST2 up-regulation. Int Immunol. 2005; 17: 1035–1046 [DOI] [PubMed] [Google Scholar]
- 15. Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004; 10: 71–84 [DOI] [PubMed] [Google Scholar]
- 16. Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011: 8: 388–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009; 30: 475–487 [DOI] [PubMed] [Google Scholar]
- 18. Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006; 10: 233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007; 7: 353–364 [DOI] [PubMed] [Google Scholar]
- 20. Gao N, Kumar A, Guo H, Wu X, Wheater M, Yu FS. Topical flagellin-mediated innate defense against Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2011; 52: 3074–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao N, Kumar A, Jyot J, Yu FS. Flagellin-induced corneal antimicrobial peptide production and wound repair involve a novel NF-kappaB-independent and EGFR-dependent pathway. PLoS One. 2010; 5: e9351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar A, Gao N, Standiford TJ, Gallo RL, Yu FS. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 2010; 12: 978–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008; 76: 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar A, Yin J, Zhang J, Yu FS. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest Ophthalmol Vis Sci. 2007; 48: 4664–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008; 320: 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijay-Kumar M, Aitken JD, Sanders CJ, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol. 2008; 180: 8280–8285 [DOI] [PubMed] [Google Scholar]
- 27. Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010; 201: 534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor-5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun. 2011; 79: 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miao EA, Alpuche-Aranda CM, Dors M, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006; 7: 569–575 [DOI] [PubMed] [Google Scholar]
- 30. Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007; 29: 275–288 [DOI] [PubMed] [Google Scholar]
- 31. Uematsu S, Akira S. Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection. J Gastroenterol. 2009; 44: 803–811 [DOI] [PubMed] [Google Scholar]
- 32. Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 2010; 4: 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campodonico VL, Llosa NJ, Grout M, Doring G, Maira-Litran T, Pier GB. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect Immun. 2010; 78: 746–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prince A. Flagellar activation of epithelial signaling. Am J Respir Cell Mol Biol. 2006; 34: 548–551 [DOI] [PubMed] [Google Scholar]
- 35. Li G, Domenico J, Jia Y, Lucas JJ, Gelfand EW. NF-kappaB-dependent induction of cathelicidin-related antimicrobial peptide in murine mast cells by lipopolysaccharide. Int Arch Allergy Immunol. 2009; 150: 122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pistolic J, Cosseau C, Li Y, et al. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway. J Innate Immun. 2009; 1: 254–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang X, Barrett RP, McClellan SA, Hazlett LD. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005; 46: 4209–4216 [DOI] [PubMed] [Google Scholar]
- 38. Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003; 44: 4247–4254 [DOI] [PubMed] [Google Scholar]
- 39. Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006; 8: 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stirnweiss A, Ksienzyk A, Klages K, et al. IFN regulatory factor-1 bypasses IFN-mediated antiviral effects through viperin gene induction. J Immunol. 2010; 184: 5179–5185 [DOI] [PubMed] [Google Scholar]
- 41. Zaheer RS, Proud D. Human rhinovirus-induced epithelial production of CXCL10 is dependent upon IFN regulatory factor-1. Am J Respir Cell Mol Biol 2010; 43: 413–421 [DOI] [PubMed] [Google Scholar]
- 42. Koetzler R, Zaheer RS, Newton R, Proud D. Nitric oxide inhibits IFN regulatory factor 1 and nuclear factor-kappaB pathways in rhinovirus-infected epithelial cells. J Allergy Clin Immunol. 2009; 124: 551–557 [DOI] [PubMed] [Google Scholar]
- 43. Zaheer RS, Proud D. Human rhinovirus-induced epithelial production of CXCL10 is dependent upon IFN regulatory factor-1. Am J Respir Cell Mol Biol. 2010; 43: 413–421 [DOI] [PubMed] [Google Scholar]
- 44. Gao N, Yin J, Yoon GS, Mi QS, Yu FS. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am J Pathol. 2011; 179: 2243–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon JM. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med. 2012; 18: 270–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-gamma production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol. 2005; 35: 1567–1575 [DOI] [PubMed] [Google Scholar]
- 47. Hazlett LD, Li Q, Liu J, McClellan S, Du W, Barrett RP. NKT cells are critical to initiate an inflammatory response after Pseudomonas aeruginosa ocular infection in susceptible mice. J Immunol. 2007; 179: 1138–1146 [DOI] [PubMed] [Google Scholar]
- 48. Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007; 37: 306–309 [DOI] [PubMed] [Google Scholar]
- 49. Honda K, Taniguchi T. Toll-like receptor signaling and IRF transcription factors. IUBMB Life. 2006; 58: 290–295 [DOI] [PubMed] [Google Scholar]
- 50. Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010; 59: 489–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Battistini A. Interferon regulatory factors in hematopoietic cell differentiation and immune regulation. J Interferon Cytokine Res. 2009; 29: 765–780 [DOI] [PubMed] [Google Scholar]
- 52. Aly S, Mages J, Reiling N, et al. Mycobacteria-induced granuloma necrosis depends on IRF-1. J Cell Mol Med. 2009; 13: 2069–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamada H, Mizuno S, Sugawara I. Interferon regulatory factor 1 in mycobacterial infection. Microbiol Immunol. 2002; 46: 751–760 [DOI] [PubMed] [Google Scholar]
- 54. Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001; 167: 623–627 [DOI] [PubMed] [Google Scholar]
- 55. Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Curr Opin Immunol. 2007; 19: 24–30 [DOI] [PubMed] [Google Scholar]
- 56. Mohan K, Cordeiro E, Vaci M, McMaster C, Issekutz TB. CXCR3 is required for migration to dermal inflammation by normal and in vivo activated T cells: differential requirements by CD4 and CD8 memory subsets. Eur J Immunol. 2005; 35: 1702–1711 [DOI] [PubMed] [Google Scholar]
- 57. Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001; 167: 623–627 [DOI] [PubMed] [Google Scholar]
- 58. Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SM. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect Immun. 2009; 77: 3264–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, Fleiszig SM. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci. 2011; 52: 1368–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Augustin DK, Heimer SR, Tam C, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011; 79: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang LC, Petkova TD, Reins RY, Proske RJ, McDermott AM. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci. 2006; 47: 2369–2380 [DOI] [PubMed] [Google Scholar]
- 62. Gao N, Sang Yoon G, Liu X, et al. Genome-wide transcriptional analysis of differentially expressed genes in flagellin-pretreated mouse corneal epithelial cells in response to Pseudomonas aeruginosa: involvement of S100A8/A9. Mucosal Immunol. 2013; 6: 993–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Quaranta MG, Napolitano A, Sanchez M, Giordani L, Mattioli B, Viora M. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: different outcome of CD56(dim) and CD56(bright) NK cell subsets. FASEB J. 2007; 21: 2323–2334 [DOI] [PubMed] [Google Scholar]
- 64. Goodridge JP, Lathbury LJ, John E, Charles AK, Christiansen FT, Witt CS. The genotype of the NK cell receptor, KIR2DL4, influences INFgamma secretion by decidual natural killer cells. Mol Hum Reprod. 2009; 15: 489–497 [DOI] [PubMed] [Google Scholar]
- 65. Schoenborn JR, Wilson CB. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007; 96: 41–101 [DOI] [PubMed] [Google Scholar]
- 66. Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004; 23: 1–30 [DOI] [PubMed] [Google Scholar]
- 67. Yu FS, Cornicelli MD, Kovach MA, et al. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol. 2010; 185: 1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]