Abstract
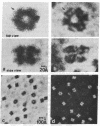
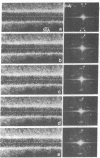
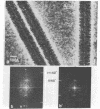
A scanning transmission electron microscope (STEM) equipped with a field emission gun has been employed for the examination of biological macromolecules at high resolution. The quality of micrographs obtained with the STEM is dependent upon the quality of the substrate used to support biological objects because the image contrast in dark field is proportional to the mass density of the specimen. In order to reduce deleterious effects of the substrates on the image quality, we have developed a method of fabricating substrates consisting of very thin, very clean carbon films supported on very clean fenestrated plastic films. These films are approximately 15 Å thick. Well-known biological macromolecules such as glutamine synthetase and tobacco mosaic virus (both stained) and low-density lipoprotein and ferritin (both unstained were placed on these substrates and examined with the STEM by using various modes of contrast. The micrographs obtained by using the dark field mode of contrast employing an annular detector were free from phase contrast, as expected. Using this contrast mode, we have been able to directly observe (in-focus) 2.5- to 4.4-Å lattice spacings in the ferritin core. The effect of electron radiation damage on the helical structure of tobacco mosaic virus was also examined. Micrographs as well as corresponding optical diffraction patterns obtained with moderately low doses showed very clear helical structure from both sides of the virus. In addition, the (11.5 Å)-1 layer lines indicated the effective resolution attained on these particles.
Keywords: phase contrast, low-dose electron microscopy, unstained biological macromolecules, dark field contrast, thin carbon films
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engel A., Dubochet J., Kellenberger E. Some progress in the use of a scanning transmission electron microscope for the observation of biomacromolecules. J Ultrastruct Res. 1976 Dec;57(3):322–330. doi: 10.1016/s0022-5320(76)80120-7. [DOI] [PubMed] [Google Scholar]
- Fukami A., Adachi K. A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microsc (Tokyo) 1965;14(2):112–118. [PubMed] [Google Scholar]
- Klug A., De Rosier D. J. Optical filtering of electron micrographs: reconstruction of one-sided images. Nature. 1966 Oct 1;212(5057):29–32. doi: 10.1038/212029a0. [DOI] [PubMed] [Google Scholar]
- Massover W. H., Cowley J. M. The ultrastructure of ferritin macromolecules. The lattice structure of the core crystallites. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3847–3851. doi: 10.1073/pnas.70.12.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottensmeyer F. P., Bazett-Jones D. P., Rust H. P., Weiss K., Zemlin F., Engel A. Radiation exposure and recognition of electron microscopic images of protamine at high resolution. Ultramicroscopy. 1978;3(2):191–202. doi: 10.1016/s0304-3991(78)80026-6. [DOI] [PubMed] [Google Scholar]
- Unwin P. N. Electron microscopy of the stacked disk aggregate of tobacco mosaic virus protein. II. The influence of electron irradiation of the stain distribution. J Mol Biol. 1974 Aug 25;87(4):657–670. doi: 10.1016/0022-2836(74)90076-x. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wall J., Langmore J., Isaacson M., Crewe A. V. Scanning transmission electron microscopy at high resolution. Proc Natl Acad Sci U S A. 1974 Jan;71(1):1–5. doi: 10.1073/pnas.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]