Abstract
Background
Most hemodialysis patients worldwide are treated with bicarbonate dialysis using sodium bicarbonate as the base. Few studies have assessed outcomes of patients treated with different dialysate bicarbonate levels, and the optimal concentration remains uncertain.
Study Design
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is an international prospective cohort study.
Setting & Participants
This study included 17,031 patients receiving thrice-weekly in-center hemodialysis from 11 DOPPS countries (2002–2011).
Predictor
Dialysate bicarbonate.
Outcomes
All-cause and cause-specific mortality and first hospitalization, using Cox regression to estimate the effects of dialysate bicarbonate concentration, adjusting for potential confounders.
Measurements
Demographics, comorbid conditions, laboratory values, and prescriptions were abstracted from medical records.
Results
Mean dialysate bicarbonate was 35.5 ± 2.7 (SD) mEq/L, ranging from 32.2 ± 2.3 mEq/L in Germany to 37.0 ± 2.6 mEq/L in the US. Prescription of high dialysate bicarbonate (≥38 mEq/L) was most common in the US (45% of patients). Approximately 50% of DOPPS facilities used a single dialysate bicarbonate concentration. 3,913 patients (23%) died during follow-up. Dialysate bicarbonate concentration was positively associated with mortality (adjusted HR, 1.08 per 4 mEq/L higher [95% CI, 1.01–1.15]; HR for dialysate bicarbonate ≥38 vs. 33–37 mEq/L, 1.07 [95% CI, 0.97–1.19]). Results were consistent across levels of pre-dialysis session serum bicarbonate and between facilities that used a single dialysate bicarbonate concentration and those that prescribed different concentrations to individual patients. The association of dialysis bicarbonate with mortality was stronger in patients with longer dialysis vintage.
Limitations
Due to the observational nature of the current study, we cannot rule out that the reported associations may be biased by unmeasured confounders.
Conclusions
High dialysate bicarbonate, especially prolonged exposure, may contribute to adverse outcomes, likely through development of post-dialysis metabolic alkalosis. Additional studies are warranted to identify the optimal dialysate bicarbonate concentration.
Keywords: hemodialysis, outcomes, statistics, DOPPS, observational
The intended function of dialysate fluid is to correct the composition of uremic blood to physiological levels, both by reducing the concentration of uremic toxins and by correcting electrolyte and acid-base abnormalities. Metabolic acidosis is common in patients receiving maintenance hemodialysis (HD) and plays an important role in the development of bone and protein-energy wasting via increased protein degradation1–4. The advent of bicarbonate dialysis in the 1980s was one of the most important technical advances in HD5; currently, the vast majority of HD patients are treated with bicarbonate rather than acetate dialysis. In bicarbonate dialysis, proportioning pumps in the dialysis machine mix purified water with separate “acid” and bicarbonate concentrates. The “acid” concentrate contains electrolytes, glucose, and 2–8 mEq/L of acetate (which is metabolized into bicarbonate in the liver) to prevent calcium precipitation. The optimal dialysate bicarbonate concentration is one that prevents acidosis at the beginning of the next HD session while avoiding post-dialysis alkalosis. Few studies have assessed outcomes of patients treated with different dialysate bicarbonate levels. Higher dialysate bicarbonate has been associated with correction of acidosis6 and improvement of nutritional markers7–9, but also with hemodynamic instability10–11, and prolongation of the corrected (QTc) interval on ECGs 12.
No data on hospitalization and mortality have been published and a recent report concluded that there were insufficient data for a meta-analysis13. We postulated that high dialysate bicarbonate may contribute to rapid electrolyte shifts during the HD session and to the development of post-dialytic alkalosis, and thus contribute to adverse clinical outcomes. In this study, we describe the variation in dialysate bicarbonate prescription and assess the associations of dialysate bicarbonate with mortality and hospitalizations in the international Dialysis Outcomes and Practice Patterns Study (DOPPS).
METHODS
The DOPPS is an international prospective cohort study of in-center HD patients ≥ 18 years of age. Patients were randomly selected from a representative sample of dialysis facilities within each country 14–15. In this analysis, data from participants in DOPPS phase 2 (2002–2004), phase 3 (2005–2008), and phase 4 (2009–2011) in Australia, Belgium, Canada, France, Germany, Italy, New Zealand, Spain, Sweden, the United Kingdom, and the United States were used. Demographics, comorbid conditions, laboratory values, and prescriptions were abstracted from medical records. Mortality and hospitalization events and their primary causes were collected during study follow-up. All variables were collected using uniform and standardized data collection tools for all DOPPS participants in all countries.
Patients dialyzing three times per week and having dialysate and serum bicarbonate values within plausible limits (20–45 mEq/L for dialysate and 10–36 mEq/L for serum) were eligible for this analysis. Patients with acetate as the predominant dialysate base/buffer (<1%) were excluded. Facilities with fewer than five eligible patients and facilities that measured serum bicarbonate in < 60% of patients were also excluded. The large dialysis organization facilities present in DOPPS phase 4 in the United States were excluded due to some incomplete data, such as dialysate bicarbonate.
Dialysate birbonate was treated both continuously and as a categorical variable with three groups: ≤32 mEq/L (low); 33–37 mEq/L (medium); and ≥38 mEq/L (high). Since the majority of patients (80%) did not change dialysate bicarbonate groups during follow-up, we used dialysate bicarbonate reported at study entry as the exposure variable. Non-individualized dialysate bicarbonate facilities were defined as those that prescribed a uniform dialysate bicarbonate level to 90% or more of their patients. The remaining facilities were defined as individualized.
Cox regression was used to estimate the association between dialysate bicarbonate and all-cause mortality, stratified by country and phase, and accounting for facility clustering using robust sandwich covariance estimators. Covariate adjustment was made for the following variables at baseline: demographics and comorbid conditions listed in Table 1, residual kidney function (RKF), catheter use, blood flow rate, treatment time, laboratory values (single-pool Kt/V, serum albumin, creatinine, hemoglobin, ferritin, white blood cell count, calcium, and phosphorus), dialysate electrolytes (potassium and calcium), and facility characteristics (percent catheter use and percent Kt/V < 1.2). Time at risk started at study enrollment and ended at the time of death, seven days after leaving the facility due to transfer or change in renal replacement therapy modality, loss to follow-up, transplantation, end of study phase, or the most recent date of data availability (whichever event occurred first). Proportional hazards for dialysate bicarbonate were confirmed by examination of log-log and Kaplan-Meier survival plots, and by testing the interaction between log-time and dialysate bicarbonate treated as a continuous variable (p=0.9).
Table 1.
Baseline patient characteristics by category of dialysate bicarbonate
| characteristic | All patients | Dialysate Bicarbonate | ||
|---|---|---|---|---|
| ≤ 32 mEq/L | 33–37 mEq/L | ≥38 mEq/L | ||
| No. of patients | 17,031 | 2,351 | 10,851 | 3,829 |
| Age (y) | 63.5 ± 15.0 | 65.2 ± 14.6 | 63.8 ± 14.9 | 61.7 ± 15.3 |
| Male sex | 57.8 | 58.0 | 57.7 | 57.8 |
| Dialysis vintage (y) | 3.6 ± 5.0 | 3.8 ± 5.3 | 3.6 ± 5.1 | 3.5 ± 4.6 |
| Body mass index (kg/m2) | 26.4 ± 6.1 | 26.0 ± 5.7 | 26.3 ± 6.0 | 27.0 ± 6.7 |
| Comorbid conditions | ||||
| Coronary artery disease | 48.0 | 45.6 | 47.0 | 52.2 |
| Cerebrovascular disease | 17.7 | 19.7 | 17.6 | 16.8 |
| Other cardiovascular disease | 36.1 | 38.7 | 37.0 | 31.8 |
| Peripheral vascular disease | 31.5 | 33.9 | 31.5 | 30.1 |
| Congestive heart failure | 35.1 | 36.8 | 34.1 | 36.9 |
| Hypertension | 85.5 | 88.3 | 84.4 | 87.1 |
| Diabetes | 41.4 | 38.2 | 40.0 | 47.6 |
| COPD | 15.4 | 16.0 | 15.2 | 15.6 |
| Gastrointestinal bleeding | 5.7 | 4.5 | 5.9 | 5.9 |
| Neurologic disease | 12.4 | 13.5 | 12.0 | 13.0 |
| Psychiatric disorder | 20.6 | 18.0 | 20.0 | 23.9 |
| Cancer (nonskin) | 14.7 | 15.7 | 14.5 | 14.5 |
| Recurrent cellulitis | 10.4 | 11.0 | 10.4 | 9.8 |
| Serum Bicarbonate (mEq/L) | 22.9 ± 3.5 | 22.2 ± 3.3 | 23.0 ± 3.4 | 23.1 ± 3.9 |
| Albumin (g/dL) | 3.67 ± 0.51 | 3.71 ± 0.56 | 3.66 ± 0.51 | 3.67 ± 0.50 |
| Hemoglobin (g/dL) | 11.4 ± 1.5 | 11.4 ± 1.5 | 11.4 ± 1.5 | 11.5 ± 1.5 |
| Calcium (mg/dL) | 9.1 ± 0.8 | 9.0 ± 0.8 | 9.1 ± 0.8 | 9.1 ± 0.9 |
| Predialysis SBP (mm Hg) | 143 ± 25 | 140 ± 25 | 143 ± 25 | 146 ± 26 |
| Postdialysis SBP (mm Hg) | 135 ± 25 | 133 ± 24 | 135 ± 25 | 136 ± 25 |
| Catheter use | 30.9 | 26.7 | 31.1 | 32.8 |
| Residual kidney function | 38.5 | 50.9 | 37.6 | 33.3 |
| Single-pool Kt/V | 1.48 ± 0.33 | 1.45 ± 0.35 | 1.49 ± 0.33 | 1.50 ± 0.31 |
| Treatment time (min) | 232 ± 39 | 243 ± 40 | 233 ± 39 | 224 ± 37 |
| Blood Flow Rate (mL/min) | 345 ± 75 | 308 ± 60 | 342 ± 74 | 379 ± 73 |
| Dialysate calcium (mEq/L) | 2.8 ± 0.4 | 2.9 ± 0.3 | 2.8 ± 0.4 | 2.6 ± 0.3 |
| Dialysate sodium (mEq/L)* | 139.6 ± 1.8 | 139.2 ± 1.7 | 139.6 ± 1.7 | 139.9 ± 2.1 |
| Dialysate potassium (mEq/L) | 2.1 ± 0.7 | 2.3 ± 0.7 | 2.1 ± 0.7 | 2.1 ± 0.6 |
| Sodium modeling | 18.2 | 8.3 | 16.1 | 30.5 |
| Oral sodium bicarbonate prescription | 9.3 | 10.9 | 9.8 | 6.7 |
Note: Unless otherwise indicated, values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation. Conversion factors for calcium in mg/dL to mmol/L, x0.2495.
COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure.
Excluding sodium modeling
Because serum bicarbonate and other indicators of patient health status may affect dialysate bicarbonate prescription, we also estimated the dialysate bicarbonate effect on all-cause mortality separately among patients treated at individualized and non-individualized facilities, i.e., dialysis facilities where the same dialysate bicarbonate is prescribed for most or all patients, hence minimizing treatment by indication bias.
The crude number of deaths, by primary cause of death, was enumerated by category of dialysate bicarbonate. Adjusted Cox models were used to analyze cause-specific mortality for (1) sudden death, (2) cardiovascular causes (including sudden death), and (3) infections. Cox models were also used to model time to first hospitalization for total inpatient admissions and cause-specific admissions for (1) arrhythmias, (2) cardiovascular causes (including arrhythmia), and (3) infections.
To investigate the possibility of pre-dialysis serum bicarbonate as an intermediate or confounder in the relationship of dialysate bicarbonate with mortality, standard statistical methods may not be sufficient because of the cross-sectional nature of these data. Instead, possible bidirectional causality between dialysate bicarbonate and serum bicarbonate was first assessed, followed by the relationship between serum bicarbonate and mortality. First, the effect of dialysate bicarbonate on serum bicarbonate was estimated separately among non-individualized and individualized facilities. Similar to the Cox models, the non-individualized facilities were used to estimate an effect of dialysate bicarbonate that is less vulnerable to bias. A test for interaction of dialysate bicarbonate with facility individualization assessed the degree of confounding by indication (i.e., the effect of serum bicarbonate on dialysate bicarbonate prescription). The relationship of serum bicarbonate with mortality was then assessed in individualized and non-individualized facilities and additionally adjusting for dialysate bicarbonate.
Interactions were assessed between dialysate bicarbonate and the following variables (considered to plausibly mediate the association of dialysate bicarbonate with mortality): dialysate potassium, dialysate calcium, dialysate sodium, use of sodium modeling, hemoglobin, serum calcium (pre- and post-dialysis), serum potassium (pre- and post-dialysis), systolic blood pressure ( pre- and post-dialysis), treatment time, RKF, dialysis vintage, oral sodium bicarbonate, calcium-acetate based phosphate binder use, and COPD.
Intradialytic hypotension was defined at baseline as a predialysis to postdialysis drop in systolic blood pressure of ≥ 30 mmHg and a post-dialysis systolic pressure of ≤ 100 mmHg16. The generalized estimating equation method with logit link function was used to examine the association of dialysate bicarbonate with intradialytic hypotension, accounting for clustering at the facility level17. An adjusted linear mixed model with intradialytic weight loss (used as a surrogate for intradialytic weight gain) as the outcome was used to examine the association of dialysate bicarbonate with intradialytic weight gain. Adjusted linear mixed models with serum bicarbonate as the outcome were used to examine the association of oral sodium bicarbonate medications, calcium acetate-based phosphate binders, and sevelamer with serum bicarbonate.
Japan was not included in the analysis because there was minimal overlap of dialysate bicarbonate distribution with worldwide data: 83% of dialysate bicarbonate values were ≤30 mEq/L, compared to only 3% in other DOPPS countries.
Missing covariate values were multiply imputed using the chained equation method18 by IVEware19. Missing values were sequentially updated using the bootstrap or Markov Chain Monte Carlo method, based on multiple regression models with other variables as covariates. This procedure was carried out for 10 cycles, thereby constructing an imputed data set. Results from five such imputed data sets were combined for the final analysis using Rubin’s formula20. The proportion of missing data was below 10% for all imputed covariates, with the exception of Kt/V (21%) and ferritin (14%). All analyses used SAS software, version 9.2 (SAS Institute Inc, Cary, NC). The authors have consulted the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement guidelines21 for reporting observational studies.
RESULTS
Dialysate and Serum Bicarbonate Distributions
This analysis included 17,031 in-center HD patients from 11 countries in DOPPS phases 2–4 (2002–2011). Median follow-up time was 16.4 (interquartile range, 8.3–27.2) months, during which 3,913 (23%) patients died (mortality rate, 0.16 per patient-year).
Mean ± standard deviation dialysate bicarbonate was 35.5 ± 2.7 (standard deviation) mEq/L, and ranged from 32.2 ± 2.3 mEq/L in Germany to 37.0 ± 2.6 mEq/L in the United States (Figure 1). Over the 10-year study period, no trends in dialysate bicarbonate were observed. Mean serum bicarbonate measured pre-dialysis session was 22.9 ± 3.5 mEq/L, and ranged from 21.4 ± 3.3 mEq/L in Italy to 24.4 ± 3.4 mEq/L in Canada.
Figure 1. Distribution of dialysate bicarbonate prescription, by country.
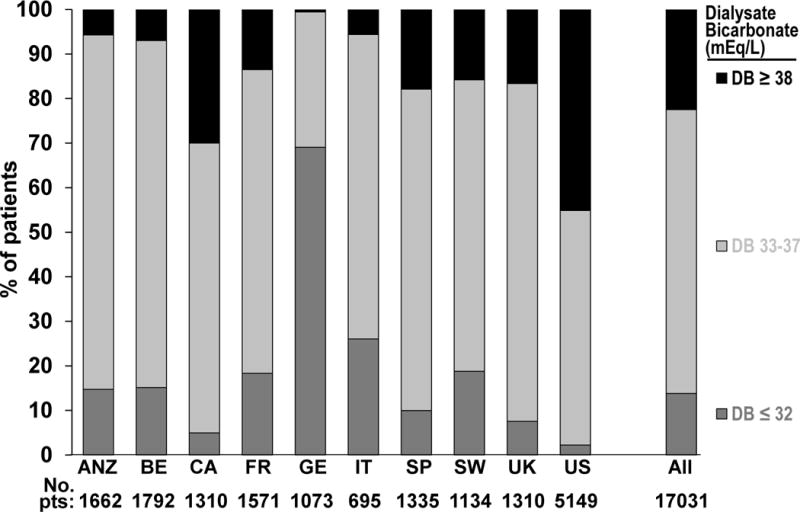
ANZ=Australia and New Zealand; BE= Belgium; CA= Canada; FR= France; GE= Germany; IT: Italy; SP= Spain; SW= Sweden; UK= United Kingdom; US= United States.
Of the 554 dialysis facilities included in these analyses, 266 (48%) were classified as “non-individualized” facilities (i.e., the same dialysate bicarbonate concentration prescribed for most or all patients). The proportion of non-individualized facilities ranged from 9% in Germany to 76% in the UK and was 54% in the United States. Mean dialysate bicarbonate was 35.7 ± 2.2 mEq/L in non-individualized facilities and 35.3 ± 3.1 mEq/L in individualized facilities. Among the 288 individualized facilities, 27% used two different dialysate bicarbonates, 23% used three, 20% used four, and 29% used five or more different dialysate bicarbonate prescriptions.
Demographic and clinical characteristics of the study cohort across the three dialysate bicarbonate categories are shown in Table 1. Patients with higher dialysate bicarbonate were older and had less residual kidney function. Many other crude group differences were driven by country effects.
Dialysate Bicarbonate and Clinical Outcomes
Dialysate bicarbonate was associated with all-cause mortality and extensive adjustment for patient health status did not markedly affect this association (Figure 2). Results were consistent in models not adjusted (hazard ratio [HR], 1.08 per 4 mEq/L higher dialysate bicarbonate; 95% confidence interval [CI], 1.01–1.15) (Model 5) and adjusted (Model 6) for serum bicarbonate. A sensitivity analysis adjusting for dialysate sodium (excluding Na modeling patients) yielded consistent results (HR, 1.09). Results in the United States (HR, 1.08) were consistent with the other 10 countries in the main analysis (HR, 1.08).
Figure 2. Association between prescribed dialysate bicarbonate (per 4 mEq/L higher) and all-cause mortality, by levels of adjustment.
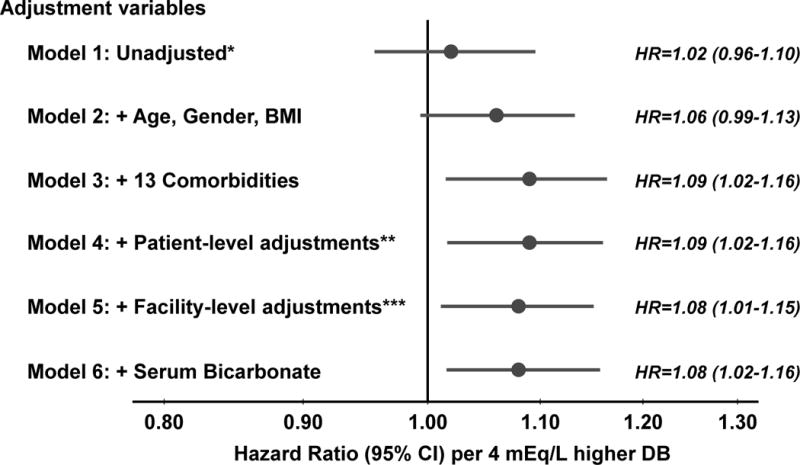
*Unadjusted Cox model stratified by DOPPS phase and country and accounted for facility clustering; **Patient-level adjustments: vintage, residual kidney function, catheter use, single pool Kt/V, prescribed blood flow rate, treatment time, serum albumin, ferritin, creatinine, calcium, phosphorus, white blood cell count, hemoglobin, dialysate Ca, and dialysate K; ***Facility-level adjustments: % catheter use and % Kt/V < 1.2; DB=Dialysate Bicarbonate, BMI=Body Mass Index.
The association between higher dialysate bicarbonate (per 4 mEq/L higher) and mortality was very similar in non-individualized facilities (HR, 1.08; 95% CI, 0.96–1.22) and in individualized facilities (HR, 1.09; 95% CI, 1.01–1.17). Additional adjustment for serum bicarbonate did not affect the results within either facility subgroup. Only weak interaction effects (p>0.05) were found between dialysate bicarbonate and all potential effect modifiers tested, with the exception of dialysis vintage (p=0.005). The HRs for all-cause mortality were 0.86 (95% CI, 0.73–1.00) for vintage of < 90 days, 1.03 (95% CI, 0.89–1.19) for 90 days to 1 year, 1.09 (95% CI, 0.97–1.22) for 1–3 years, and 1.13 (95% CI, 1.03–1.25) for ≥ 3 years.
The crude number of deaths, by primary cause of death, was enumerated by category of dialysate bicarbonate (Table S1). In adjusted analyses, the association between dialysate bicarbonate and both cardiovascular mortality and sudden death were in alignment with the all-cause mortality model (Figure 3). Surprisingly, the strongest association was for infection-related mortality (HR per 4 mEq/L higher bicarbonate concentration, 1.39 [95% CI, 1.15–1.67]). This association was similar in the United States compared with the other 10 countries and in patients using a catheter (vs. graft or fistula). About 90% of the 502 deaths from infections were attributed to septicemia or pneumonia. Dialysate bicarbonate was associated with all-cause (HR per 4 mEq/L higher bicarbonate concentration, 1.08; 95% CI, 1.03–1.14) and cardiovascular hospitalizations (HR per 4 mEq/L, 1.09; 95% CI, 1.01–1.19); hospitalization due to arrhythmias and infections showed a weaker association with dialysate bicarbonate.
Figure 3. Association between prescribed dialysate bicarbonate (per 4 mEq/L higher) and various clinical outcomes.
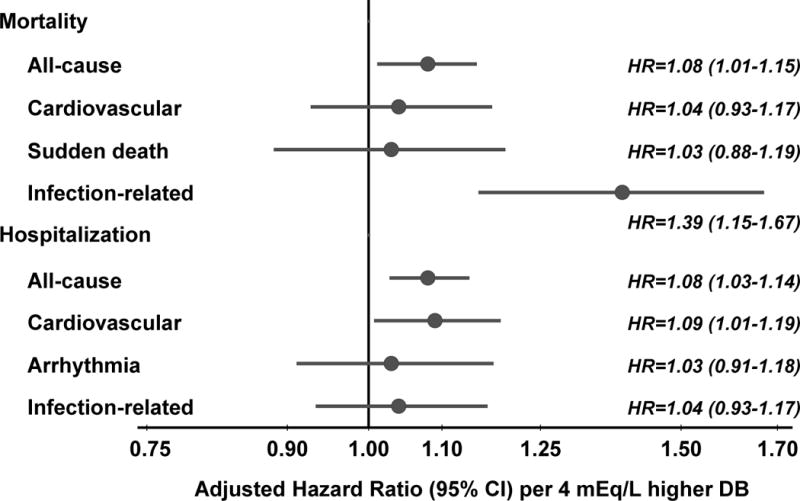
Cox models adjusted for age, sex, body mass index, 13 summary comorbid conditions, vintage, residual kidney function, catheter use, single pool Kt/V, prescribed blood flow rate, treatment time, serum albumin, ferritin, creatinine, calcium, phosphorus, white blood cell count, hemoglobin, dialysate Ca, dialysate K, facility % catheter use, and facility % Kt/V <1.2, stratified by DOPPS phase and country, and accounted for facility clustering effects. DB=Dialysate Bicarbonate.
Categorizing dialysate bicarbonate into three groups yielded consistent results (Figure 4). Compared to patients with dialysate bicarbonate 33–37 mEq/L, the HRs of all-cause mortality were 1.07 (95% CI, 0.97–1.19) for patients with high values (≥38 mEq/L) and 0.90 (95% CI, 0.80–1.01) for patients with low values (≤32 mEq/L). A linear pattern of association between dialysate bicarbonate and mortality was suggested that was consistent within each category of serum bicarbonate.
Figure 4. Association between 3 categories of prescribed dialysate bicarbonate and all-cause mortality, overall and by serum bicarbonate.
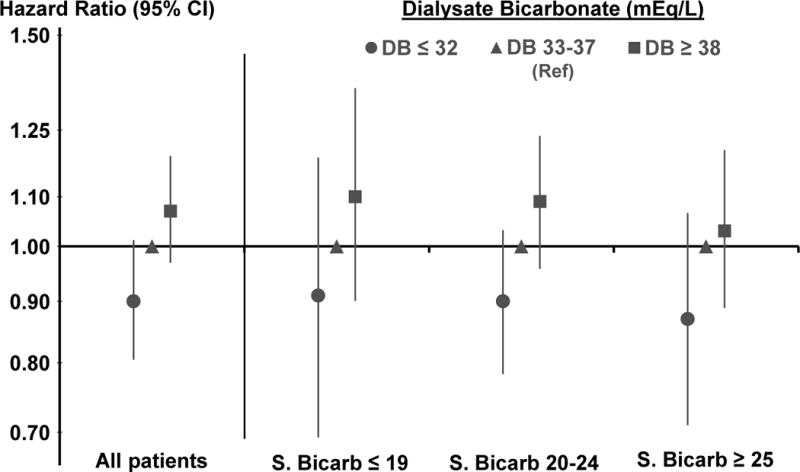
Two separate Cox models adjusted for age, sex, body mass index, 13 summary comorbid conditions, vintage, residual kidney function, catheter use, single pool Kt/V, prescribed blood flow rate, treatment time, serum albumin, ferritin, creatinine, calcium, phosphorus, white blood cell count, hemoglobin, dialysate Ca, dialysate K, facility % catheter use, and facility % Kt/V <1.2, stratified by DOPPS phase and country, and accounted for facility clustering effects; S.Bic=Serum Bicarbonate.
There was a positive association between dialysate bicarbonate and the incidence of intradialytic hypotension (HR per 4 mEq/L higher bicarbonae concentration, 1.12 [95% CI, 0.96–1.32). However, only 3% of the patients fit the criteria for intradialytic hypotension, limiting statistical power. Dialysate bicarbonate was also associated with interdialytic weight gain (0.09 [95% CI, 0.05–0.13] kg increase per 4 mEq/L higher bicarbonate concentration).
Predialysis Serum Bicarbonate and Mortality
Serum bicarbonate measured before the HD session was not strongly correlated with dialysate bicarbonate levels (Spearman correlation, 0.09). In an adjusted linear mixed model, the association between dialysate and pre-dialysis serum bicarbonate was slightly stronger at “non-individualized” (0.4 [95% CI, 0.3–0.6] mEq/L higher serum bicarbonate for 4 mEq/L higher dialysate bicarbonate) than “individualized” facilities (0.2 [95% CI, 0.1–0.3] mEq/L), but the interaction effect between dialysate bicarbonate and facility individualization provided little evidence of treatment-by-indication (p=0.7). The association between serum bicarbonate and mortality in individualized and non-individualized facilities is shown in Figure 5. The HR (per 4 mEq/L higher serum bicarbonate) for all-cause mortality was 0.94 (95% CI, 0.90–0.98) in all patients and was similar in non-individualized and individualized facilities, with and without adjustment for dialysate bicarbonate.
Figure 5. Association between categories of serum bicarbonate and all-cause mortality, in non-individualized and individualized facilities.
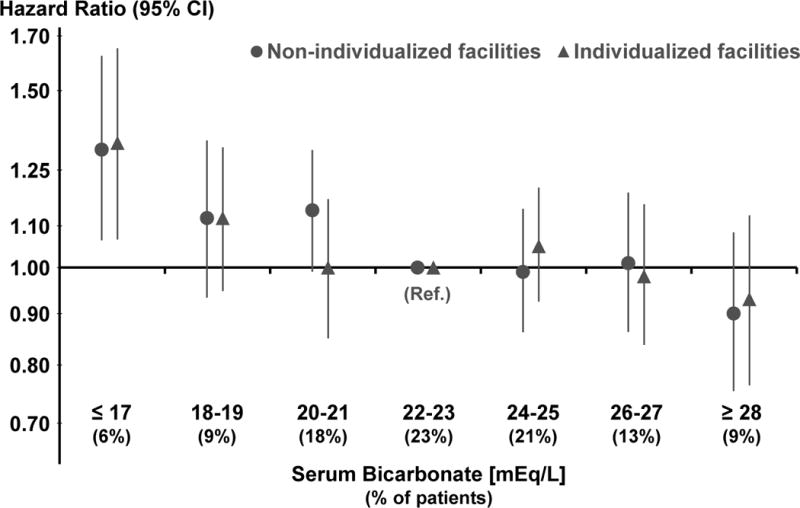
Two separate Cox models adjusted for age, sex, body mass index, 13 summary comorbid conditions, vintage, residual kidney function, catheter use, single pool Kt/V, prescribed blood flow rate, treatment time, serum albumin, ferritin, creatinine, calcium, phosphorus, white blood cell count, hemoglobin, dialysate bicarbonate, facility % catheter use, and facility % Kt/V <1.2, stratified by DOPPS phase and country, and accounted for facility clustering effects. Distribution of serum bicarbonate categories is shown overall; distributions differ minimally between individualized and non-individualized facilities.
Oral Medications That Affect Acid-Base Status
Study participants on oral sodium bicarbonate (9%) had 0.3 mEq/L lower (range, −0.5 to −0.1) serum bicarbonate level, likely reflecting the indication for the prescription. Sodium bicarbonate therapy was not associated with all-cause mortality (HR, 1.00; 95% CI, 0.87–1.15). Serum bicarbonate was higher in patients prescribed calcium acetate as a phosphate binder (0.2 [95% CI, 0.0 to 0.3] mEq/L) and lower in those on sevelamer (−0.7 [95% CI, −0.8 to −0.5] mEq/L).
DISCUSSION
The detailed DOPPS data set allowed us to provide the first comprehensive report on dialysate bicarbonate prescription and its association with clinical outcomes. The variability in dialysate bicarbonate across DOPPS countries was likely related to differences in other clinical practices (e.g., oral bicarbonate prescription) and dialysis equipment (e.g. centralized bicarbonate delivery system). Patient characteristics did not differ across dialysate bicarbonate categories, indicating that dialysate bicarbonate was prescribed independently of patient health status and largely based on common practice at a given facility.
Our study is the first to report higher mortality in patients treated with higher dialysate bicarbonate. The 2000 NKF-KDOQI (National Kidney Foundation–Kidney Disease Outcomes Quality Initiative) guidelines for nutrition in chronic kidney failure state, “Higher concentrations of bicarbonate in hemodialysis (> 38 mmol/L) have been shown to safely increase pre-dialysis serum bicarbonate concentrations”3. Our results question the safety of such high levels of dialysate bicarbonate. In fact, the NKF-KDOQI statement was based on earlier studies that did not test the safety of high dialysate bicarbonate, but rather assessed the impact of correction of metabolic acidosis on nutritional outcomes in dialysis22–27 patients.
Higher dialysate bicarbonate (ranging from 35 to 42 mEq/L) has been associated with correction of acidosis6, improvement of protein turnover 7, triceps fold thickness8, and serum branched-chain amino acids levels9 as well as with hemodynamic instability10–11, and prolongation of the QTc interval in ECGs 12.
It is reasonable to postulate that higher mortality in patients treated with higher dialysate bicarbonate may be mediated by development of metabolic alkalosis during and/or post HD sessions. Given that post-dialysis serum bicarbonate levels approach the concentration in the dialysate 28, patients treated with higher dialysate bicarbonate are more likely to develop post-dialysis alkalosis, independent of respiratory function 29 and acid-base status, and presumably are alkalotic for some time after the end of an HD session. High prevalence (54%) of metabolic alkalosis (serum bicarbonate, >30 mEq/L) was demonstrated among patients treated with a dialysate with high alkali concentration (35 mEq/L bicarbonate solution + 8 mEq/L of acetate in the acid concentrate) at hospital admissionl30. Unfortunately, post-dialysis serum bicarbonate levels are not available in the DOPPS data set, precluding us form further testing this hypothesis. Interestingly, the association of high dialysate bicarbonate with mortality was stronger for patients with longer vintage, suggesting that cumulative exposure over time to high levels of dialysate bicarbonate may be harmful.
Several mechanisms secondary to metabolic alkalosis induced by high dialysate bicarbonate may contribute to adverse outcomes. During HD, a more rapid change in serum bicarbonate drives potassium from the extra- to the intra-cellular space, leading to a faster decrease in serum potassium, which in turn can result in the development of cardiac arrhythmias31. Dialysate bicarbonate and post-dialysis plasma bicarbonate levels have been associated with prolongation of the QTc interval on post-dialysis ECGs 12, which was likely mediated by the rapid drop in serum potassium and ionized calcium. However, we did not find any interaction between dialysate bicarbonate and serum and dialysate potassium and calcium. Intradialytic metabolic alkalosis also results in increased neuromuscular excitability, reduced cerebral blood flow, and respiratory suppression32–33. Finally, alkalosis-mediated vasodilatation may contribute to hemodynamic instability11, 34, 35, which is consistent with results from our study, indicating a weak association between dialysate bicarbonate and intradialytic hypotension (HR=1.12). High dialysate bicarbonate was also associated with greater interdialytic weight gain, which could be due either to exposure to higher sodium levels during HD or to fluid administration in patients who developed intradialytic hypotension. Post-dialysis alkalosis may result in precipitation of calcium phosphate in soft tissues, including vessel walls25, 36, 37 especially in the presence of high serum calcium levels (e.g., due to high dialysate calcium or treatment with 1–25 vitamin D) and may contribute to the pathogenesis of cardiovascular disease. Our finding of an association of cardiovascular hospitalization with high dialysate bicarbonate is supportive of such mechanisms, while cardiovascular mortality was not confirmatory.
One interesting finding was the strong association between high dialysate bicarbonate and mortality due to infections (primarily sepsis and pneumonia) both in patients with a permanent vascular access and with a catheter. Extracellular acidosis has been reported to improve dendritic cells antigen-presenting capacity38. One could speculate that this mechanism may be impaired in the setting of metabolic alkalosis, hence providing a potential mechanism contributing to the association between high dialysate bicarbonate and infections. However, additional studies are needed to better understand this relationship.
While tailoring of dialysate bicarbonate to a specific patient acid-base status has been proposed as beneficial 39, we found no survival advantage for patients in individualized versus non-individualized facilities (adjusted HR, 0.98; 95% CI, 0.89–1.07) and no interaction between dialysate bicarbonate and pre-dialysis serum bicarbonate.
Our results indicate a weak association between dialysate bicarbonate and pre-dialysis serum bicarbonate levels. While dialysate bicarbonate clearly has a major impact on post-dialysis serum bicarbonate, its contribution to pre-dialysis serum bicarbonate levels may be diluted by other factors, such as protein intake, physical activity, respiratory function, and residual kidney function.
In agreement with prior reports 40, 41, 42, low pre-dialysis serum bicarbonate was strongly associated with mortality. Severe pre-dialysis acidosis may contribute to protein-energy wasting1, 2 and greater intradialytic electrolyte shifts, and thus lead to higher mortality. In the present analysis, serum bicarbonate levels above 23 mEq/L were not associated with increased mortality. This contrasts with a previous analysis of participants in the first DOPPS phase 40 and in a US cohort of HD patients 41. It is possible that the discrepancy with these prior analyses based on data from 10–20 years ago may be in part due to changes in practices over time, as well as differences in analytic methods. Our findings are in agreement with results from a more recent analysis that reported no association between serum bicarbonate > 22 mEq/L and mortality after adjustment for nutritional and inflammatory markers 42.
Due to the observational nature of the current study, we cannot rule out that the reported associations may be biased by unmeasured confounders. To address this, we assessed the association of dialysate bicarbonate with clinical outcomes in “non-individualized” facilities. Dialysate bicarbonate prescription in non-individualized facilities is independent of patient characteristics that may impact mortality risk, both measured and unmeasured. Hence, the likelihood of confounding by indication bias is non-existent, though the possibility of facility-level confounders still remains. Because the association between dialysate bicarbonate and mortality was very consistent in non-individualized and individualized facilities, dialysate bicarbonate prescription is likely not influenced by patient health status. We also acknowledge that we studied the prescribed concentration of sodium bicarbonate, and not the total alkali concentration in the dialysate. Since most dialysate baths also contains some acetate, our “dialysate bicarbonate” levels likely underestimated the actual alkali exposure. This reflects current clinical practice, since providers typically prescribe a certain dialysate bicarbonate concentration, while mostly ignoring the acetate present in the acid bath. However, after it has been metabolized, the acetate contributes 2–8 additional mEq/L of bicarbonate and may abet the development of post-dialysis alkalosis. This issue was recently the object of a safety communication issued by the US Food and Drug Administration that reminded clinicians to consider the impact of acetate and other sources of alkali when ordering a dialysate prescription43.
Our study raises the possibility that higher dialysate bicarbonate levels may be associated with mortality and hospitalization. Further studies are needed to confirm our findings and to identify the dialysate bicarbonate concentration at which HD patients experience the lowest rates of adverse clinical outcomes.
Supplementary Material
Table S1: Number of deaths by primary cause and by category of dialysate bicarbonate.
Acknowledgments
The authors thank Dean Hiett for providing his expertise in dialysis technology and Jennifer McCready-Maynes (an employee of Arbor Research Collaborative for Health) for editing this manuscript.
Support: The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbott (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications. F. Tentori is supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (1K01DK087762-01A1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Financial Disclosure: F Tentori, A Karaboyas, B Robinson, J Zhang, and FK Port are employees of Arbor Research Collaborative for Health. F. Tentori has received honoraria from Amgen, Dialysis Clinic Inc, and Renal Research Institute. TA İkizler is a consultant for DSI Corp. R Fissell has a consulting agreement with Medtronic. R Vanholder receives research grants from Fresenius Medical Care, Baxter Health Care, Gembro, and Bellco. The other authors declare that they have no other relevant financial interests.
References
- 1.Franch HA, Mith WE. Catabolism in uremia: the impact of metabolic acidosis. J Am Soc Nephrol. 1998 Dec;9(12 Suppl):S78–81. [PubMed] [Google Scholar]
- 2.Uribarri J, Levin NW, Delmez J, et al. Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis. 1999 Sep;34(3):493–9. doi: 10.1016/s0272-6386(99)70077-6. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation; K/DOQI Workgroup. K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am J Kidney Dis. 2000;35(6):s17–s104. doi: 10.1053/ajkd.2000.v35.aajkd03517. Supplement. [DOI] [PubMed] [Google Scholar]
- 4.Eknoyan G, Levin A, Levin NW. Bone metabolism and disease in chronic kidney disease/KDOQI guidelines. Am J Kidney Dis. 2003;42(Supplement 3):1–201. [Google Scholar]
- 5.Locatelli F, Covic A, Chazot C, Leunissen K, Luño J, Yaqoob Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant. 2004 Apr;19(4):785–96. doi: 10.1093/ndt/gfh102. [DOI] [PubMed] [Google Scholar]
- 6.Oettinger CW, Oliver JC. Normalization of uremic acidosis in hemodialysis patients with a high bicarbonate dialysate. J Am Soc Nephrol. 1993 May;3(11):1804–7. doi: 10.1681/ASN.V3111804. [DOI] [PubMed] [Google Scholar]
- 7.Graham KA, Reaich D, Channon SM, Downie S, Goodship TH. Correction of acidosis in hemodialysis decreases whole-body protein degradation. J Am Soc Nephrol. 1997 Apr;8(4):632–7. doi: 10.1681/ASN.V84632. [DOI] [PubMed] [Google Scholar]
- 8.Williams AJ, Dittmer ID, McArley A, Clarke J. High bicarbonate dialysate in haemodialysis patients: effects on acidosis and nutritional status. Nephrol Dial Transplant. 1997 Dec;12(12):2633–7. doi: 10.1093/ndt/12.12.2633. [DOI] [PubMed] [Google Scholar]
- 9.Kooman JP, Deutz NE, Zijlmans P, et al. The influence of bicarbonate supplementation on plasma levels of branched-chain amino acids in haemodialysis patients with metabolic acidosis. Nephrol Dial Transplant. 1997 Nov;12(11):2397–401. doi: 10.1093/ndt/12.11.2397. [DOI] [PubMed] [Google Scholar]
- 10.Graziani G, Casati S, Passerini P, Crepaldi M, Campise M, Ambroso G. Pathophysiology and clinical consequences of metabolic alkalosis in hemodialyzed patients. Arch Ital Urol Nefrol Androl. 1987 Dec;59(2):105–11. [PubMed] [Google Scholar]
- 11.Gabutti L, Ferrari N, Giudici G, Mombelli G, Marone C. Unexpected haemodynamic instability associated with standard bicarbonate haemodialysis. Nephrol Dial Transplant. 2003 Nov;18(11):2369–76. doi: 10.1093/ndt/gfg383. [DOI] [PubMed] [Google Scholar]
- 12.Di Iorio B, Torraca S, Piscopo C, et al. Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: pilot study of single dialysis effects. J Nephrol. doi: 10.5301/jn.5000036. [published online ahead of print 2011] [DOI] [PubMed] [Google Scholar]
- 13.Roderick PJ, Willis NS, Blakeley S, Jones C, Tomson C. Correction of chronic metabolic acidosis for chronic kidney disease patients. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD001890.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int. 2000;57(suppl 74):S-74–S-81. [Google Scholar]
- 15.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner M, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study: Design, data elements, and methodology. Am J Kidney Dis. 2004;44(Suppl 2):S7–S15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Saran R, Bragg-Gresham JL, Wizemann V, et al. Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 17.SAS/STAT User’s Guide Version 8. Cary, North Carolina: SAS Institute; 2000. [Google Scholar]
- 18.Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Raghunathan Trivellore E, Solenberger Peter W, Van Hoewyk John. IVEware: Imputation and Variance Estimation Software. [Google Scholar]
- 20.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Brady JP, Hasbargen JA. Correction of metabolic acidosis and its effect on albumin in chronic hemodialysis patients. Am J Kidney Dis. 1998;31(1):35–40. doi: 10.1053/ajkd.1998.v31.pm9428449. [DOI] [PubMed] [Google Scholar]
- 23.Sonikian M, Gogusev J, Zingraff J, et al. Potential effect of metabolic acidosis on beta 2-microglobulin generation: in vivo and in vitro studies. J Am Soc Nephrol. 1996;7(2):350–356. doi: 10.1681/ASN.V72350. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Pagel M, Vizzo J, Scribner BH. Effect of the normalization of acid-base balance on postdialysis plasma bicarbonate. Trans Am Soc Artif Intern Organs. 1980;26:318–22. [PubMed] [Google Scholar]
- 25.Harris DC, Yuill E, Chesher DW. Correcting acidosis in hemodialysis: effect on phosphate clearance and calcification risk. J Am Soc Nephrol. 1995;6:1607–1612. doi: 10.1681/ASN.V661607. [DOI] [PubMed] [Google Scholar]
- 26.Mitch WE, Clark AS. Specificity of the effects of leucine and its metabolites on protein degradation in skeletal muscle. Biochem J. 1984;222(3):579–586. doi: 10.1042/bj2220579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avram MM, Goldwasser P, Erroa M, Fein PA. Predictors of survival in continuous ambulatory peritoneal dialysis patients: the importance of prealbumin and other nutritional and metabolic markers. Am J Kidney Dis. 1994;23(1):91–98. doi: 10.1016/s0272-6386(12)80817-1. [DOI] [PubMed] [Google Scholar]
- 28.Graham KA, Hoenich NA, Goodship TH. Pre and interdialytic acid-base balance in hemodialysis patients. Int J Artif Organs. 2001;24(4):192–6. [PubMed] [Google Scholar]
- 29.Alfakir M, Moammar MQ, Ali MI, et al. Pulmonary gas exchange during hemodialysis: a comparison of subjects with and without COPD on bicarbonate hemodialysis. Ann Clin Lab Sci. 2011 Fall;41(4):315–20. [PubMed] [Google Scholar]
- 30.Pande S, Raja R, Bloom E, Chewaproug D, Dissanayake I. Effect of dialysate baths on serum bicarbonate levels in hemodialysis patients. Am J Kidney Dis. 2011;57(4):A75. (Abstract #234) [Google Scholar]
- 31.Heguilen RM, Sciurano C, Bellusci AD, et al. The faster potassium-lowering effect of high dialysate bicarbonate concentrations in chronic haemodialysis patients. Nephrol Dial Transplant. 2005 Mar;20(3):591–7. doi: 10.1093/ndt/gfh661. [DOI] [PubMed] [Google Scholar]
- 32.Sethi D, Curtis JR, Topham DL, Gower PE. Acute metabolic alkalosis during haemodialysis. Nephron. 1989;51(1):119–20. doi: 10.1159/000185265. [DOI] [PubMed] [Google Scholar]
- 33.Kaye M, Somerville PJ, Lowe G, Ketis M, Schneider W. Hypocalcemic tetany and metabolic alkalosis in a dialysis patient: an unusual event. Am J Kidney Dis. 1997 Sep;30(3):440–4. doi: 10.1016/s0272-6386(97)90292-4. [DOI] [PubMed] [Google Scholar]
- 34.Sam R, Vaseemuddin M, Leong WH, Rogers BE, Kjellstrand CM, Ing TS. Composition and clinical use of hemodialysates. Hemo Int. 2006;10(1):15–28. doi: 10.1111/j.1542-4758.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 35.Gabutti L, Ross V, Duchini F, Mombelli G, Marone C. Does bicarbonate transfer have relevant hemodynamic consequences in standard hemodialysis? Blood Purif. 2005;23(5):365–72. doi: 10.1159/000087193. [DOI] [PubMed] [Google Scholar]
- 36.Ibels LS. The pathogenesis of metastatic calcification in uraemia. Prog Biochem Pharmachol. 1980;17:242–50. [PubMed] [Google Scholar]
- 37.Uribarri J. Moderate metabolic acidosis and its effects on nutritional parameters in hemodialysis patients. Clin Nephrol. 1997;48(4):238–40. [PubMed] [Google Scholar]
- 38.Vermeulen M, Giordano M, Trevani AS, et al. Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J Immunol. 2004 Mar 1;172(5):3196–204. doi: 10.4049/jimmunol.172.5.3196. [DOI] [PubMed] [Google Scholar]
- 39.Uribarri J. How should dialysis fluid be individualized for the chronic hemodialysis patient? Bicarbonate. 2008 May-Jun;21(3):221–3. doi: 10.1111/j.1525-139X.2008.00427.x. Semin Dial. [DOI] [PubMed] [Google Scholar]
- 40.Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44(4):661–671. [PubMed] [Google Scholar]
- 41.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990 May;15(5):458–82. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 42.Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006 Jan;1(1):70–8. doi: 10.2215/CJN.00010505. [DOI] [PubMed] [Google Scholar]
- 43.http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm305477.htm. Accessed August 16, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Number of deaths by primary cause and by category of dialysate bicarbonate.


