Abstract
Purpose.
Intraocular pressure in the sitting position changes minimally during sleep, although aqueous humor flow decreases by 50% or more at night. The explanation for this apparent discrepancy has been unclear. This study investigated the roles of outflow facility, episcleral venous pressure (EVP), and uveoscleral outflow in maintaining IOP at night.
Methods.
Forty-two eyes of 21 healthy subjects (age 47–76 years, mean 59 years) were studied. Aqueous humor flow rate, IOP in the sitting position, outflow facility, and EVP were measured in each eye during the middiurnal period (2:00–4:00 PM). Uveoscleral flow was calculated from the other variables by using the modified Goldmann equation. These variables were remeasured during the midnocturnal period (2:00–4:00 AM) and compared with those measured during the diurnal period by using generalized estimating equation models.
Results.
Intraocular pressure did not change from the middiurnal period (13.9 ± 3.0 mm Hg) to the midnocturnal period (13.0 ± 1.8 mm Hg, mean ± SD, P = 0.07), although aqueous humor flow rate decreased from 2.48 ± 0.96 μL/min to 1.27 ± 0.63 μL/min (P < 0.001). Outflow facility decreased from 0.23 ± 0.06 μL/min/mm Hg to 0.20 ± 0.06 μL/min/mm Hg (P = 0.004), and EVP was unchanged from the middiurnal period (7.4 ± 1.8 mm Hg) to the midnocturnal period (7.4 ± 2.2 mm Hg, P = 0.95). Uveoscleral outflow decreased 93%, from 0.94 ± 1.26 μL/min during the middiurnal period to 0.07 ± 0.78 μL/min (P = 0.008) during the midnocturnal period.
Conclusions.
The nocturnal decrease in aqueous humor flow rate in older, healthy subjects is compensated by a small decrease in outflow facility and a large decrease in uveoscleral outflow to maintain a stable IOP.
Keywords: aqueous humor dynamics, uveoscleral pathway, aqueous flow, outflow facility, episcleral venous pressure
This study investigated the circadian changes in aqueous humor dynamics in healthy subjects. A large nocturnal decrease in aqueous humor flow rate is compensated by a large decrease in uveoscleral flow rate and a small decrease in outflow facility, resulting in a relatively stable IOP.
Introduction
Intraocular pressure measured in the habitual positions (sitting while awake and supine during sleep) is highest during sleep in most subjects.1–5 When IOP is measured at night in the sitting position, it remains close to or slightly less than IOP during the day.1–5 This behavior is the opposite of that of aqueous humor flow, which decreases by as much as 50% during sleep.6–8 Because IOP is directly related to aqueous humor flow, the dramatic decrease in flow rate must be compensated by a decrease in outflow facility, an increase in episcleral venous pressure, or a diminished nonconventional outflow during sleep.
In an earlier study, outflow facility decreased somewhat at night, but the decrease was not enough to compensate entirely for the typical decrease in aqueous humor flow rate9 and we were not able to detect this pattern in young, healthy subjects.10 An increase in episcleral venous pressure (EVP) at night, if such an increase exists, might compensate for the decreased flow rate.9,10 Two studies found an increased EVP at night when measured with the subject supine at night and seated during the day.11,12 However, one of these studies found that EVP remained consistent if the subject did not change positions.11 As well, subjective endpoints used for previous measurements of EVP have left the nocturnal pattern of EVP unclear.13
Differences in uveoscleral flow might also explain the minimal IOP differences between day and night. Uveoscleral flow cannot be measured directly but must be calculated from IOP, aqueous humor flow, outflow facility, and EVP measured at approximately the same time. One study estimated a large decrease in nighttime uveoscleral flow in healthy subjects, when using seated IOP, tonographic outflow facility, and an assumed nighttime EVP based on EVP measured during the day.9 However, the lack of objective measurement of EVP left the calculation of uveoscleral flow rate uncertain. An understanding of the relatively unchanged IOP at night, a time when aqueous humor production is naturally low, will require measurement of all variables of aqueous humor dynamics during the night as well as during the day. In this study, we investigated the circadian variation of aqueous humor dynamics in older, healthy subjects.
Methods
Study Subjects
Twenty-one participants in good general health, between the ages of 47 and 76 years (mean 59.0 ± 9.3 years, ± SD), with regular sleep schedules were recruited from employees and patients of Mayo Clinic, or local area residents. Patients with chronic disease (e.g., hypertension) were included if their condition was stable, and they did not require treatment other than medications. None of the participants were using systemic steroids or beta-adrenergic blockers, or had any history of ocular pathology including intraocular surgery or trauma, narrow angles, glaucoma, diabetic eye disease, uveitis, high myopia (>6 diopters [D]), or high hyperopia (>4 D). Each subject gave informed consent to participate after discussion of the nature and possible consequences of the study. This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board at Mayo Clinic.
Measurements
Intraocular Pressure.
Intraocular pressure was measured in the sitting and supine positions by using a pneumatonometer (Model 30 Classic; Medtronic Ophthalmics, Jacksonville, FL) after instilling a topical anesthetic (proparacaine, 0.5%). Subjects remained seated or supine for at least 5 minutes prior to measurements. The mean of three consecutive measurements was recorded as the IOP.
Aqueous Humor Flow Rate.
The aqueous humor flow rate was calculated from the clearance of fluorescein from the combined cornea and anterior chamber, as described previously14:
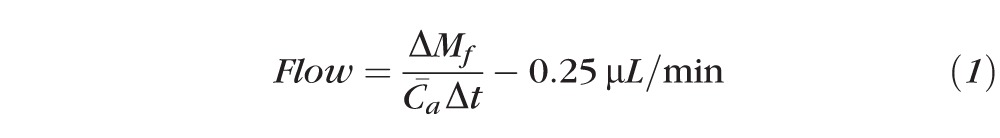 |
where ΔMf is the mass of fluorescein lost from the combined cornea and anterior chamber during interval Δt,
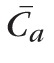 is the average concentration of fluorescein in the anterior chamber during the same interval (estimated from the initial and final concentrations, assuming an exponential decay), and 0.25 μL/min is the flow rate equivalent to the assumed diffusional loss of fluorescein from the eye.15–18 Fluorescein concentrations in the anterior chamber and cornea were measured by using a scanning ocular fluorophotometer,19–21 and the mass in each compartment was the product of its volume and the mean fluorescein concentration. Anterior chamber volume was measured photogrammetrically,22 and cornea volume was assumed to be 70 μL in all subjects.14,23
is the average concentration of fluorescein in the anterior chamber during the same interval (estimated from the initial and final concentrations, assuming an exponential decay), and 0.25 μL/min is the flow rate equivalent to the assumed diffusional loss of fluorescein from the eye.15–18 Fluorescein concentrations in the anterior chamber and cornea were measured by using a scanning ocular fluorophotometer,19–21 and the mass in each compartment was the product of its volume and the mean fluorescein concentration. Anterior chamber volume was measured photogrammetrically,22 and cornea volume was assumed to be 70 μL in all subjects.14,23
Outflow Facility.
Outflow facility was measured by using a custom digital tonometer based on an existing electronic Schiøtz tonometer (Berkeley Bio-Engineering, Inc., San Leandro, CA).24 A 4-minute tracing with a 5.5-g weight was recorded. Outflow facility was calculated from the beginning and end points of a second order polynomial, fitted to the pressure decay curves by a least-squares method, and standard tables.25 Facility was measured at the end of the middiurnal and midnocturnal periods. Prior to tonography, topical proparacaine 0.5% was instilled in the eye as an anesthetic.
Episcleral Venous Pressure (EVP).
Episcleral venous pressure was measured by using a novel device that has been described in detail elsewhere.13,26 In brief, the technique determined the pressure that was required to initiate collapse of an episcleral vein. A high-definition video camera captured images of the vein as it was compressed by an air chamber with a flexible and clear wall. The pressure in the chamber was increased linearly and the pressure associated with each video frame was recorded by a computer as the vein collapsed.
Episcleral veins were identified in the video stream as being straighter and deeper than the conjunctival vessels and were often bifurcated. The vein was assumed to be uncollapsed prior to increasing the pressure in the balloon above ambient pressure. As the chamber inflated, the vein began to collapse, and this was seen in the video sequence as a reduction of vessel intensity and sometimes as vessel narrowing. Image processing techniques were used to identify the frame where the vessel just began to collapse, and we assumed that the pressure associated with that frame corresponded to EVP.
Uveoscleral Flow Rate.
Uveoscleral flow rate cannot be measured directly in humans, but must be estimated from the modified Goldmann equation:
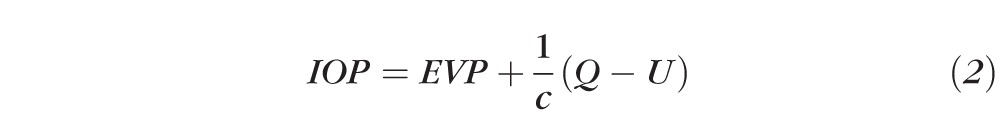 |
where Q is the aqueous humor flow rate, c is the conventional (trabecular) outflow facility, and U is the pressure-insensitive uveoscleral outflow rate. Uveoscleral outflow can be estimated by rearranging equation 2:
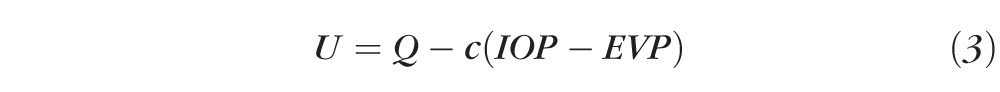 |
This expression represents the difference between the production rate of aqueous humor measured by fluorophotometry and the estimate of trabecular flow from outflow facility and the pressure difference that moves aqueous humor out of the eye.
Blood Pressure.
Blood pressure was measured with an automated blood pressure cuff (Model HEM-780; Omron Healthcare, Inc., Vernon Hills, IL). The cuff was placed on the right arm, with the subject in the seated position, immediately after the seated IOP measurements.
Experimental Protocol
All subjects received a dilated eye examination and an updated medical history was recorded. One-week prior to the study session, subjects were asked to maintain a regular sleep cycle of 8-hours sleep and 16-hours wake. Subjects were equipped with a wrist actigraph to monitor physical activity (Actiwatch AW-L; Mini Mitter, Bend, OR). They were also asked to maintain a log with sleep and wake times. Subjects' sleep patterns were used to adjust the actual time of the measurements during the study, so that the nocturnal period for each subject was equivalent to 11 PM to 7 AM. Contact lens wearers (3 subjects) were asked to stop wearing their contact lenses one-week prior to the study in order to minimize any potential effect on permeability of the corneal epithelium to fluorescein and provide a consistent initial depot of fluorescein in the stroma.
On the day of the study the subjects were asked to maintain a regular schedule with normal activities. The subjects instilled 2 to 3 drops of topical 2% fluorescein in each eye between 1 AM and 2 AM on the study day, and carefully cleaned excess fluorescein from their lashes and lid margins prior to returning to sleep.
Subjects reported to the Clinical Research Unit (CRU) at Mayo Clinic at 1 PM on the day of the study. Meals were provided and subjects continued normal activities while at the CRU, except that they were asked to refrain from exercise. They were permitted to leave the CRU for brief periods but were asked to remain in the hospital.
At the beginning of the middiurnal period (2:00 PM), fluorescein concentration was measured in the anterior segment of each eye by using fluorophotometry. This measurement was repeated at end of the middiurnal period (4:00 PM). Episcleral venous pressure was measured immediately after fluorophotometry, and this was followed by measurement of IOP and blood pressure. Outflow facility was measured at the end of the diurnal measurements. Immediately after tonography, subjects instilled another 2 to 3 drops of 2% fluorescein in each eye, depending on the measured anterior chamber fluorescein concentration. After cleaning excess fluorescein from the lashes and lid margins, subjects lay supine for 20 minutes with eyes closed to ensure a uniform distribution of the fluorescein in the cornea. Subjects were asked to go to sleep at their regular times based on their sleep patterns from the previous week.
The subjects were awakened at the beginning of the mid-nocturnal period (2:00 AM) for a third set of fluorophotometry measurements, after which subjects returned to sleep. At the end of the midnocturnal period (4:00 AM), anterior segment fluorescence was measured a fourth and final time. This was followed by measurement of EVP, IOP, blood pressure, and outflow facility. During the nocturnal period, all measurements were performed under reduced light conditions (approximately 10 lux white light). Previous reports indicated that a moderate light exposure at night does not affect the nocturnal IOP pattern.27 Total time for all measurements in the midnocturnal period was 20 to 30 minutes.
Statistical Analysis
Mean IOP, aqueous humor flow rates, EVP, outflow facility, and uveoscleral flow rates from the midnocturnal period were compared with the same variables from the middiurnal period. Both eyes were included in statistical analysis, and diurnal and nocturnal data were compared by using generalized estimating equation models to account for possible correlation between the two eyes of each subject. Blood pressure in the middiurnal period was compared with blood pressure in the mid-nocturnal period using paired t-tests. Differences were considered significant if P was less than 0.05.
Our sample size was estimated to detect a 20% difference between diurnal and nocturnal outflow facilities. If diurnal outflow facility in our sample were 0.25 ± 0.10 μL/min/mm Hg (mean ± SD), similar to that of other healthy subjects,8,10,28–30 34 eyes would be required to have a 90% chance of detecting a 20% difference between diurnal and nocturnal outflow facility if such a difference actually exists (paired t-test, α = 0.05, β = 0.10).
Results
Experimental Results
Forty-two eyes of 21 subjects were enrolled in the study and analyzed. The ages of the subjects ranged from 47 to 76 years (58.5 ± 9.1 years, mean ± SD). The baseline characteristics of subjects are shown in Table 1. One subject was being treated for hypertension with hydrochlorothiazide, eight subjects were taking daily aspirin, and six were being treated with simvastatin.
Table 1.
Study Population Baseline Characteristics
|
Parameter |
Value |
| Number of patients | 21 |
| Number of eyes | 42 |
| Age, y ± SD | 58.5 ± 9.1 |
| Range | 47–76 |
| Sex | |
| Female, number | 12 |
| Male, number | 9 |
| IOP, mm Hg ± SD, at screening | 14.7 ± 2.9 |
| Refractive error, diopters ± SD | −0.30 ± 1.0 |
| Range | 1.8 to −3.4 (spherical equivalent) |
| Central corneal thickness, μm ± SD | 541.9 ± 26.2 |
| Range | 488–629 |
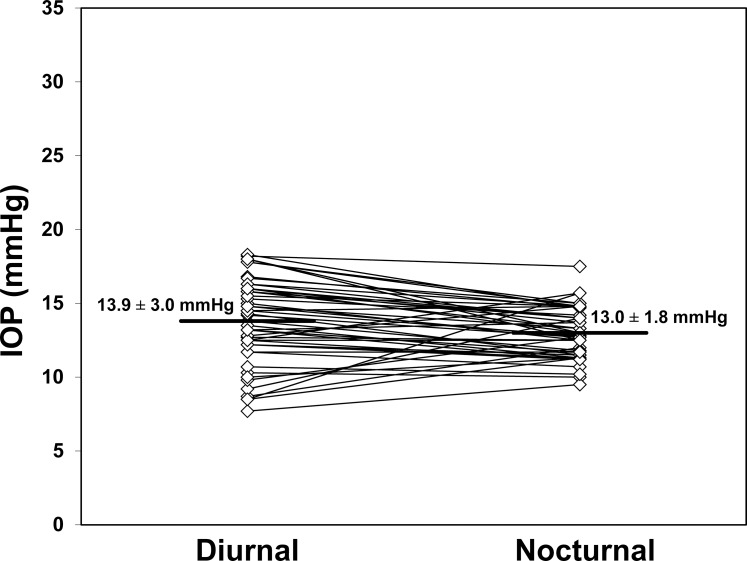
Intraocular pressure in the sitting position (Fig. 1) did not change from the middiurnal period (13.9 ± 3.0 mm Hg, mean ± SD) to the midnocturnal period (13.0 ± 1.8 mm Hg, P = 0.07). Intraocular pressure in the supine position (Fig. 2) also did not change from the middiurnal period (18.1 ± 3.1 mm Hg) to the midnocturnal period (17.8 ± 2.3 mm Hg, P = 0.42). During both the middiurnal and midnocturnal periods, the supine IOP was higher than the sitting IOP (P < 0.001).
Figure 1.
Intraocular pressure measured in the sitting position did not change from the middiurnal period (13.9 ± 3.0 mm Hg) to the midnocturnal period (13.0 ± 1.8 mm Hg, mean ± SD, P = 0.07).
Figure 2.
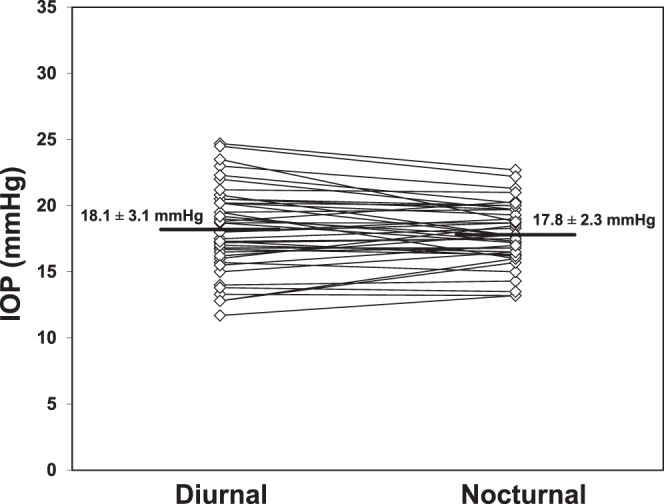
Intraocular pressure measured in the supine position did not change from the middiurnal period (18.1 ± 3.1 mm Hg) to the midnocturnal period (17.8 ± 2.3 mm Hg, mean ± SD, P = 0.42).
Seated systolic blood pressure decreased from 130.5 ± 14.0 mm Hg during the middiurnal period to 121.0 ± 10.4 mm Hg during the midnocturnal period (P = 0.006). Seated diastolic blood pressure did not change, from the middiurnal period (83.5 ± 9.1 mm Hg) to the midnocturnal period (80.7 ± 7.8 mm Hg, P = 0.562)
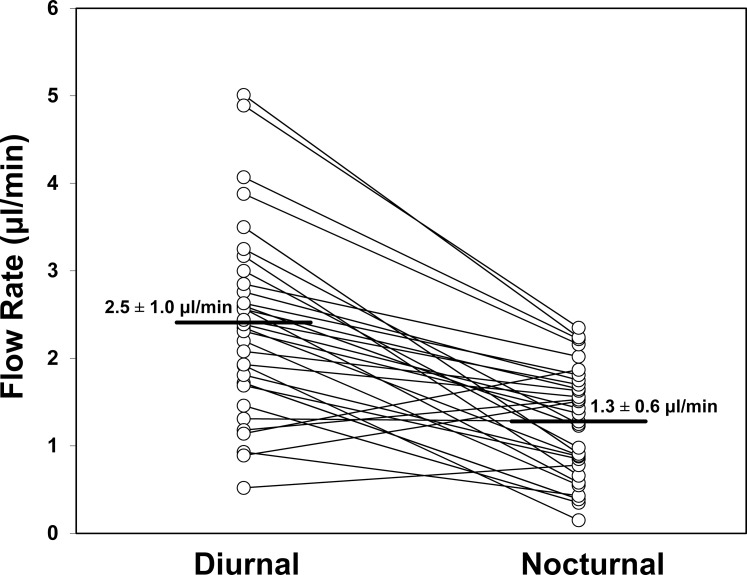
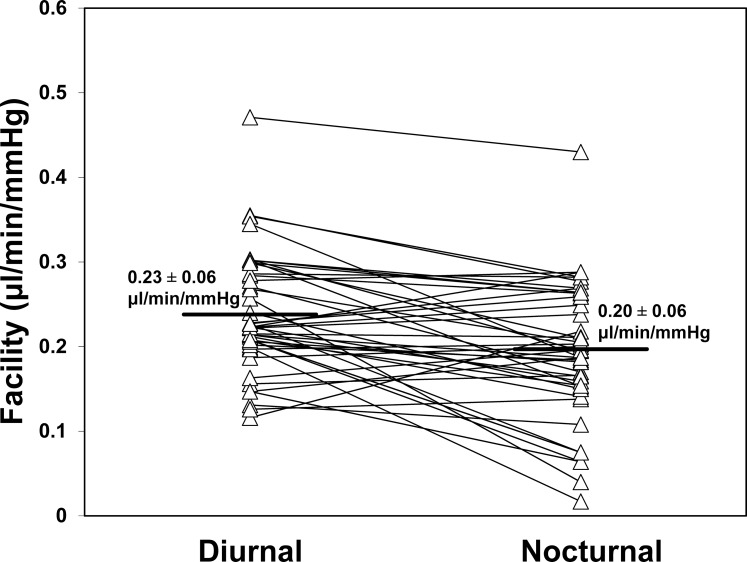
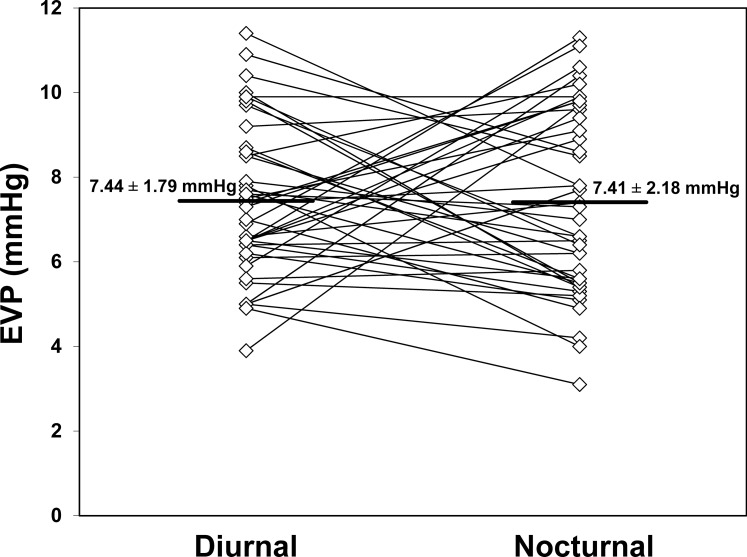
Aqueous humor flow rate (Fig. 3) decreased from 2.48 ± 0.96 μL/min during the middiurnal period to 1.27 ± 0.63 μL/min (P < 0.001) during the midnocturnal period. The mean outflow facility (Fig. 4) was 0.23 ± 0.06 μL/min/mm Hg during the middiurnal period, and decreased to 0.20 ± 0.06 μL/min/mm Hg (P = 0.004) during the midnocturnal period. Episcleral venous pressure (Fig. 5) did not change from the middiurnal period (7.44 ± 1.79 mm Hg) to the midnocturnal period (7.41 ± 2.18 mm Hg, P = 0.95)
Figure 3.
Aqueous humor flow rate decreased from 2.5 ± 1.0 μL/min (mean ± SD) during the middiurnal period to 1.3 ± 0.6 μL/min during the midnocturnal period (P < 0.001).
Figure 4.
Outflow facility measured by using tonography decreased significantly from 0.23 ± 0.06 μL/min/mm Hg (mean ± SD) during the middiurnal period to 0.20 ± 0.06 μL/min/mm Hg during the midnocturnal period (P = 0.004).
Figure 5.
Episcleral venous pressure measured by using objective venomanometry did not change from the middiurnal period (7.44 ± 1.79 mm Hg) to the midnocturnal period (7.41 ± 2.18 mm Hg, mean ± SD, P = 0.95).
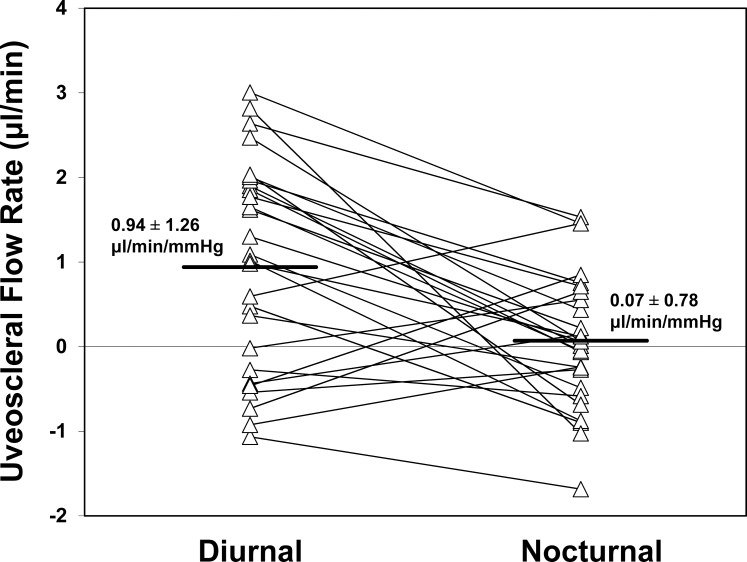
The uveoscleral outflow rate (Fig. 6) calculated by using Equation 3 was 0.94 ± 1.26 μL/min during the middiurnal period and decreased to 0.07 ± 0.78 μL/min during the midnocturnal period (P = 0.008, Table 2), a reduction of 93%. Uveoscleral outflow rate comprised 38.0% of total aqueous humor flow during the middiurnal period, and 5.6% of total flow during the midnocturnal period.
Figure 6.
Uveoscleral outflow rate calculated by using the modified Goldmann equation decreased from 0.94 ± 1.26 μL/min during the middiurnal period to 0.07 ± 0.78 μL/min during the midnocturnal period (P = 0.008), a reduction of 93%.
Table 2.
Aqueous Humor Dynamics Parameters During the Middiurnal and Midnocturnal Periods (± SD)
|
Sitting IOP, mm Hg |
Aqueous Humor Flow Rate, μL/min |
Outflow Facility, μL/min/mm Hg |
EVP, mm Hg |
Uveoscleral Outflow, μL/min |
|
| Diurnal | 13.9 ± 3.0 | 2.5 ± 1.0 | 0.23 ± 0.06 | 7.4 ± 1.8 | 0.94 ± 1.26 |
| Nocturnal | 13.0 ± 1.8 | 1.3 ± 0.6 | 0.20 ± 0.06 | 7.4 ± 2.2 | 0.07 ± 0.78 |
| P | 0.07 | <0.001 | 0.004 | 0.95 | 0.008 |
Discussion
Diurnal rhythms in IOP have been well documented, starting with the work of Drance in the 1960s.31 Subsequent investigators examined the 24-hour pattern of IOP variation and found that glaucoma patients exhibited a drop in IOP during the night.32,33 However, these studies measured IOP in the sitting position by Goldmann applanation tonometry. Liu et al.1,2,5 demonstrated that IOP measured in the habitual positions (sitting while awake and supine while asleep) was significantly higher during the nocturnal period than the diurnal period. When IOP was measured in the supine position during the day and night, glaucoma patients showed a slight decrease in IOP at night,5 while healthy subjects showed a slight increase.1,2 In the present study, we did not find any change in the nocturnal IOP compared with diurnal IOP when both were measured in the same body position. However, when measured in habitual positions (sitting while awake, supine during sleep), nocturnal IOP was significantly higher than diurnal IOP, consistent with the work of Liu et al.1,2,5
In this study, outflow facility decreased somewhat from day to night, and this is similar to the decrease in nocturnal outflow facility in healthy subjects reported by Lui et al.9 and ocular hypertensive subjects reported by Fan et al.12 It is not clear why outflow facility decreases at night. Acute changes in outflow facility can be induced with parasympathomimetics, such as pilocarpine.34 However, nocturnal parasympathetic activity is typically higher than diurnal activity,35 as is ciliary muscle tone.36 This would be expected to increase outflow facility rather than decrease it as we observed. As well, our previous study of younger, healthy subjects did not find a change in outflow facility from day to night. It is possible that as subjects get older, the day–night differences in outflow facility may increase, as suggested by Liu et al.9 The mean age of subjects in the previous study was 29 years, which was younger than the mean age of 59 years in this study. However, the reasons for decreases in outflow facility at night are unknown at this time.
Episcleral venous pressure did not change from day to night, and this is consistent with recent work by Lui et al.9 Uveoscleral outflow, calculated from the other measured variables, decreased by over 90% from the middiurnal period to the midnocturnal period. However, the reason for this decrease is unclear because our understanding of uveoscleral outflow continues to evolve. One possible explanation is related to the increase in ciliary muscle tone at night.36 Pilocarpine decreases uveoscleral flow in monkeys, likely by closing intramuscular spaces in the ciliary body, and as a consequence decreasing flow through this path.37 It is also possible that active contractions of the ciliary muscle are required to move fluid through the uveoscleral system, similar to microlymphatic vessels, which lack muscular walls and any significant pressure gradient and requires muscle contraction to move fluid.38 If this is the case, then increased ciliary muscle tone at night may reduce active contractions, reducing movement of aqueous humor through the uveoscleral path. However, the nature of uveoscleral outflow and its changes at night continue to be elucidated.
The circadian change in aqueous humor dynamics is important for glaucoma therapy if similar patterns exist in glaucoma patients. For example, aqueous humor flow decreases at night most likely because of the nocturnal lack of sympathetic tone. Beta-adrenergic antagonists cannot reduce aqueous humor flow rate further at night because there is not sufficient sympathetic stimulation to block.39,40 Unlike beta-antagonists, prostaglandin analogs reduce pressure at night, but not as well as they do during the day.41–44 Prostaglandin analogs, however, may reduce pressure through two actions, one that improves outflow facility (although this has not been a universal finding) and the other that increases uveoscleral flow.45–47 A study by Gulati et al.48 suggested that latanoprost might increase uveoscleral outflow at night, although their study was not sufficiently powered to show a statistically significant difference. If prostaglandin analogs increase uveoscleral flow by a percentage, rather than by a fixed amount, then the nocturnal effect on absolute uveoscleral flow rate would be small, because uveoscleral flow is markedly reduced at night. This small change in absolute uveoscleral flow rate would be more difficult to detect, consistent with the findings of Gulati et al.48 In contrast, outflow facility decreased by only approximately 15% at night in our subjects, which suggests that improving outflow facility may be a possible mechanism for reducing nocturnal IOP. It is possible that prostaglandin analogs, which have persistent nocturnal efficacy, may use this mechanism. Other medications that improve outflow facility, such as parasympathomimetics, may also be good options for lowering IOP for similar reasons, but their nocturnal efficacy needs to be investigated.
Uveoscleral outflow was calculated from the difference in trabecular flow, estimated from our measurements of outflow facility, IOP, and EVP, and the total aqueous humor flow calculated from fluorescein clearance, and this difference is sensitive to all of the variables measured. At night, the estimate of aqueous humor flow rate is accurate because it is based on clearance of fluorescein during the 2-hour interval that subjects slept, and aqueous humor flow is independent of body position.49 However, IOP, EVP, and outflow facility were measured during a short time and could be affected by transients shortly after subjects were awoken. For example, the process of waking can transiently elevate IOP with a rapid decay to baseline over several minutes. It is unclear if the nocturnal IOP that we measured was affected by this transient or was representative of true IOP during sleep. Subjects were awake for approximately 5 to 10 minutes during fluorophotometry before EVP measurements, and even longer before IOP and outflow facility measurements, sufficient time for transients to return to baseline in most subjects. However, the question of whether these measurements represent a sleeping, awake, or a transient state cannot be answered at this time. Any systematic difference between our estimate of IOP, EVP, or outflow facility, and the true value during sleep could systematically shift the estimate of flow through the trabecular meshwork and this would influence our estimate of uveoscleral flow. Ideally, we would measure uveoscleral outflow by a method independent of the other variables, although such a noninvasive method, suitable for use in human volunteers, is not available.
In summary, aqueous humor flow rate decreases by approximately 50% during the midnocturnal period as compared with the middiurnal period. In contrast, IOP remains remarkably similar from day to night after accounting for differences in body position. This stable pressure seems to be maintained by a small reduction in outflow facility accompanied by a large reduction in uveoscleral flow rate. Further research is required to determine if glaucoma patients experience similar changes.
Acknowledgments
Supported in part by the American Glaucoma Society Physician-Scientist Grant (AJS), BrightFocus Foundation (AJS), National Institutes of Health Grant UL1 TR000135—Center for Translational Science Activities, the Mayo Foundation for Medical Education and Research, and a Schaub Special Scholar award from Research to Prevent Blindness (AJS).
Disclosure: C.B. Nau, None; M. Malihi, None; J.W. McLaren, None; D.O. Hodge, None; A.J. Sit, None
References
- 1. Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998; 39: 2707–2712 [PubMed] [Google Scholar]
- 2. Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999; 40: 2912–2917 [PubMed] [Google Scholar]
- 3. Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in young adults with moderate to severe myopia. Invest Ophthalmol Vis Sci. 2002; 43: 2351–2355 [PubMed] [Google Scholar]
- 4. Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003; 44: 4439–4442 [DOI] [PubMed] [Google Scholar]
- 5. Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003; 44: 1586–1590 [DOI] [PubMed] [Google Scholar]
- 6. Reiss GR, Lee DA, Topper JE, Brubaker RF. Aqueous humor flow during sleep. Invest Ophthalmol Vis Sci. 1984; 25: 776–778 [PubMed] [Google Scholar]
- 7. Larsson LI, Rettig ES, Sheridan PT, Brubaker RF. Aqueous humor dynamics in low-tension glaucoma. Am J Ophthalmol. 1993; 116: 590–593 [DOI] [PubMed] [Google Scholar]
- 8. Larsson LI, Rettig ES, Brubaker RF. Aqueous flow in open-angle glaucoma. Arch Ophthalmol. 1995; 113: 283–286 [DOI] [PubMed] [Google Scholar]
- 9. Liu H, Fan S, Gulati V, et al. Aqueous humor dynamics during the day and night in healthy mature volunteers. Arch Ophthalmol. 2011; 129: 269–275 [DOI] [PubMed] [Google Scholar]
- 10. Sit AJ, Nau CB, McLaren JW, Johnson DH, Hodge D. Circadian variation of aqueous dynamics in young healthy adults. Invest Ophthalmol Vis Sci. 2008; 49: 1473–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blondeau P, Tetrault JP, Papamarkakis C. Diurnal variation of episcleral venous pressure in healthy patients: a pilot study. J Glaucoma. 2001; 10: 18–24 [DOI] [PubMed] [Google Scholar]
- 12. Fan S, Hejkal JJ, Gulati V, Galata S, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in volunteers with ocular hypertension. Arch Ophthalmol. 2011; 129: 1162–1166 [DOI] [PubMed] [Google Scholar]
- 13. Sit AJ, McLaren JW. Measurement of episcleral venous pressure. Exp Eye Res. 2011; 93: 291–298 [DOI] [PubMed] [Google Scholar]
- 14. Brubaker RF. Clinical evaluation of the circulation of aqueous humor. In: Duane TD. ed Clinical Ophthalmology. Philadelphia, PA: Harper and Row; 1986: 1–11 [Google Scholar]
- 15. Brubaker RF. The flow of aqueous humor in the human eye. Trans Am Ophthalmol Soc. 1982; 80: 391–474 [PMC free article] [PubMed] [Google Scholar]
- 16. Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci. 1991; 32: 3145–3166 [PubMed] [Google Scholar]
- 17. McLaren JW, Ziai N, Brubaker RF. A simple three-compartment model of anterior segment kinetics. Exp Eye Res. 1993; 56: 355–366 [DOI] [PubMed] [Google Scholar]
- 18. McLaren JW, Brubaker RF. Measurement of fluorescein and fluorescein monoglucuronide in the living human eye. Invest Ophthalmol Vis Sci. 1986; 27: 966–974 [PubMed] [Google Scholar]
- 19. McLaren JW, Brubaker RF. A two-dimensional scanning ocular fluorophotometer. Invest Ophthalmol Vis Sci. 1985; 26: 144–152 [PubMed] [Google Scholar]
- 20. Brubaker RF, McLaren JW. Uses of fluorophotometry in glaucoma research. Ophthalmology. 1985; 92: 884–890 [DOI] [PubMed] [Google Scholar]
- 21. Maus TL, McLaren JW, Brubaker RF. A comparison of two methods of measuring aqueous flow in humans: fluorophotometry and flare measurement. Curr Eye Res. 1993; 12: 621–628 [DOI] [PubMed] [Google Scholar]
- 22. Johnson SB, Coakes RL, Brubaker RF. A simple photogrammetric method of measuring anterior chamber volume. Am J Ophthalmol. 1978; 85: 469–474 [DOI] [PubMed] [Google Scholar]
- 23. McLaren JW, Herman DC, Brubaker RF, et al. Effect of ibopamine on aqueous humor production in normotensive humans. Invest Ophthalmol Vis Sci. 2003; 44: 4853–4858 [DOI] [PubMed] [Google Scholar]
- 24. Selvadurai D, Hodge D, Sit AJ. Aqueous humor outflow facility by tonography does not change with body position. Invest Ophthalmol Vis Sci. 2010; 51: 1453–1457 [DOI] [PubMed] [Google Scholar]
- 25. Grant WM. Tonographic method for measuring the facility and rate of aqueous flow in human eyes. Arch Ophthalmol. 1950; 44: 204–214 [DOI] [PubMed] [Google Scholar]
- 26. Sit AJ, Ekdawi NS, Malihi M, McLaren JW. A novel method for computerized measurement of episcleral venous pressure in humans. Exp Eye Res. 2011; 92: 537–544 [DOI] [PubMed] [Google Scholar]
- 27. Liu JH, Kripke DF, Hoffman RE, et al. Elevation of human intraocular pressure at night under moderate illumination. Invest Ophthalmol Vis Sci. 1999; 40: 2439–2442 [PubMed] [Google Scholar]
- 28. Teitelbaum CS, Podos SM, Lustgarten JS. Comparison of standard and computerized tonography instruments on human eyes. Am J Ophthalmol. 1985; 99: 403–410 [DOI] [PubMed] [Google Scholar]
- 29. Feghali JG, Azar DT, Kaufman PL. Comparative aqueous outflow facility measurements by pneumatonography and Schiotz tonography. Invest Ophthalmol Vis Sci. 1986; 27: 1776–1780 [PubMed] [Google Scholar]
- 30. Takeda Y, Azuma I. Diurnal variations in outflow facility. Ann Ophthalmol. 1978; 10: 1575–1580 [PubMed] [Google Scholar]
- 31. Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960; 64: 494–501 [DOI] [PubMed] [Google Scholar]
- 32. Kitazawa Y, Horie T. Diurnal variation of intraocular pressure in primary open-angle glaucoma. Am J Ophthalmol. 1975; 79: 557–566 [DOI] [PubMed] [Google Scholar]
- 33. Konstas AG, Mantziris DA, Cate EA, Stewart WC. Effect of timolol on the diurnal intraocular pressure in exfoliation and primary open-angle glaucoma. Arch Ophthalmol. 1997; 115: 975–979 [DOI] [PubMed] [Google Scholar]
- 34. Kaufman PL, Barany EH. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976; 15: 793–807 [PubMed] [Google Scholar]
- 35. Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001; 10: 253–264 [DOI] [PubMed] [Google Scholar]
- 36. Berggren L, Walinder PE. Tonus of the ciliary muscle during sleep. Acta Ophthalmol (Copenh). 1969; 47: 1149–1155 [DOI] [PubMed] [Google Scholar]
- 37. Crawford K, Kaufman PL. Pilocarpine antagonizes prostaglandin F2 alpha-induced ocular hypotension in monkeys. Evidence for enhancement of Uveoscleral outflow by prostaglandin F2 alpha. Arch Ophthalmol. 1987; 105: 1112–1116 [DOI] [PubMed] [Google Scholar]
- 38. Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990; 70: 987–1028 [DOI] [PubMed] [Google Scholar]
- 39. McCannel CA, Heinrich SR, Brubaker RF. Acetazolamide but not timolol lowers aqueous humor flow in sleeping humans. Graefes Arch Clin Exp Ophthalmol. 1992; 230: 518–520 [DOI] [PubMed] [Google Scholar]
- 40. Rettig ES, Larsson LI, Brubaker RF. The effect of topical timolol on epinephrine-stimulated aqueous humor flow in sleeping humans. Invest Ophthalmol Vis Sci. 1994; 35: 554–559 [PubMed] [Google Scholar]
- 41. Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol. 2004; 138: 389–395 [DOI] [PubMed] [Google Scholar]
- 42. Sit AJ, Asrani S. Effects of medications and surgery on intraocular pressure fluctuation. Surv Ophthalmol. 2008; 53 (suppl 1); S45–S55 [DOI] [PubMed] [Google Scholar]
- 43. Sit AJ, Weinreb RN, Crowston JG, Kripke DF, Liu JH. Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am J Ophthalmol. 2006; 141: 1131–1133 [DOI] [PubMed] [Google Scholar]
- 44. Tung JD, Tafreshi A, Weinreb RN, Slight JR, Medeiros FA, Liu JH. Twenty-four-hour effects of bimatoprost 0.01% monotherapy on intraocular pressure and ocular perfusion pressure. BMJ Open. 2012; 2 p iii: e001106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002; 47 (suppl 1); S53–S64 [DOI] [PubMed] [Google Scholar]
- 46. Bahler CK, Howell KG, Hann CR, Fautsch MP, Johnson DH. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008; 145: 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim KS, Nau CB, O'Byrne MM, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008; 115: 790–795 e794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gulati V, Fan S, Zhao M, Maslonka MA, Gangahar C, Toris CB. Diurnal and nocturnal variations in aqueous humor dynamics of patients with ocular hypertension undergoing medical therapy. Arch Ophthalmol. 2012; 130: 677–684 [DOI] [PubMed] [Google Scholar]
- 49. Carlson KH, McLaren JW, Topper JE, Brubaker RF. Effect of body position on intraocular pressure and aqueous flow. Invest Ophthalmol Vis Sci. 1987; 28: 1346–1352 [PubMed] [Google Scholar]