Abstract
Rationale
Neurosteroids are steroids synthesized within the brain with rapid effects on neuronal excitability. Allopregnanolone, allotetrahydrodeoxycorticosterone, and androstanediol are three widely explored prototype endogenous neurosteroids. They have very different targets and functions compared to conventional steroid hormones. Neuronal GABAa receptors are one of the prime molecular targets of neurosteroids.
Objective
This review provides a critical appraisal of recent advances in the pharmacology of endogenous neurosteroids that interact with GABAa receptors in the brain. Neurosteroids possess distinct, characteristic effects on the membrane potential and current conductance of the neuron, mainly via potentiation of GABAa receptors at low concentrations and direct activation of receptor chloride channel at higher concentrations. The GABAa receptor mediates two types of inhibition, now characterized as synaptic (phasic) and extrasynaptic (tonic) inhibition. Synaptic release of GABA results in the activation of low-affinity γ2-containing synaptic receptors, while high-affinity δ-containing extrasynaptic receptors are persistently activated by the ambient GABA present in the extracellular fluid. Neurosteroids are potent positive allosteric modulators of synaptic and extrasynaptic GABAa receptors and therefore enhance both phasic and tonic inhibition. Tonic inhibition is specifically more sensitive to neurosteroids. The resulting tonic conductance generates a form of shunting inhibition that controls neuronal network excitability, seizure susceptibility, and behavior.
Conclusion
The growing understanding of the mechanisms of neurosteroid regulation of the structure and function of the synaptic and extrasynaptic GABAa receptors provide many opportunities to create improved therapies for sleep, anxiety, stress, epilepsy, and other neuropsychiatric conditions.
Keywords: GABA, GABAa receptor, neurosteroid, allopregnanolone, δ-subunit, tonic inhibition, phasic inhibition, extrasynaptic receptors
Introduction
Neurosteroids are steroids synthesized within the brain with rapid effects on neuronal excitability. The term neurosteroid was coined in 1981 by the French endocrinologist Étienne-Émile Baulieu to refer to steroids that are synthesized de novo in the nervous system from cholesterol independently of the peripheral steroidogenic endocrine glands (Baulieu, 1981). Subsequently, the term neuroactive steroid has been widely used to describe natural or synthetic steroids that rapidly alter the excitability of neurons by binding to membrane-bound receptors (Paul and Purdy, 1992; Kulkarni and Reddy, 1995; Reddy, 2003a). It has been known since the 1940s, from the pioneering work of Hans Selye, that naturally occurring steroids such as the ovarian steroid progesterone and the adrenal steroid deoxycorticosterone can exert anesthetic and anticonvulsant actions (Selye, 1941). Recognizing that some steroids could produce such acute central nervous system (CNS) effects, researchers at the pharmaceutical company Glaxo identified the synthetic steroid alphaxolone as having anesthetic properties. In the early 1970s, alphaxolone was marketed as a component of the intravenous anesthetic agent Althesin, which was withdrawn later due to solvent toxicity (Clarke et al., 1973). Several years later, the mechanism of action of alphaxolone was identified. Alphaxolone was found to enhance synaptic inhibition via an action on γ-aminobutyric acid (GABA) type A receptors in the brain (Scholfield, 1980; Harrison and Simmonds, 1984).
A major advance occurred when 5α-reduced metabolites of progesterone and deoxycorticosterone were found to enhance GABAa receptor function (Majewska et al., 1986; Kulkarni and Reddy, 1995). It was speculated that the anesthetic and anticonvulsant properties of progesterone and deoxycorticosterone, known since the time of Selye, were due to their conversion to allopregnanolone (3α-hydroxy-5α-pregnane-20-one) and allotetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one; THDOC), respectively (Reddy, 2003b) (Fig.1). The androgenic neurosteroid androstanediol (5α-androstan-3α,17β-diol) is synthesized from testosterone (Reddy, 2004a,b; 2008; Reddy et al., 2005a). At the time, it was recognized that the enzymes required for the conversion of the steroid hormone precursors to their active 5α-reduced metabolites are present in the brain and therefore their synthesis occurs locally (Kulkarni and Reddy, 1995). There is now compelling evidence that all of the enzymes required for the biosynthesis of the neurosteroids from cholesterol are present in the brain (Do Rego et al., 2009). Therefore, allopregnanolone, THDOC, and androstanediol came to be referred to as neurosteroids (Reddy, 2011). Since neurosteroids are highly lipophilic and can readily cross the blood-brain barrier, neurosteroids synthesized in peripheral tissues accumulate in the brain. Pregnenolone, its sulfate ester, commonly referred as pregnenolone sulfate (PS) and dehydroepiandrosterone (DHEA) and its sulfate ester, DHEAS, are also present in the brain and could elicit rapid effects in enhancing neuronal excitability.
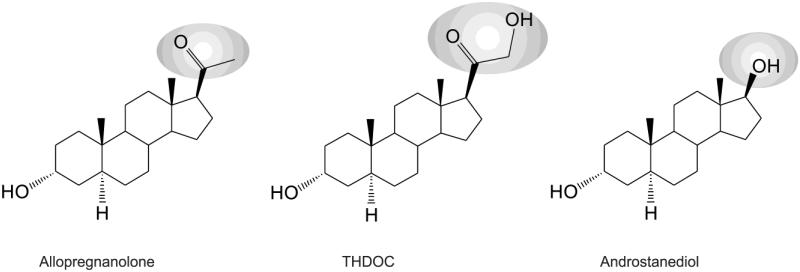
Fig. 1. Chemical structures of three prototype endogenous neurosteroids.
THDOC differs from allopregnanolone by a 21β-hydroxyl group, while androstane differs from allopregnanolone by a 17β-hydroxyl group instead of 17β-methyl-carbonyl group. Synthetic analogs of neurosteroids are prepared by additional moieties at C3-position (ganaxolone), C11-position (alphaxolone), and C2- and C11-positions (minaxolone).
Increasing evidence points to the vast array of potent effects that endogenous neurosteroids have on GABAa receptors in modulating inhibition within the brain (Reddy, 2013a,b). Given that GABAa receptors are the main mediators of inhibition in the brain, properties that influence this inhibition exert significant regulatory control over neuronal excitability. GABAa receptors are membrane-bound, ligand-gated ion channels which, when activated by GABA, hyperpolarize neurons through influx of negatively charged chloride ions in the adult brain. At birth, the chloride driving force is positive such that GABA transmission causes depolarization. As the brain matures, a negative shift in the chloride ion reversal potential induces the electrochemical gradient to allow for inward current flow when the GABAa receptor channel is opened (Rivera et al., 1999). The hyperpolarizing current serves to reduce neuronal excitability and short-circuits action potential firing. Inhibitory GABAa receptors are heterogenic, possessing a high degree of variability in their structural features and subunit composition. In addition, there is a large catalog of agonists and antagonists for these receptors, and each ligand has distinct affinity and efficacy (Mitchell et al., 2008; Uusi-Oukari and Korpi, 2010). Inhibitory network control is essential to maintain proper neuronal function for motor actions, cognition, autonomic activity, and nearly every other function of the brain.
This review aims to elucidate current perceptions on the interaction between neurosteroids and GABAa receptors. It aims to provide a critical appraisal of recent advances in the pharmacology of neurosteroids, in conjunction with emerging information on new mechanisms underlying the regulation of receptor subunit plasticity, tonic inhibition and their applications for improved understanding of normal and abnormal neuronal excitability conditions in the brain. For the purposes of this review, discussion will be centered on neurosteroid and GABAa receptor interaction as it pertains to limbic structures.
An overview of neurosteroid pharmacology
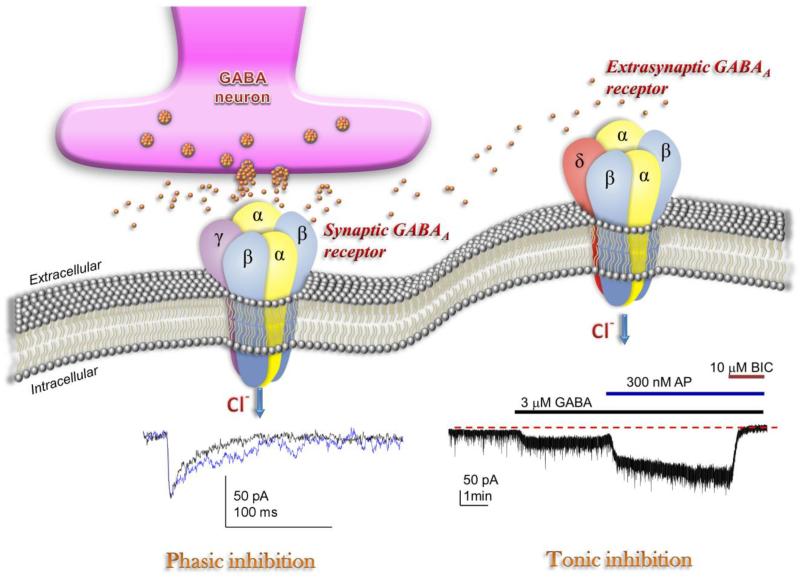
Emerging understanding of GABAa receptor interaction with neurosteroids has yielded many interesting developments in neuronal network physiology and clinical application. In the 1980s, alphaxolone and structurally similar steroids were found to enhance GABA agonist activity, and this action was unaffected by benzodiazepine antagonist activity (Harrison and Simmonds, 1984; Harrison et al., 1987). Since that time, both natural and synthetic neurosteroids have been identified which have more potent effects on GABAa receptors to enhance inhibitory activity within the brain. Consequently, it is clear that two distinct types of GABAergic inhibition –phasic or synaptic inhibition and tonic inhibition—control excitability in the brain (Fig.6). Phasic inhibition is attributed to the inhibitory postsynaptic current (IPSC) resultant from membrane receptor opening in response to rapid release of GABA across the synapse and binding GABAa receptors as a traditional neurotransmitter (Farrant and Nusser, 2005). Vesicular release of GABA into the synapse generates a local peak concentration of GABA that may reach millimolar levels. Tonic inhibition is persistent inhibitory current mediated by perisynaptic or extrasynaptic receptors in response to ambient or extracellular GABA (Glykys and Mody, 2007). Tonic current enables shunting inhibition to control gain of neuronal excitability (Mitchell and Silver, 2003). Both synaptic and extrasynaptic receptors possess sensitivity to neurosteroids and contribute to the overall significance of neurosteroid modulation of inhibition. Higher sensitivity to neurosteroid contributes to the extrasynaptic receptors’ functional divergence from synaptic receptors in modulating inhibition (Bai et al., 2001; Belelli et al., 2009).
Fig. 6. Neurosteroid modulation of synaptic and extrasynaptic GABAa receptors.
Postsynaptic GABAa receptors, which are pentameric chloride channels composed of 2α2βγ subunits, mediate the phasic portion of GABAergic inhibition, while extrasynaptic GABAa receptors, pentamers composed of 2α2βδ subunits, primarily contribute to tonic inhibition in the dentate gyrus. Neurosteroids activate both synaptic and extrasynaptic receptors and enhance the phasic and tonic inhibition, and thereby promote maximal net inhibition. The trace illustrating phasic inhibition shows an IPSC produced by endogenous GABA release (black) or in the presence of 300 nM allopregnanolone (blue). Neurosteroids enhance the IPSCs by prolonging the deactivation/decay kinetics. The trace illustrating tonic inhibition shows tonic conductance activated by GABA that was further enhanced by application of allopregnanolone.
Neurosteroids play a significant role in the electrical and molecular control of neurons through GABAa receptor binding and interaction. Even in physiological concentrations, they are potent, allosteric modulators of GABAa receptors. Benzodiazepines, barbiturates, alcohols, and neurosteroids all interact with GABAa receptors to influence channel activity, but they have major differences in binding sites, potency and efficacy (Fig.2). Different types of positive allosteric modulators bind the GABAa receptor at unique sites. Each of the allosteric modulators can also modify the molecular components, location, and expression of receptors (Uusi-Oukari and Korpi, 2010). They are also involved in the plastic receptor expression changes that occur to adjust balance between inhibition and excitability. GABAa receptor assembly and turn-over occur through several regulatory mechanisms involving expressional and distributional changes to the synaptic and extrasynaptic membrane physiology (Luscher and Keller, 2004). Extrasynaptic receptors can be transported to synaptic sites in response to requirements for stability at the synapse (Bogdanov et al., 2006) and fine adjustment to recover inhibitory sensitivity through diffusional trafficking (Thomas et al., 2005).
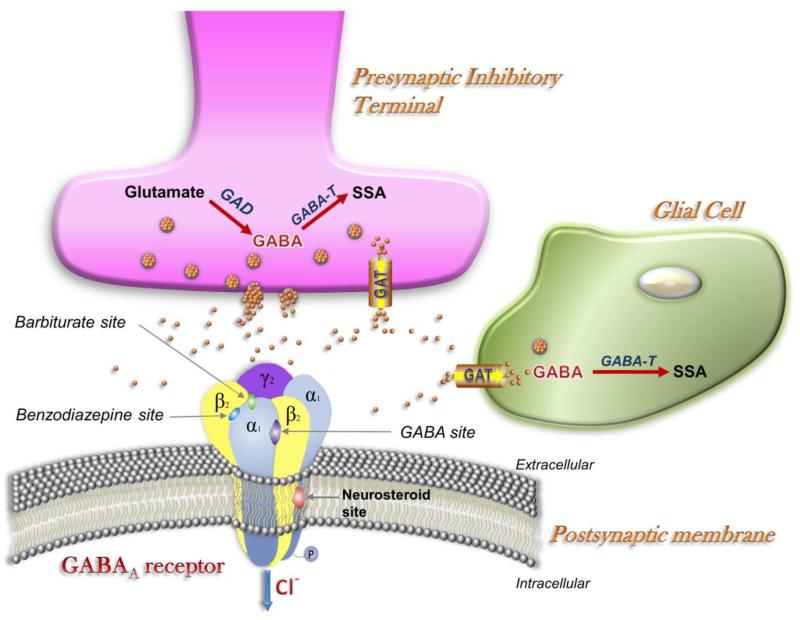
Fig. 2. The GABAergic synapse.
GABA, which is released from presynaptic vesicles, serves as the main fast inhibitory neurotransmitter in the brain by activation of postsynaptic GABAa receptors. GABAa receptors are pentameric in structure, with the five subunits arranged around a central Cl selective pore. A variety of chemical compounds are capable of acting on GABAa receptors to modulate its channel function. Apart from GABA sites, the receptor has specific sites for benzodiazepines, barbiturates, and neurosteroids, which are allosteric sites for modulation of GABA currents or Cl conductance. GABA transporters in neurons (GAT-1) and glia (GAT-2/3) remove synaptically released GABA, thereby limiting or terminating its inhibitory action. Reuptake into terminals permits immediate recycling by vesicular uptake, whereas reuptake into astrocytes leads to metabolism via GABA-transaminase (GABA-T) and succinic semialdehyde (SSA) and glutamate. Tiagabine, which is highly selective for GAT-1, inhibits GABA reuptake. Binding of neurosteroids to their binding site(s) enhances the effect of GABA by increasing the frequency and duration of channel opening.
Neurosteroid biosynthesis and regulation in the brain
Neurosteroid biosynthesis
Neurosteroids are synthesized de novo in the brain or derived in peripheral tissues from metabolism of classical steroids such as progesterone, deoxycorticosterone, and testosterone. Reduction reactions are catalyzed by 5α-reductase and subsequently 3α-hydroxysteroid dehydrogenase (or 3α-hydroxysteroid oxidoreductase, 3α-HSOR) (Fig.4). Allopregnanolone and THDOC are classified as pregnane steroids, and they are the most commonly studied endogenous neurosteroids (Reddy and Kulkarni, 2000; Belelli and Lambert, 2005). Allopregnanolone is synthesized from progesterone and THDOC is synthesized from deoxycorticosterone. Pregnenolone, progesterone, and deoxycorticosterone are pregnane steroid precursors and also exhibit neuroactive effects. The androstane class of neurosteroids, including androstanol and androstanediol, are derived from testosterone (Reddy, 2004b; Kaminski et al., 2006; Reddy, 2008). Both pregnane and androstane neurosteroids can positively potentiate GABAa receptor-mediated current and thereby enhance inhibitory function in neurons. Estradiol has neuroactive effects in modulating GABAergic substrates and regulation of neuronal plasticity to promote excitability, especially within the hippocampus (Wójtowicz et al., 2008; Herzog, 2009). Synthesis of estradiol can occur through the aromatase-driven modification of testosterone, which could serve as an endogenous regulator of neuronal excitability in men, as well as women (Herzog, 1999).
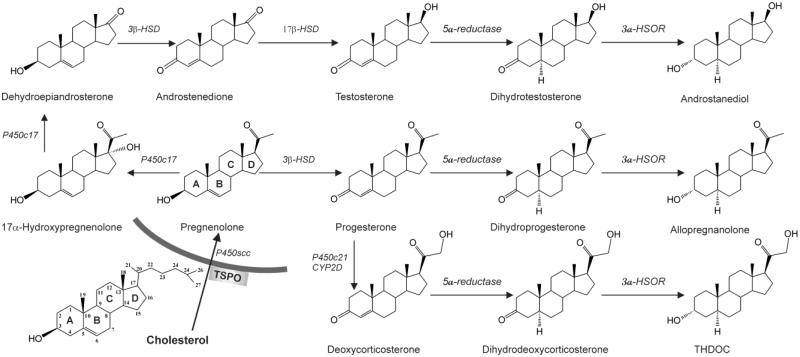
Fig. 4. Biochemical pathways of neurosteroid biosynthesis.
Enzymatic pathways for the production of three prototype neurosteroids allopregnanolone, THDOC, and androstanediol are illustrated from cholesterol and intermediate steroid precursors. Cholesterol is converted to pregnenolone by P450scc in the inner mitochondrial membrane. Pregnenolone is the precursor for progesterone and other neurosteroids. Progesterone, deoxycorticosterone and testosterone undergo two sequential A-ring reduction steps catalyzed by 5α-reductase and 3α-HSOR to form the 5α, 3α-reduced neurosteroids. The conversion of progesterone, deoxycorticosterone or testosterone into neurosteroids occurs in several regions within the brain. The 5α-reductase, 3α-HSOR and other enzymes are present in the brain. Legend: P450scc, cholesterol side-chain cleavage enzyme; 3β HSD, 3β-hydroxysteroid dehydrogenase; 3α-HSOR, 3α-hydroxysteroid oxidoreductase; 17π-HSD, 17β-hydroxysteroid dehydrogenase; P450c21, cytochrome P450 21-hydroxylase.
Neurosteroids reach neuronal GABAa receptors from the periphery or local brain tissue. Precursor steroids may enter the brain from the blood circulation and can be converted to neurosteroids (Agís-Balboa et al., 2006). In addition, neurosteroids are very lipophilic molecules and easily cross the blood brain barrier from peripheral sources. Subcutaneous injection of progesterone rapidly elevates plasma and brain levels of allopregnanolone in rodents, even in the case of animals in which the progesterone receptor gene is completely absent (Reddy and Mohan, 2011), indicating the efficiency of the neurosteroid biosynthetic system. The drug finasteride is used as a tool to inhibit endogenous neurosteroid biosynthesis, as it blocks the activity of 5α-reductase conversion of steroids into their 5α-dihydro-reduced form (Reddy, 2010; Reddy and Ramanathan, 2012).
In addition to peripheral production and humoral delivery, it is clear that neurosteroids can be formed locally in the brain from steroid hormone precursors. 5α-Reductase activity has been identified in both neurons and glia in rodent and sheep brain within regions such as the neocortex and hippocampus (Melcangi et al., 1998; Petratos et al., 2000). 3α-HSOR is also expressed widely in the brain (Khanna et al., 1995). In humans, both enzymes have been found in neocortex and hippocampus (Stoffel-Wagner et al., 2000; 2003; Stoffel-Wagner, 2001). Steroid precursors readily enter the brain, so pools of peripherally synthesized precursors are available for local neurosteroid biosynthesis. Taken together, all of the necessary enzymes required for steroid synthesis are region-specific, cell-specific, and available within the neural tissue, both in neurons and glia (Compagnone and Mellon, 2000). Therefore, de novo synthesis of neurosteroids may occur within the brain.
Regulation of neurosteroidogenesis and the role of TSPO in neurosteroid biosynthesis
The initial key step in steroidogenesis is the conversion of cholesterol to pregnenolone by the mitochondrial enzyme P450scc (cytochrome P450 cholesterol side-chain cleavage enzyme). Access of cholesterol to P450scc requires StAR (steroidogenic acute regulatory protein), which functions to transfer cholesterol from the outer mitochondrial membrane to the inner membrane where P450scc is located (Stoffel-Wagner et al., 2003). Translocator protein 18 kD (TSPO), formerly called peripheral or mitochondrial benzodiazepine receptor, likely functions as a complex with StAR and VDAC (voltage-dependent anion channel) at the outer mitochondrial membrane, but this role is not completely clear (Korneyev et al., 1993; Jefcoate, 2002; Papadopoulos et al., 2006; Midzak et al., 2011b). The drug PK11195 is used to verify the TSPO mediated neurosteroid production. TSPO is highly expressed in tissue where steroidogenesis occurs including the brain (Papadopoulos et al., 2006). It transports cholesterol from the outer mitochondrial membrane to the inner membrane, and it promotes production of neurosteroid. Activation of TSPO by certain ligands facilitates increased neurosteroid production (Kita and Furukawa, 2008; Rupprecht et al., 2009). Other TSPO ligands may influence the synthesis of pregnenolone from cholesterol, and once synthesized, neurosteroids are likely released from microglial and glial cells in a paracrine fashion to act on GABAa receptor targets (Papadopoulos et al., 2006; Nothdurfter et al., 2012). While this assumption remains valid, reduced steroids are locally synthesized within neurons in a wide distribution throughout the brain (Saalmann et al., 2007). Additionally, recent findings suggest that neurosteroids or biochemically similar compounds may have a role in controlling their own biosynthesis through negative feedback loops to interact with TSPO and inhibit cholesterol utilization during steroidogenesis (Midzak et al., 2011a). Mechanisms pertaining to neurosteroid transport may be key in identifying how regional levels of neurosteroid are controlled within the brain to exert modulation over neural networks.
Neurosteroid synthesis in principal neurons
Emerging evidence suggests that production of neurosteroids in the brain occurs in principal neurons. In studies with rodent brain, in situ hybridization with mRNA probes to 5α-reductase and 3α-HSOR indicates that the two mRNAs colocalize to glutamatergic principal neurons and not GABAergic inhibitory neurons or glial cells within neocortex, hippocampus, amygdala, and other brain regions (Agis-Balboa et al., 2006). Immunohistochemistry with an antiserum against allopregnanolone, that also recognizes THDOC, confirms that the neurosteroids are concentrated in principal neurons, predominantly in cell bodies and thick dendrites (Saalman et al., 2007). The highly restricted distribution of neurosteroids to principal neurons suggests that they are mainly derived from local synthesis and not from the circulation, although it is clear that peripheral neurosteroids, as previously noted, readily cross the blood-brain barrier. It is remarkable that brain neurosteroids are localized to the neurons that contain their targets. This observation is consistent with the notion that neurosteroids function in an autocrine fashion in which they reach their targets by lateral membrane diffusion (Chisari et al., 2010). However, the rates of production and their specific control in different regions remain unclear.
GABAa receptor structure and subunit composition
The human brain contains about 100 billion neurons. An estimated 20–30% of the neurons in the CNS are GABAergic. Activation of neuronal GABA receptors typically results in hyperpolarization, and thus GABA is the major inhibitory neurotransmitter in the CNS. In the general scheme of feed-forward and feed-back interactions between principal and interneurons, inhibitory interneurons play critical role in regulating network excitability. By controlling spike timing and sculpting neuronal rhythms, inhibitory GABAergic interneurons play a key role in regulating neuronal circuits. Two pharmacologically distinct classes of GABA receptors have been identified: GABAa receptors (heteropentameric ligand-gated chloride channels) and GABAb receptors (heterodimeric Gi/Go protein-coupled receptors). There is little evidence that neurosteroids interact with GABAb receptors, which are not covered in this article.
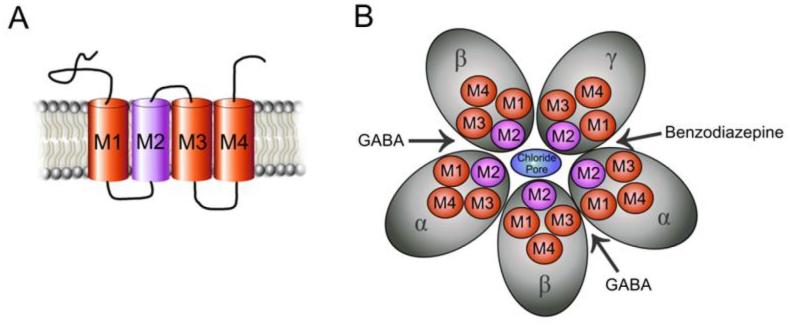
GABAa receptors are pentameric in structure, and each of the five subunits has four transmembrane domains (M1-M4) (Fig.3). The pentamer arrangement of subunits forms a selective channel for chloride ion passage when gated and opened by GABA. The second transmembrane domain lines the internal wall of the channel pore. There are 19 variants of GABAa receptor subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π, ρ1-3), of which α, β, γ, and δ subtypes are most prominently studied (Sieghart and Sperk, 2002). The discussion is focused on receptors consisting of α, β, γ, and δ subunits as they pertain to neurosteroid interaction. GABAa receptors are typically composed of two α subunits, two β subunits, and the fifth subunit incorporated is most often γ, which is present in approximately 90% of GABAa receptors (Sieghart, 2006). The δ subunit can be assembled as the fifth subunit in the place of γ, and there is a high incidence of its occurrence within the cerebellum, thalamus, and hippocampus (Pirker et al., 2000). The functional stoichiometry forming the synaptic channel pore is 2α:2β:1γ (Baumann et al., 2002; Ernst et al., 2003). Similarly, α4β2δ receptors only form functional channels with a stoichiometry of 2α:2β:1δ (Shu et al., 2012). Due to the large degree of variety in receptor arrangements, it has been a large undertaking to further classify the physiology and pharmacology for different GABAa receptor pentamer isoforms.
Fig. 3. GABAa receptor structure and subunit families.
GABAa receptors are heteropentamers made up from 19 known subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π and ρ1–3) with an integral channel that is permeable to Cl− ions. (A) A cross-section of the intramembrane structure shows the Cl− channel pore formed by M2 helical elements. Each subunit has four transmembrane segments, with both the amino and carboxy terminal located extracellularly. (B) A top view of GABAa receptor channel, illustrated as five cylinders arranged to form the Cl− ionophore. The receptor is pentameric, being composed of two α, two β, and one γ subunit. The δ subunit may be assembled instead of γ. Binding of GABA in the two binding sites at the interface between and subunits open the receptor-associated Cl− channel.
The GABAa receptor subunit compositions and location are listed in Table 1. Approximately 60% of all GABAa receptors have the subunit combination α1β2γ2, ~15–20% have the α2β3γ2 combination, ~10–15% have the α3βxγ2 combination, ~ 5% have the α4βxγ or α4βxδ, combination, less than 5% have the α5β2γ2 combination and, likewise, less than 5% have the α6β2/3γ2 combination (Mohler et al., 2002; Rudolph and Knoflach, 2011).
TABLE 1.
GABAA receptor subunit compositions and distribution in the brain.
| Subunit Composition |
Brain Location | GABA potency (EC50)* |
Specific Pharmacology Sensitivity** |
|---|---|---|---|
| Synaptic | |||
| α1 β2 γ2 | ubiquitous1,3,9 | 6.6 μM | Benzodiazepines5,7 |
| α 1 β 3 γ 2 | ubiquitous | 2.1 μM | Benzodiazepines5 |
| α 2 β 3 γ 2 | hippocampus, amygdala, lateral septum, caudate putamen1,3,9 |
13.4 μM | Benzodiazepines5 |
| α 3 β 2/3 γ 2 | cerebral cortex, thalamus | 12.5 μM | Benzodiazepines5 |
| α 4 β 2/3 γ 2 | hippocampus, thalamus2 | 2.1 μM | Furosemide, Ro15-451310 |
| α 6 β 3 γ 2 | cerebellum | 0.17 μM | Furosemide, Ro15-451310 |
| Extrasynaptic | |||
| α 4 β 2/3 δ | dentate gyrus granule layer8,11, hippocampus (limited)8,11, thalamus11 |
0.91 – 1.7 μM | Furosemide10, THIP15 |
| α 1 β δ | dentate gyrus molecular layer14,16, hippocampal interneurons16 |
3.7 μM | |
| α 5 β 3 γ 2 | cerebral cortex12, CA1-3 hippocampus6,12 |
1.4 μM | L-655,7086,12 |
| α 6 β 2/3 δ | cerebellum granule layer4 | 0.17 μM | Furosemide10, THIP15 |
Subunit source citations:
Persohn et al., 1992;
Wisden et al., 1991;
Wisden et al., 1992;
Korpi et al., 1995;
Sieghart et al., 1995;
Quirk et al., 1996;
Wafford et al., 1996;
Sperk et al., 1997;
Pirker et al., 2000;
Brown et al., 2002;
Peng et al., 2002;
Caraiscos et al., 2004;
Pillai et al., 2004;
Sun et al., 2004;
Boehm et al., 2006;
Glykys et al., 2007
potency data based on recombinantly expressed receptors in HEK293 cells (Mortensen et al., 2012)
see text for pharmacology profiles
Parsing isoform configurations at extrasynaptic/perisynaptic and synaptic sites is essential to understand GABAergic inhibition and its pharmacological modulation by various ligands. The distribution of GABAa receptors within the hippocampus is diverse, but there are expressional patterns that emerge from immunocytochemistry (Sperk et al., 1997). Synaptic GABAa receptors primarily consist of α, β, and γ2 subunits. The α-subunit expression within synaptic receptors varies, but they are typically benzodiazepine-sensitive (Hajos et al., 2000). Particular α-subtypes contribute to synaptic or tonic inhibition differentially in hippocampal neurons (Prenosil et al., 2006). Extrasynaptic receptors are most often characterized by the inclusion of a δ subunit rather than γ, together with β2/3 and a particular isoform of the α subunit, dependent on the tissue and region; α4-containing profiles are found in dentate gyrus granule cells (DGGC) and thalamic neurons, α5 is primarily located in CA1-3 neurons (although coupled with γ rather than δ), and α6 expression is pervasive within cerebellar granule cells (CGC) (Persohn et al., 1992; Benke et al., 1997; Pirker et al., 2000; Wei et al., 2003; Sun et al., 2004; Mangan et al., 2005; Zheleznova et al., 2009). These profiles mentioned are commonly found, but much cross-over exists in the variety of subunit assembly found within regions and cell-specificity of neurons.
In the hippocampus DGGCs, α4βδ receptors are expressed primarily extrasynaptically, while α1βγ2 & α2βγ2 receptors are located within the synaptic sites. However, α1, α4, γ2, and δ are all present in the extrasynaptic or perisynaptic location in the hippocampus (Sun et al., 2004). According to immunostaining studies, a population of interneurons in the molecular layer of the dentate gyrus also express the δ-subunit, but the α4 subunit is not present (Sun et al., 2004). These receptors have been found to coexpress and colocalize α1 and δ subunits extrasynaptically with different kinetics and pharmacology than α4/α6βδ receptors (Glykys et al., 2007). Therefore, principal cells and interneurons in the hippocampus may have quite distinct extrasynaptic receptor composition. Further investigations into β subunit-containing receptors have identified that β2 is a component of benzodiazepine-insensitive receptors contributing to extrasynaptic current in DGGCs, while β3-containing receptors are predominantly assembled in synaptic receptors associated with phasic current (Herd et al., 2008).
Pharmacological tools continue to develop in effort to explain the observed interactions between neurosteroids and receptors at the molecular level. It is important to supplement such evidence with principal brain network physiology in order to elucidate the properties of GABAergic inhibition. A recent study has rigorously investigated receptor isoforms that pertain to either synaptic or extrasynaptic current in order to identify properties of specific subunits that confer GABA potency (Mortensen et al., 2012; see Table 1).
Neurosteroid modulation of GABAa receptors
A variety of GABAa receptor modulatory neurosteroids are known to be synthesized endogenously (Fig.4). Neurosteroids are bimodal modulators of GABAa receptor function depending upon their structural features. The best recognized of these are the pregnane neurosteroids allopregnanolone, THDOC, and androstanediol. Generally, sulfated analogs, such as PS and DHEAS, as well as 3β-hydroxysteroids exhibit negative allosteric modulatory properties.
Positive modulation
Allopregnanolone, THDOC, and androstanediol are positive allosteric modulators of GABAa receptors, which modify the natural affinity and/or efficacy of GABA and thereby exert control over neuronal excitability. At concentrations in the range 10–500 nM, allopregnanolone and THDOC enhance the activation of GABAa receptors by GABA (Harrison et al., 1987; Kokate et al., 1994; Reddy and Rogawski, 2002). At higher concentrations, the steroids directly activate the receptor in the absence of GABA. Like other positive allosteric modulators of GABAa receptors, neurosteroids exert allosteric effects on these receptors such that there is enhancement of the binding of [3H]flunitrazepam, a benzodiazepine receptor agonist, and [3H]muscimol, a specific GABA-site agonist, as well as inhibition of the binding of [35S]t-butylbicycloorthobenzoate (TBPS), a cage convulsant and noncompetitive GABAa receptor antagonist (Gee et al., 1988; Lan et a., 1991). Unlike benzodiazepines (which enhance the channel open frequency) or barbiturates (which increase the channel open duration), neurosteroid enhancement of GABAa receptors occurs in a hybrid fashion, increasing both the frequency and duration of the chloride channel opening (Twyman and Macdonald, 1992; Hosie et al., 2007; 2009; Lambert et al., 2009; Ramakrishnan and Hess, 2010). Thus, neurosteroids greatly enhance the probability of GABAa receptor chloride channel opening, thereby enhancing GABAa receptor-mediated inhibition.
The molecular nature of neurosteroid binding sites is under intense scrutiny. The effects of neurosteroids on GABAa receptors occur by binding to discrete sites on the receptor-channel complex that are located within the transmembrane domains of the α and β subunits (Hosie et al., 2007) (Fig.2). The binding sites for neurosteroids are distinct from the recognition sites for GABA, benzodiazepines, and barbiturates. Having the ability to potentiate GABAergic current at 10 – 500 nM concentrations and to autonomously, directly induce receptor channel opening at larger concentrations (> 500 nM) (Belelli and Lambert, 2005), neurosteroids appear to operate on a wider array of receptor isoforms and are thus less specific in binding as compared with benzodiazepines. In fact, the neurosteroid enhancement of binding is thought to be due to allosteric interaction with an altogether different site on the receptor. It has been proposed that neurosteroids may bind receptors from intracellular access or at a site within the neuronal plasma membrane (Akk et al., 2009). Nevertheless, the location of this site is uncertain and warrants further characterization. Neurosteroid binding interfaces are discussed in further detail throughout this review.
Consistent with potentiation effects at the cellular level, endogenous neurosteroids like allopregnanolone and THDOC are capable of sedative, anxiolytic, and anticonvulsant behavioral effects on the CNS (Reddy and Kulkarni, 1997; Reddy, 2003a; Reddy and Zeng, 2007; Reddy, 2011). It is appropriate to consider the system-wide pharmacological effects of treatments that involve neuromodulatory agents. Because of the capacity of these steroids to affect the neuronal network in profound ways, behavioral measures are of importance to develop neurosteroids for therapeutic applications.
Structure-activity studies of neurosteroid potentiation of GABA-gated currents revealed unique structural features of neurosteroids (Harrison et al., 1987; Gee et al., 1988). A range of steroid structures have activity as positive modulators of GABAa receptors in line with the hydrophobic surface binding site model. Nevertheless, there are certain strict structural requirements for neurosteroid positive modulation. A hydrogen bond-donating 3α-hydroxy group on the steroid A-ring and a hydrogen bond-accepting group (typically a keto moiety) on the D-ring at either C20 of the pregnane steroid side chain or C17 of the androstane ring system are critical for positive modulatory activity at GABAa receptors (Purdy et al., 1990; Lambert et al., 2003). The orientation of the C5 hydrogen group only modestly influences potency (Kokate et al., 1994).
The structural configurations of α and β neurosteroid epimers have highly differential effects on GABAa receptor function, owing to the stereoselectivity of binding. The 5β-isomers of allopregnanolone and THDOC have GABAa receptor modulatory activity that is only modestly less potent than that of the corresponding 5α-epimers. Behavioral, electrophysiological, and pharmacological data substantiate that 5α-reduced steroids, but not 5β-reduced steroids possess a high level of enantiospecificity and selectivity in function of GABAa receptor modulation and anesthetic efficacy (Covey et al., 2000). 5α-Reduced neurosteroids have greater anticonvulsant activity in conjunction with inhibitory current potentiation compared to their 5β-epimers (Kokate et al., 1994).
The neurosteroids that have androstane skeleton and that lack the pregnane 17β-ethyl moiety, such as 5α-androstanediol, androsterone, and etiocholanolone, can be considered androstane neurosteroids. Androsterone and etiocholanolone also have GABAa receptor-positive modulatory activity and represent endogenous neurosteroids (Kaminski et al., 2005). Substantial amounts of androstenol, the 16-unsaturated form of 5α-androstanediol, are present in mammals, including humans. This compound is considered to be a pheromone that increases sexual receptivity in pigs and possibly other species. Androstenol also is a GABAa receptor-positive modulator that has similar efficacy but is modestly less potent than allopregnanolone (Kaminski et al., 2006).
The androgenic steroid testosterone differs from progesterone by virtue of a 17-hydroxyl group that replaces the 17-acetyl in progesterone. Testosterone is a substrate for both 5α-reductase and 5β-reductase isoenzymes (Reddy, 2004a,b; Reddy, 2008). The product of 5α-reduction of testosterone, 5α-dihydrotestosterone, is hormonally more active than testosterone itself. However, subsequent 3α-reduction leads to 5α-androstanediol (5α-androstane-3α,17β-diol). 5α- and 5β-androstanediol are further metabolized by 17β-hydroxysteroid dehydrogenase to androsterone and etiocholanolone, respectively. The synthetic enantiomers of androsterone and etiocholanone have been found to have more efficacy than their naturally occurring counterparts (Katona et al., 2008). This is in contrast to ent-pregnane steroids, which are less potent than allopregnanolone or pregnanolone. In vitro electrophysiology on CA1 pyramidal neurons demonstrates divergent androstane 3-epimer activity on GABA receptors as well; co-application of 3α-androstanediol (reduced by 3α-hydroxy dehydrogenase) and GABA induce a greater hyperpolarizing current than that of GABA alone, while 3β-androstanediol (reduced by 3β-hydroxy dehydrogenase) co-applied with GABA shows no significant effect in enhancing the GABA response (Reddy and Jian, 2010). In animal studies, half-maximal values for seizure protection in mice by androstanediol are within the concentration range of androstanediol-induced potentiation (Reddy and Jian, 2010). The enhanced potentiation by 3α-androstanediol, rather than the 3β epimer, exhibits protective effects against kindled seizure excitability, and this confirm that the neuroactive effect of the neurosteroid are attributed to the α epimer structure, connecting in vitro and in vivo findings across several studies and methods.
Neurosteroids act as autocrine factors in principal neurons or interneurons. Although neurosteroids are viewed as high potency modulators of GABAa receptors since they are effective at concentrations in the nanomolar range in aqueous solution, recent studies indicate that neurosteroid binding to the GABAa receptor is actually of low affinity (true membrane EC50, ~1 mM) (Chisari et al., 2010). The high effective potency of neurosteroids results from partitioning of the lipophilic steroids within the plasma membrane, such that the concentrations accessible to the receptor are orders of magnitude greater. Neurosteroids access the GABAa receptor from the lipophilic plasma membrane. The nonspecific accumulation and removal of the neurosteroids from the membrane are the major factors determining the rates of neurosteroid action when applied to cells via aqueous solution; rates of binding and unbinding to the receptor are only secondary factors (Chisari et al., 2009). It is noteworthy that intracellular delivery through the plasma membrane is compatible with the autocrine mechanism discussed above, in which the neurosteroids act on the GABAa receptors in the same neurons in which they are produced (Saalmann et al., 2007).
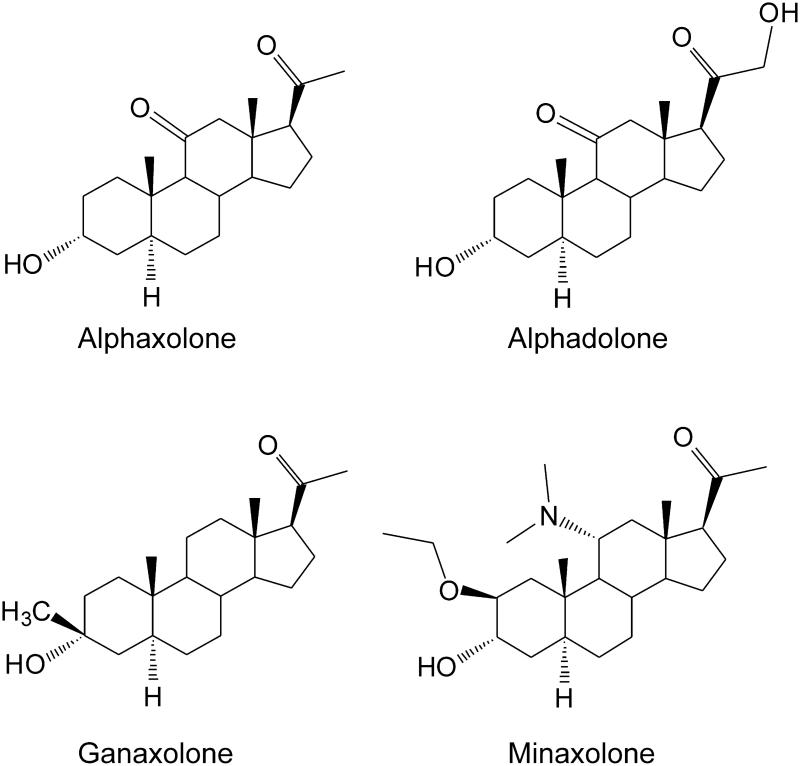
Endogenous neurosteroids often have low availability due to rapid inactivation or conversion into other steroidal products. To overcome these limitations, synthetic neurosteroids analogs have been developed for therapeutic use (Reddy and Kulkarni, 2000; Fig.5). Analogs also serve as pharmacological tools to better understand specific ligand binding characteristics of neurosteroids based on their conserved lipophilic characteristics. Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one) is an analog of allopregnanolone which is altered to include a β-methyl group at the C3 position (Carter et al., 1997; Reddy and Woodward, 2004). It possesses sedative and anticonvulsant effects and positively modulates GABAa receptors allosterically, similarly to allopregnanolone (Reddy and Rogawski, 2010). It is the only neurosteroid analog thus far to be considered in human clinical trials for the treatment of epilepsy (Nohria et al., 2010). Alphaxolone is a general anesthetic that has been well classified in GABAa receptor binding characteristics. The synthetic derivative minaxolone has been shown to induce an extrasynaptic, GABAergic conductance, similar to allopregnanolone at 1 μM concentrations (Mitchell et al., 2007). These analogs have the ability to potentiate GABAa receptors and induce sedative, anticonvulsant, anesthetic, or anxiolytic actions, very similar to the endogenous neurosteroids. Their positive interaction with receptors verifies that neurosteroid structural selectivity is reliant on conserved sites to bind and enhance GABAergic function.
Fig. 5.
Chemical structures of synthetic neurosteroids.
Negative modulation
Sulfated neurosteroids such as DHEAS and PS can inhibit GABAergic activity and may serve as proconvulsants (Baker et al., 2010; Gartside et al., 2010). As negatively-modulating steroids, they have been shown to antagonize GABAa receptors in a noncompetitive fashion. They inhibit channel activity in either a closed state prior to GABA agonist activation or in an open state, blocking activity in instances where GABA is bound (Akk et al., 2001). In addition to GABAergic antagonism, PS and DHEAS have been implicated in potentiating N-methyl-D-aspartate (NMDA) receptors to induce excitability (Friess et al., 2000; Horak et al., 2004), and PS modulation of NMDA is regulated by phosphorylation pathways (Petrovic et al., 2009). Interestingly, PS does not exhibit an effect on the rapid kinetics of channel opening, closing, or desensitization in the presence of high concentrations of GABA (1 mM), however the inhibition of receptors occurs as a slow, gradual block (Akk et al., 2001). The exact mechanism of antagonism is not well understood in the presence of low levels of extracellular GABA responsible for physiological tone. A high concentration of GABA reflects synaptic neurotransmission of GABA release, but may not explain a negatively-modulating mechanism at extrasynaptic sites of lower GABA concentrations.
While studies have thus far revealed that the sulfated neurosteroid binding site and mechanism of action differs from that of positively potentiating neurosteroids, pharmacological study of sulfated neurosteroids has been limited to the context of synaptic receptor interaction. In pharmacological studies, PS and DHEAS promote learning and memory (Reddy and Kulkarni, 1998a). Due to their intrinsic ability to block GABAergic inhibition, there is a basis for this group of neurosteroids to serve as a homeostatic balance to the positively potentiating class of endogenous neurosteroids. Recently, sulfated steroid antagonism of GABAa receptors was found to be similar on recombinantly expressed δ-containing and γ-containing receptors (Wang et al., 2002; Shu et al., 2012). Pregnane sulfated steroids display diastereoselectivity in antagonism as well.
PS and DHEAS are proconvulsant steroids and can induce seizures when administered systemically or directly into the brain (Reddy and Kulkarni, 1998b; Kokate et al., 1999; Williamson et al., 2004). These pharmacological effects are consistent with their negative modulation effect on the GABAa receptor function (Wu et al., 1991; Majewska, 1992). The proconvulsant actions of PS are evident at concentrations which are 100 to 500-fold higher than its levels in the brain. Thus, it is highly unlikely that endogenous PS by itself can trigger seizures. However, PS can decrease GABAergic inhibitory transmission at physiological concentrations via a presynaptic action (Teschemacher et al., 1997; Mtchedlishvili and Kapur, 2003). Allopregnanolone blocks the seizure facilitating effects of PS and DHEAS, and consequently, these sulfated neurosteroids could contribute to seizure susceptibility when allopregnanolone and THDOC levels are low.
3β-Hydroxysteroids possess interesting properties to negatively modulate GABAa receptors. Previous reports suggested that the 3β-diastereomer of pregnanolone, epipregnanolone, was found to act as a competitive antagonist for the neurosteroid site and this inhibited its ability to potentiate the receptor but displayed no antagonism at the GABA site (Prince and Simmonds, 1992). More recent studies have revealed that the 3β-hydroxy pregnane steroids act noncompetitively with their 3α counterparts, and furthermore act as noncompetitive GABAa receptor antagonists and share similar properties with the sulfated neurosteroids in terms of negative modulation (Wang et al., 2002). Both classes of antagonists block GABAa receptor in conditions of channel opening. 3β-Hydroxysteroids however, inhibit GABAa receptor activity in a manner independent of desensitization kinetics while PS aids in slowing the desensitization of receptors. In this context, PS is a more potent modulator than the 3β-hydroxysteroids (Wang et al., 2007).
(3α,5α)-17-Phenylandrost-16-en-3-ol (17PA) has been classified as a neurosteroid antagonist, displaying selective, negative modulation of 5α-reduced steroids with limited effect on 5β-reduced steroid, and no effect on barbiturate, or benzodiazepine potentiation (Mennerick et al., 2004). The evidence suggests that this compound may competitively antagonize the neurosteroid allosteric sites, explaining specificity of its actions. This antagonism serves to reduce allosteric potentiation of the gated receptor and inhibits the anesthetic effects of neurosteroid on the brain. 17PA exhibits higher affinity for inhibiting allopregnanolone and THDOC over alphaxolone (Kelley et al., 2007). Its diverse modulatory characteristics may be attributed to the heterogeneity of GABAa receptors and subunit configurations that dictate binding affinities for both the positive and negative neurosteroid modulators.
Other steroid metabolites could negatively modulate GABAa receptors through antagonism in reducing channel opening probability. 11-Deoxycortisol induces neuronal excitability in promoting status epilepticus by accelerating the decay time and reducing the amplitude and frequency of IPSCs that are conducted via GABAa receptors (Kaminski et al., 2011). This finding suggests a potent activity of steroids and their precursors whereby 11-deoxycortisol contributes to the dampening of GABAergic activity and could have an underpinning role in epileptogenesis.
There are a wide array of non-steroid antagonists, inverse agonists, and convulsants that also negatively affect GABAa receptor inhibition (Ebert et al., 1997; Rupprecht, 2003). They are typically used pharmacologically to study and contrast the neurosteroid effects on GABAa receptors. Bicuculline and SR95531 (gabazine) are competitive agonists which act on the GABA ligand binding site to block channel activity. Neither bicuculline nor gabazine act as competitive inhibitors of currents that are engendered by allosteric drugs, like alphaxolone or pentobarbital. They cannot fully block current elicited by these types of agents, but they reduce the channel opening probability once alphaxolone or pentobarbital has bound and thus may be classified as inverse agonists to these types of currents (Ueno et al., 1997). The β-carboline class of organic amines also has negative modulatory effect on GABAa receptors due to inverse agonist function. Some agents are able to interfere with receptors based on selectivity to specific receptor subunits or channel location (Ing and Poulter, 2007). L-655,708 is a partial inverse agonist for the α5 subunit with selectivity over a limited concentration range (Quirk et al., 1996). L-655,708 has an intrinsic efficacy for the α5 subtype rather than other α-containing isoforms, enhances long-term potentiation, but it does not display proconvulsant activity at the range of dose selective for α5 (Atack et al., 2006). Furosemide noncompetitively blocks α4/α6 mediated currents, regardless of γ2 or δ subunits (Korpi et al., 1995; Korpi and Luddens, 1997; Bosman et al., 2002). Picrotoxin and other compounds act as noncompetitive antagonists for GABAa receptors, blocking the channel and inducing convulsive effects. Along with the cage compound tert-butylbicyclophosphorothionate (TBPS), picrotoxin has been shown to act at the channel pore-forming M2 domain to block inward chloride ion flux due to specific residues in that region (Jursky et al., 2000; Buhr et al., 2001; Sedelnikova et al., 2006). Negatively functioning agents can be investigated in conjunction with positive potentiating neurosteroids to understand the mechanisms underlying GABAa receptor interaction.
Physiology of neurosteroid interactions with GABAa receptors
Physiological potentiation by neurosteroids
Low nanomolar concentrations of neurosteroid found in the plasma (Wang et al. 1996) and brain are able to substantially potentiate GABAa receptor inhibition in the presence of ambient GABA (Lerma et al., 1986; Lambert et al., 1995). Neurosteroids prolong the decay of IPSCs in CA1 pyramidal cells, dentate gyrus principal neurons, cerebellar granule cells, Purkinje neurons, hypothalamus, and cortex (Lambert et al., 2003). It is shown that 60 nM allopregnanolone is sufficient to induce a hyperpolarizing shift in GABAergic tonic current within mouse DGGCs (Rajasekaran et al., 2010). Patch clamp recordings of hippocampal neurons demonstrate a concentration-dependent enhancement of GABA-activated, hyperpolarizing potentiation by allopregnanolone (Fig.7). The endogenous levels of neurosteroid vary within circulation and the brain and depend on endocrine fluctuations due to physiological changes like development, the menstrual cycle, or stress. However, concentrations are normally greater in the CNS due to de novo synthesis (Reddy, 2003). Levels of neurosteroid in plasma and brain have been estimated to be between 10-300 nM, owing to a variety of physiological and pathophysiological conditions (Belelli et al., 2009). At pharmacological application of 10-1000 nM, neurosteroids allosterically enhance GABAa receptor function in the presence of GABA. However, at greater concentrations reaching 1 µM and above, neurosteroids are able to directly activate the gating of the chloride channel without GABA (Reddy and Rogawski, 2002; Lambert et al., 2009). It is also possible for sub-micromolar levels of neurosteroid to directly gate GABAa receptors at a slower, gradual rate due to accumulation within the plasma membrane (Shu et al., 2004; Akk et al., 2009). Direct activation has interesting implications for therapeutic application of neurosteroid when acute control of excitability is necessary (e.g. status epilepticus). Conditions in which GABA transport and uptake are augmented may also influence extrasynaptic receptor interaction (Madsen et al., 2011). The ability of neurosteroid drugs and similar positive allosteric modulators to affect tonic inhibition also depends on the concentration of extracellular GABA and GABAa receptor occupancy (Houston et al., 2012).
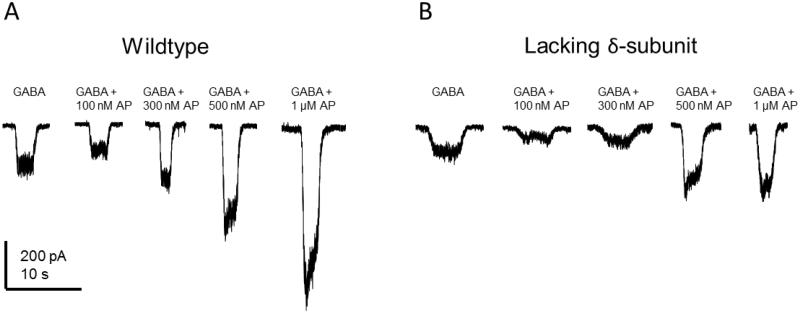
Fig. 7. Comparative efficacy of allopregnanolone on GABA-gated currents in wildtype (A) and δ-subunit knockout (B) mouse dentate gyrus granule cells.
Concentration-dependent responses of allopregnanolone (AP) potentiation of inward chloride current in the presence of 3 μM GABA in DGGCs. Recordings were at -70 mV voltage-clamp, whole-cell patch of in acutely dissociated DGGCs. GABA was first locally applied to the bath solution for 5 seconds to establish a baseline level of gated, inward chloride current. Increasing concentrations of AP were applied for 10 seconds. After 5 seconds of application, GABA perfusion was initiated, resulting in concentration-dependent potentiation by AP. AP alone did not elicit a change in current, however, at 500 nM and 1 μM concentrations, AP application produced a slight inward current.
Recent evidence suggests that allopregnanolone may potentiate presynaptic GABAa receptors, which serves to increase the frequency of spontaneous glutamate release in the hilar neuronal region (Kim et al., 2011). Therefore, neurosteroids may be responsible for presynaptic feedback control on the level of excitability and possibly even modulate tonic current presynaptically as well. Presynaptic GABAa receptors are pharmacologically distinct from extrasynaptic receptors and appear to largely contain γ subunits due to high benzodiazepine sensitivity and low zinc sensitivity (Han et al., 2009).
Within minutes of getting into circulation following systemic administation, neurosteroids enter the brain and modulate neuronal function. The acute effects of neurosteroids involve modulation of GABAergic inhibition, while chronic effects may involve modulation of steroid receptor and related pathways. Neurosteroids rapidly bind and immediately potentiate inhibitory GABAa receptors upon reaching the target neurons. Dynamic changes to GABAa receptor subunit composition provide a separate mechanism and slower time course for adjusting inhibitory inputs over hours to days. This is discussed in greater detail later in the review.
Extrasynaptic GABA
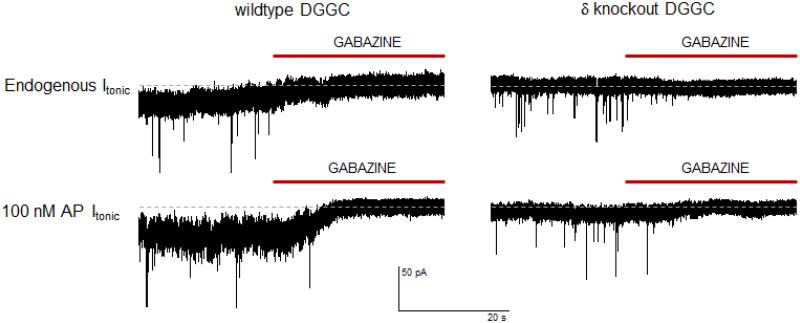
Extracellular concentration of GABA has been predicted to be 0.4 μM at resting membrane potential (−80 mV) and 3.1 μM during depolarization (+20 mV) (Attwell et al., 1993). Extracellular GABA in the rat hippocampus has been measured to be between 0.8 – 2.9 μM using microdialysis (Lerma et al., 1986). GABA transporters likely have an important and dynamic role in controlling extracellular levels of GABA responsible for tonic inhibition (Richerson and Wu, 2003). The prevalent perception is that surplus GABA spillover from vesicular synaptic release contributes to tonic current in neurons that contain extrasynaptic, δ-containing receptors like CGCs and DGGCs (Hamann et al., 2002; Wei et al., 2003; Glykys and Mody, 2007). This is challenged by the recent finding that extrasynaptic populations of receptors in CGCs and thalamic neurons have been shown to be desensitized to synaptic, transient spillover of GABA (Bright et al., 2011). There may be a role in certain neuronal networks for desensitization to high concentrations of GABA to stabilize inhibition, but this desensitization to synaptic release has not yet been observed in other brain regions. While this recent finding interestingly contrasts previously held views, determination of the precise mechanism of GABAa receptor modulation in the presence of spillover GABA and the effect of distance from the synapse on tonic function remains to be explained. The desensitization phenomenon may not be present in all areas where region-dependent tonic inhibition occurs. GABA reuptake may not be as efficient at the perisynaptic and extrasynaptic membrane, and it is reasonable that some GABA escapes into the extracellular fluid. Tonic inhibition proceeds only in the presence of GABA activation, as physiological levels of neurosteroid have not been found to induce receptor channel opening on their own.
Extrasynaptic, α4-containing receptors contain properties that limit the capability of response to prolonged GABA and/or recurring stimulations that result in higher concentrations of GABA (Lagrange et al., 2007). Houston and colleagues report decreases in tonic conductance upon manipulation by lowering the rate of vesicular GABA release (Houston et al., 2012). Sources of ambient GABA have also been proposed to be from interneurons or astrocytes within the cerebellum (Cavelier et al., 2005). This is an alternative view to synaptic spillover based on reports that blocking vesicular release of GABA had no effect on tonic inhibition, but this has not been confirmed in all brain regions with prominent tonic inhibition (Rossi et al., 2003). Reverse uptake could result from astrocytic depolarization by glutamate released from nearby neurons (Attwell et al., 1993). Recent evidence suggests that glial and astrocytic cells may modulate release of GABA and reuptake of extracellular GABA to influence the overall tonic current in CGCs via GABA-permeable channels belonging to a bestrophin family of anion channels residing in the glial membrane (Lee et al., 2010). This finding, however, has been challenged by contrary evidence concerning the ability of Bestrophin1 channels to contribute to GABAergic tonic currents (Diaz et al., 2012).
Subunit property differences
Studies of recombinant GABAA receptor isoforms indicate that neurosteroids act on most subunit configurations (Puia et al., 1990; Brown et al., 2002). This distinguishes neurosteroids from benzodiazepines, which only act on GABAa receptors that contain γ2 subunits and do not contain α4 or α6 subunits. In general, the specific α subunit type may influence neurosteroid efficacy, whereas the γ subunit type may affect both the efficacy and potency of neurosteroid modulation (Lambert et al., 2003). The γ1-containing receptors display a greater potentiating current response to allopregnanolone than γ2-containing receptors (Puia et al., 1993), however, γ1 has a distribution generally contained within the rodent basal and septal forebrain, amygdala, and basal ganglia, and is not found within the hippocampus (Pirker et al., 2000). GABA is a relatively low-efficacy agonist of GABAa receptors containing the δ subunit rather than the more common γ2 subunit, even though it binds with high affinity to such δ-subunit containing receptors (Brown et al., 2002; Glykys and Mody, 2007). These δ-containing receptors possess a significantly higher affinity for GABA than other receptor subtypes, and they are not desensitized in the constant presence of agonist (Saxena and Macdonald, 1994; Mody, 2001). Neurosteroids therefore can markedly enhance the current generated by δ-subunit-containing GABAa receptors even in the presence of saturating GABA concentrations. A recent study using concatemeric receptors expressed in oocytes reported that allopregnanolone and THDOC modulate δ-containing receptors and synaptic-like receptors at similar threshold concentrations (Shu et al., 2012). The study confirms the low-efficacy properties that drive δ-specific current gating. However, individual isoform expression is not representative of the complex composition of receptors on native neurons and does not necessarily reflect regional δ at dendritic locations responding to low, exogenous GABA. Furthermore, extrasynaptic GABAa receptors that contain the δ subunit are highly sensitive to neurosteroid-induced potentiation of GABA responses (Belelli et al., 2002; Wohlfarth et al., 2002; Meera et al., 2009).
There are evident differences in GABAa receptor synaptic strength that are regulated by specific α-subunit isoforms (Ortinski et al., 2004). In comparison with α1βγ2 receptors, α2βγ2 receptors possess faster activation kinetics, slower deactivation kinetics, and prolonged channel opening (Lavoie et al., 1997). A substantial change from α1- to α2-containing receptors within hypothalamus has been previously shown to be associated with slower GABAergic postsynaptic current decay (Brussaard et al., 1997; Brussaard and Herbison, 2000). An α2-dominant population of synaptic receptors could increase net inhibitory synaptic current while exhibiting differential neurosteroid affinity (Maitra and Reynolds, 1999). Herbison and colleagues report changes in allopregnanolone sensitivity coupled with this α-subunit plasticity in the regulation of oxytocin across pregnancy. Subunit plasticity could also have an effect on receptor desensitization in presence of neurosteroid (Smith and Gong, 2005). In study of recombinant receptors, the α2β3γ2 isoform exhibits the lowest GABAa receptor agonist binding affinity but a moderate degree of potency when compared to the other α-containing isoforms; α2β3γ2 also displays the highest binding affinity for the GABAa receptor antagonists gabazine and bicuculline (Ebert et al., 1997). The α-subunit has recently been described to have an important role in specific targeting of GABAa receptors to synaptic versus extrasynaptic sites (Wu et al., 2012). Endogenous neurosteroids may differentially bind and regulate α1- and α2-containing synaptic GABAa receptor function.
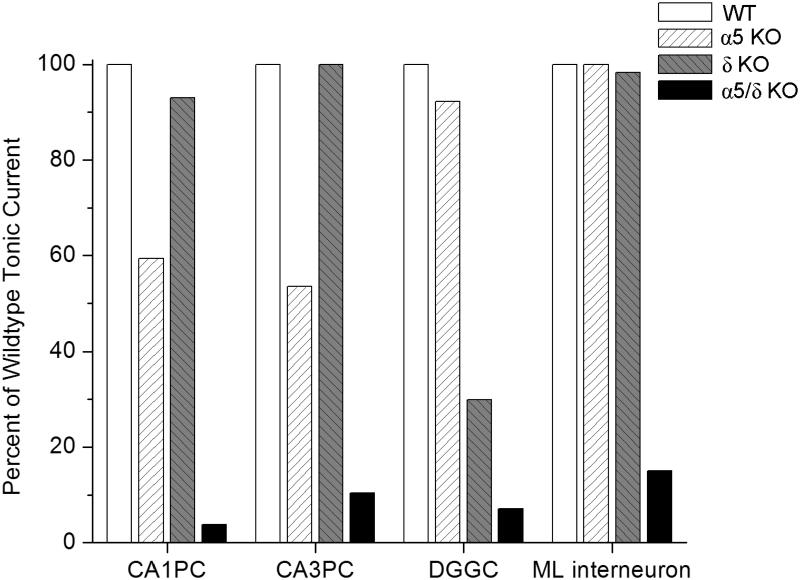
While neurosteroids are able to modulate both synaptic and extrasynaptic receptors (Fig.6), there are clear differences in efficacy between γ-containing and δ-containing receptors, (Pillai et al., 2004) as well as differences between the composition of α subunits (Belelli et al., 2002; see Table 2). The δ-containing receptors produce a much higher maximal response to neurosteroids (Brown et al., 2002). The δ-subunit-containing receptors possess unique functional actions and are found at dendrites, where there are low levels of ambient GABA (Wei et al., 2003; Semyanov et al., 2004). Therefore, receptors that are attuned to extrasynaptic concentrations of GABA can be modulated by exogenous agents like neurosteroids that can finely adjust and potentiate the level of tonic inhibition (Stell et al., 2003; Farrant & Nusser, 2005). Knockout studies reveal that α4δ and α5 subunit-containing receptors mediate the majority of tonic inhibition in DGGC and CA1 hippocampal neurons, respectively (Caraiscos et al., 2004; Glykys et al., 2008). Similarly, neurosteroids have high affinity for the α6δ-containing GABAa receptors, which are responsible for the tonic inhibition within cerebellar granule cells (Hadley and Amin, 2007). Other subunit isoforms may be expressed extrasynaptically and contribute to tonic inhibition, but this would account for a relatively small fraction of total tonic current. Co-expression of different α subtypes with β1 and γ2 delivers relatively little influence over endogenous neurosteroid modulation of GABAergic current (Belelli et al., 2002). In contrast, combinations of different α subtypes with β3γ2S present different degrees of pregnanolone potentiation; α5 isoforms have the greatest potentiation and α1 isoforms have the lowest potentiation (Smith et al., 2001). Interactions of α4-containing receptors with neurosteroid largely depend on adjacent, co-assembled subunits, as α4βδ profiles are more sensitive to neurosteroids than α4βγ receptors (Brown et al., 2002).
TABLE 2.
Neurosteroid and gaboxadol pharmacology for specific GABAAreceptors.
| Drug Interaction | Efficacy | Potency | References |
|---|---|---|---|
| Allopregnanolone & αxβ1γ2 | α4 > α1 > α6 > α5 > α3 > α2 |
α3 > α1 > α2 > α6 > α5 > α4 |
Belelli et al., 2002 |
| Allopregnanolone & αxβsδ | α6 > α4 | α4 > α6 |
Brown et al., 2002; Pillai et al., 2004 |
| THDOC | α1β2/3γ2 > α5β3γ2 | α5β3γ2 ≈ α1β3γ2 | Caraiscos et al., 2004 |
| Gaboxadol | α6β3γ2 > α1β2/3γ2 > α4β3γ2 > α4β3δ > α6β3δ |
α6β3δ > α4β3δ > α6β3γ2 > α4β3γ2 > α1β2/3γ2 |
Saarelainen et al., 2008; Mortensen et al., 2010; Meera et al., 2011 |
At the DGGC synapse, α1 and γ2 display similar distributions when observed with synaptic marker colocalization, however α1 and γ2 are also expressed extrasynaptically in these neurons (Sun et al., 2004). Receptors with γ2 subunit may have a contributing role in tonic inhibition as neurosteroids have a greater effect on α1β1γ2 than α1β1 receptors; moreover, the particular isoform of γ appears to have little or no effect on the degree of modulation (Belelli et al., 2002; Lambert et al., 2003). Consequently, δ-containing receptors participate in a distinctive role to control the excitability of the hippocampus and other neuronal tissues by setting a baseline level of inhibitory current. Evidence continues to emerge that this type of neuromodulation exhibits a great amount of plasticity in the ability of neurons to reconfigure receptor populations on the membrane. Further insight into the composition of GABAa receptor profiles, neurosteroid pharmacodynamic activity, and regional brain inhibition provide meaningful explanations of the underlying modulatory controls intrinsic to GABAa receptor and neurosteroid interaction. Overall, the robust effect of neurosteroids is likely to be due to their action on both synaptic and perisynaptic/extrasynaptic GABAa receptors.
Potency of neurosteroids in seizure models
Allopregnanolone-like neurosteroids are powerful anticonvulsants. Exogenously administered neurosteroids, like other agents that act as positive GABAa receptor modulators, exhibit broad-spectrum anticonvulsant effects in diverse rodent seizure models (Reddy, 2010). Neurosteroids display rapid anticonvulsant activity and provide protection within minutes. Neurosteroids protect against seizures induced by GABAa receptor antagonists, including pentylenetetrazol and bicuculline, and are effective against pilocarpine-induced limbic seizures and seizures in kindled animals (Kokate et al., 1994; Belelli et al., 1989; Frye, 1995; Wieland et al., 1995; Reddy et al., 2004; 2010; Reddy and Rogawski, 2010). Like other GABAergic agents, they may exacerbate generalized absence seizures (Snead, 1998; Citraro et al., 2006). As shown in Table 3, the potencies of neurosteroids in models where they confer seizure protection vary largely in accordance with their activities as positive allosteric modulators of GABAa receptors. Thus, allopregnanolone is roughly equally potent as THDOC, but androstanediol and androsterone are somewhat less potent (Reddy, 2004a; 2004b; Kaminiski et al., 2005).
TABLE 3 Antiseizure potency (ED50values) of neurosteroids in seizure models in mice.
| Seizure Model | Allopregnanolone | THDOC | Androstanediol | References |
|---|---|---|---|---|
| Kindling models: | ||||
| Amygdala kindling | 14 (8–23) | 15 (10–30) | ND | Reddy et al., 2004 |
| Hippocampus kindling | 3.5 | ND | 50 (36-64) | Reddy and Jian, 2010 |
| Electroshock models: | ||||
| Maximal electroshock model | 29 (19–44) | 48 (35–66) | ND | Kokate et al., 1994 |
| 6-Hz stimulation model | 14 (10–19) | ND | ND | Kaminski et al., 2005 |
| Chemoconvulsant models: | ||||
| Pentylenetetrazol | 12 (10–15) | 19 (77–122) | 40 (27–60) |
Kokate et al., 1994; Reddy, 2004a,b |
| Bicuculline | 12 (10–15) | 12 (10–15) | 44 (24–81) | Reddy, 2004a b |
| Picrotoxin | 10 (5–19) | 10 (5–19) | 39 (21–74) | Reddy, 2004a b |
| N-methyl-D-aspartate | >40** | >40** | >200** |
Kokate et al., 1994; Reddy, 2004a,b |
| 4-Aminopyridine | >40** | >40** | >200** |
Kokate et al., 1994; Reddy, 2004a,b |
| Status epilepticus models: | ||||
| Pilocarpine | 7 (4–13) | 7 (4–13) | 81 (45–133) | Kokate et al., 1996; Reddy, 2004a |
| Kainic acid | >40** | >40** | >200** | Kokate et al., 1996; Reddy, 2004a,b |
| Behavioral models: | ||||
| Sedation & ataxia | >30 | >30 | 146 (96-226) |
Kokate et al., 1994; Reddy, 2004a,b |
| Anesthesia | 30 (20-42) | >40 | >200 |
Reddy and Apanites, 2005; Reddy and Zeng, 2007 |
| Anxiolytic | (2-5) | (7.5-15) | ND | Reddy and Kulkarni, 2007; Reddy et al., 2005b |
The potency of neurosteroids is expressed in terms of ED5, which is the dose in mg/kg producing seizure protection in 50% of animals. Values in parentheses are 95% confidence limits. ND, not determined.
Considered as inactive because of such high (sedative or anesthetic) doses.
Like other GABAergic agents, neurosteroids are inactive or only weakly active against seizures elicited by maximal electroshock. Neurosteroids are highly active in the 6-Hz model, a better paradigm in which limbic-like seizures are induced by electrical stimulation of lower frequency and longer duration than in the maximal electroshock test (Kaminiski et al., 2004). Androstanediol, but not its 3β-epimer, produced a dose-dependent suppression of behavioral and electrographic seizures in the mouse hippocampus kindling (Reddy and Jian, 2010). The estimated concentrations of androstanediol producing 50% seizure protection in the kindling model (~10 μM) are within the range of concentrations that potentiate GABAa receptor function in CA1 pyramidal neurons. Neurosteroids are also highly effective in suppressing seizures due to withdrawal of GABAa receptor modulator drugs including neurosteroids and benzodiazepines, and also due to other types of agents such as ethanol, which may act in part through GABAa receptors, and cocaine, which does not (Tsuda et al., 1997; Devaud et al., 1996; Reddy and Rogawski, 2000a; 2001; Reddy et al., 2012).
Lack of tolerance
Benzodiazepines exhibit pharmacodynamic tolerance during prolonged use. In contrast to benzodiazepines, where utility in the chronic treatment of epilepsy is limited by reductions in efficacy over time, anticonvulsant tolerance is not obtained with neurosteroids (Kokate et al., 1998; Reddy and Rogawski, 2000b). Thus, neurosteroids have the potential to be used in the chronic treatment of epilepsy, and this has also been evident in clinical trials (Nohria et al., 2010). The mechanisms responsible for tolerance to benzodiazepines are not known. However, factors such as uncoupling of the allosteric linkage between the GABA and benzodiazepine sites and changes in receptor subunit turnover with switching of subunits may be contributing mechanisms (Bateson, 2002). Neurosteroids do not act on the benzodiazepine site of GABAa receptors, and they are able to modulate all isoforms of GABAa receptors, even those that contain benzodiazepine-insensitive α4 and α6 subunits or do not include the obligatory γ2 subunit required for benzodiazepine sensitivity. Surprisingly, chronic neurosteroid exposure does lead to cross tolerance to benzodiazepines (Reddy and Rogawki, 2000b). Thus, it appears that the same plastic changes that underlie benzodiazepine tolerance are brought into play by chronic neurosteroid exposure. However, neurosteroids act at distinct sites on GABAa receptors and exhibit effects on the full range of GABAa receptor isoforms. Therefore, the potentials side effects of neurosteroids are mostly related to their GABAergic effects (Reddy, 2013b).
GABAa receptor pharmacology and allosteric interactions
The positive or negative modification of GABAa receptors by allosteric modulators depends on their structural features. The affinity and efficacy define their overall profile. In study of the allosteric interaction of neurosteroids on receptors, an accurate understanding of the various pharmacopore interactions on isoforms of GABAa receptors is essential. Specific binding sites and overlap between pharmacological features are useful in examination of the neurosteroid pharmacopore, which is less clear than most of the other GABAa receptor modulators.
Neurosteroid interactions
While there have been early attempts to determine binding patterns for neurosteroids, the true ligand-binding properties for neurosteroid interaction remain unclear. Neurosteroids have a distinguishable binding sites from benzodiazepines, which act only on GABAa receptors containing γ2 subunits and those that do not have α4 or α6 subunits assembled. Neurosteroids have been shown to possess a different binding site from barbiturates, though they share various similarities in receptor-mediated functions. The lipophilic properties of neurosteroids influence potency and affinity for GABAa receptors (Chisari et al., 2009). Molecular evidence shows that a C3α-hydroxyl group and a C20 position ketone group on pregnane neurosteroids or a C17 ketone on androstane neurosteroids are essential for binding affinity (Mitchell et al., 2008). This suggests specificity in a binding pocket and interaction with certain residues yet to be clarified. Although the exact location of neurosteroid binding has not been mapped, it has been proposed that there are two distinct sites for neurosteroids that act as positive modulators: one for allosteric enhancement of GABA and another for direct activation of the receptor, as well as an antagonistic transduction site for sulfated neurosteroids involving the M2 region of the α subunit (Akk et al., 2001; Lambert et al., 2003; Hosie et al., 2006; 2007). Using site-directed mutagenesis, it has been shown that a highly conserved glutamine at position 241 in the M1 domain (toward the intracellular side) of the α subunit plays a key role in neurosteroid modulation of GABA responses and is believed to contribute to the binding site for modulation (Hosie et al., 2009). Additional nearby residues in the M4 domain of the same α subunit (tyrosine 410 and asparagine 407, toward the extracellular side) have also been proposed to contribute to the binding site.
Other investigators have found that mutations in serine 240 and tryptophan 245 of the α subunit interfere with neurosteroid potentiation. Multiple studies with structurally diverse steroids have led to the conclusion that the steroid binding pocket on the α subunit is more correctly viewed as a hydrophobic surface that can accommodate steroid molecules of different structures (Akk et al., 2009). Direct activation of the receptor, in contrast, has been proposed to be due to steroid binding at a site on the interface between β and α subunits formed by a threonine at position 236 in the α subunit and a tyrosine at position 284 in the β subunit (Hosie et al., 2007). However, more recent models of the GABAa receptor have questioned whether these residues reside at the β–α subunit interface. A photo-incorporable analog of the anesthetic etomidate appears to bind at the interface, but binding of this ligand is not competitively inhibited by neurosteroids (Li et al., 2009).
The β subunit has been less prominently studied than α subunit in regard to neurosteroid binding. Early reports suggested that the β subunit does not appear to possess an influential role in neurosteroid interaction with GABAa receptors (Lambert et al., 2003). However, β2 subunits are preferentially assembled in extrasynaptic channels with high neurosteroid affinity and β2 deficiency reduces tonic inhibition, while β3 subunit-containing receptors are mostly synaptic, based on dentate gyrus investigation (Herd et al., 2008). Manipulation of the M1 domain of the β2 subunit has an effect on the spontaneous activity of extrasynaptic receptors (Baker et al., 2010). The extracellular domain loop 9 of the β2 subunit has been shown to steady the closed-state gate of the α1β2γ2S synaptic GABAa receptors, and this has indirect consequences on receptor efficacy in GABA activation and the allosteric modulation by propofol and pregnanolone (Williams et al., 2010). A recent report identified a novel β3 subunit transmembrane domain involved in neurosteroid binding (Chen et al., 2012). While β isoforms have been found to influence tonic current (Herd et al., 2008), it is still unclear if they significantly affect neurosteroid modulation.
The δ subunit is integral to the high sensitivity of neurosteroid-receptor interaction (Brown et al., 2002); it is not germane to the actual neurosteroid binding site for opening of the channel but rather is involved in the transduction of the gated response subsequent to initial binding (Hosie et al., 2009). Channels of α4β2δ do not differ in GABA potency from channel pentamers composed of α4β2 without a third subunit type (Mortensen et al., 2012). Moreover, receptors modified to contain a single functional GABA agonist site and/or a single proposed allosteric site for steroidal binding are adequate in eliciting potentiation by allopregnanolone, yielding to the potent sensitivity of neurosteroid activity on receptors (Bracamontes et al., 2011). While this recombinant GABAa receptor study was conducted to disassociate the two known sites for neurosteroid binding, the physiologically existent receptors have much more diverse kinetics relative to direct and allosteric neurosteroid binding, and the details surrounding those binding targets remain unclear.
The δ-subunit does however, possess an intrinsic property within receptors to increase channel opening probability in potentiating current. Therefore, the α-β interface without δ may permit neurosteroid affinity, but the δ subunit incorporation provides a potentiating shift for longer channel opening and gating of current (Bianchi et al., 2003). Mutation in the M2 region of δ-containing receptors decreases the potency of THDOC and the positive modulator tracazolate, which typically potentiate GABAa receptors at low efficacy (Zheleznova et al., 2008). In addition, the S238-V264 domain of the δ subunit (including M1 and the intracellular loop connecting to M2) has been identified to influence the high level of agonist sensitivity to α4β3δ receptors apparent in both measures of efficacy and potency (You and Dunn, 2007). Binding of receptor does not likely occur at these δ subunit domains by either GABA or neurosteroid, but these domains nevertheless have profound transducing effects on channel gating.
Despite the fact that neurosteroid function diverges from the classical steroid role of diffusion across the membrane to act through nuclear receptors, the lipophilic structure of neurosteroids permits their movement in cells and the plasma membrane similarly to other steroids. Potentiation of GABAa receptor channels occurs upon application of allopregnanolone to the inner leaflet of transfected membrane in an inside-out patch configuration (Akk et al., 2005). In conjunction with other data showing the ability of neurosteroids to reach GABAa receptors through lateral diffusion or from intracellular paths, this substantiates that neurosteroids may be able to potentiate current through routes besides extracellular access which is representative of most ligand-gated ion channels (Akk et al., 2009). Access via a hydrophobic pocket of the receptor within the plasma membrane bilayer therefore remains a feasible biochemical explanation for neurosteroid binding.
Benzodiazepine interactions: Comparison with neurosteroids
Benzodiazepines non-selectively and allosterically bind to GABAa receptors containing the α1, α2, α3 or α5 subunits and thereby potentiate synaptic GABAergic inhibition. They have been in clinical use for decades and are still among the most widely prescribed drugs for the treatment of insomnia, anxiety, epilepsy, and status epilepticus. However, their use is limited by side effects such as sedation, cognitive impairments, tolerance and the risk of drug dependence. Due to their wide array of differential binding affinity, benzodiazepine-site interactions with GABAa receptors are quite dynamic in comparison to neurosteroid allosteric interactions.
GABAa receptors have a separate allosteric binding site for the benzodiazepines located at the α-γ interface, which is an altogether distinct site from the barbiturate binding interface as well (Sancar and Czajkowski, 2011). Thus, for each γ-containing receptor, there is one ligand binding site for benzodiazepine molecules. The pharmacology is mainly governed by α and γ subunit isoforms at the binding pocket and has been well classified through classical 1,4-benzodiazepine study. Furthermore, α subunit diversity has been implicated for the varying anticonvulsant, anxiolytic, or anesthetic activities by diazepam and similar benzodiazepines on GABAa receptors (Rudolph and Mohler, 2004; Olsen and Sieghart, 2009). Representative of α subunit heterogeneity, zolpidem binds with high affinity for receptors with α1, partial agonism for α2 and α3, and low affinity for α5 (Smith et al., 2001). Although not a benzodiazepine itself, zolpidem is an imidazopyridine which binds to GABAa receptors in the same allosteric location as benzodiazepines and is functional only on receptors with γ2 subunits (Wulff et al., 2007).
Ro 15-1788 (flumazenil, benzodiazepine antagonist), Ro16-6028 (bretazenil, partial agonist) and Ro15-4513 have been shown to have high affinity at α4 and α6 receptors that are otherwise insensitive to diazepam and imidazenil (Wisden et al., 1991; Knoflach et al., 1996; Wafford et al., 1996; Benke et al., 1997). In addition, flumazenil and Ro15-4513 modify the efficacy of α4 and α6 receptors to act as positive allosteric modulators. These compounds differ from 1,4-benzodiazepines, which only bind to GABAa receptors containing either α1, α2, α3, or α5 subunits. Ro15-4513 is a partial inverse agonist that binds to the α-γ benzodiazepine site of GABAa receptors (Benson et al., 1998). It has high binding affinity and efficacy for modulating GABAergic current of α4β3γ2 receptors, but does not directly modify or affect α4β3δ channel activity (Brown et al., 2002). A recent autoradiography study reported that α4/6β3δ receptors also have a high-affinity binding site for Ro15-4513 (Hancar et al., 2006). DGGC tonic current enhancement by Ro15-4513 is signified by decline of its potentiating effects in α4 knockout mice (Liang et al., 2008). These findings expand on the fact that γ-containing receptors are located extrasynaptically as well, albeit in lower quantities.
Receptors containing α4 or α6 subunits are insensitive to classical 1,4-benzodiazepines, and interestingly, receptors with these α isoforms are most commonly found in extrasynaptic receptors. Chimeric GABAa receptor mutations have confirmed that specific residues in the α-subunit impart altered binding affinity that affects benzodiazepine sensitivity (Derry et al., 2004). The receptor structure fits a motif which functionally divides receptors not only based on location relative to the synapse, but substrate-ligand interface affinity as well. Generally, receptors containing α1, α2, α3, or α5 subunits are diazepam-sensitive whereas receptors containing α4 or α6 subunits are diazepam-insensitive. This information suggests that receptor subtype-selective compounds could overcome the limitations of classical benzodiazepines such as sedation. Subtype-specific agents are undergoing clinical studies as non-sedative anxiolytics (Rudolf and Knoflach, 2011). In contrast to benzodiazepines, neurosteroids exhibit preferential modulation of extrasynaptic α4/δ-containing receptors.
GABA site agonist interactions
It is generally accepted that GABA binds to the α-β interface to allow channel opening such that two GABA molecules may gate the receptor, given that each receptor has two α-β subunit interfaces. The two α-β interfaces differ in local environment based on the adjacent subunits (γ and β or α and γ) and therefore display some differences in agonist preferences (Baumann et al., 2003). Muscimol is a potent agonist for the GABA binding site, and is often used to quantify pharmacology regarding allosteric binding of GABAa receptors. 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (gaboxadol; THIP) is a hypnotic agent and a partial agonist for GABAa receptors with a higher selectivity for δ-containing receptors (Ebert et al., 1997; Wafford and Ebert, 2006; Herd et al., 2008), but displays different degrees of agonist binding that is dependent on the α subunit isoform incorporated (Stórustovu and Ebert, 2003). The involvement of the δ-subunit in GABAa receptors confers a high gaboxadol potency (Meera et al., 2011). GABA and gaboxadol have higher potency on α4β3δ than α4β3γ2L recombinant receptors expressed in Xenopus oocytes (You and Dunn, 2007). Furthermore, gaboxadol has a higher efficacy than GABA for δ-containing receptors, but gaboxadol has lower efficacy than GABA for γ2-containing receptors (Stórustovu and Ebert, 2006; Saarelainen et al., 2008). As potent receptor ligands, gaboxadol and muscimol exhibit different degrees of agonist response in interaction with extrasynaptic GABAa receptors. Gaboxadol is known to selectively enhance extrasynaptic function with high potency at α4β3δ and α6β3δ, which display similar extrasynaptic functional properties (Brown et al., 2002); high concentrations of gaboxadol on α4β3δ receptors allow for longer and more frequent channel openings, while muscimol binding reduces the desensitization of the receptor (Mortensen et al., 2010). In comparison of α4β2δ and α4β3δ receptors, there does not seem to be a significant difference in the potency of GABA binding, signifying a less imperative role for β subunit variety to dictate agonist binding properties (Mortensen et al., 2012).
Other pharmacological ligands
The pervasive and abundant amino sulfonic acid, taurine, has been detailed as a partial agonist at GABAa receptors. Taurine also enhances GABAergic tonic current inhibition and displays pharmacological properties that are consistent with extrasynaptic, δ-containing receptors (Jia et al., 2008). The possibility of interplay between taurine and neurosteroids in the brain has implications in GABAa receptor plasticity and functional characteristics. Taurine may change αβγ receptors to become low-efficacy, and in effect alter binding patterns to allow for neurosteroid to selectively bind and potentiate receptors that are primarily synaptic in location (Bianchi and Macdonald, 2003). In a similar manner, β-alanine increases the efficacy of αβδ receptors; altering concentrations of this amino acid can reduce neurosteroid selectivity for extrasynaptic receptors, having the greater effect of reduced baseline inhibition. Induction of seizure in rats with the convulsant pentylenetetrazol increases the levels of taurine in the hippocampus. In addition, these animals exhibit decreased extracellular levels of GABA, involving a shift in excitatory/inhibitory amino acid levels that could have profound effects on the overall neuronal circuitry and channel current (Szyndler et al., 2008).
Transition metal zinc cations (Zn2+) have a potent ability to inhibit GABAa receptor function. GABAa receptors exhibit differing sensitivities to Zn2+, which can negatively modulate the receptors through noncompetitive antagonism. There are three sites that coordinate Zn2+ interaction including one within the ion channel, and two sites on amino-terminal residues on the αβ interface; for this reason, zinc potently inhibits subunits of αβ and αβδ composition with high sensitivity while αβγ isoforms have a lesser amount of interaction and sensitivity (Nagaya and Macdonald, 2001; Hosie et al., 2003). GABAa receptors responsible for tonic inhibition are highly sensitive to blockade by Zn2+ (Mortensen and Smart, 2010). Based on Timm’s staining for heavy metals, zinc is pervasive throughout hippocampal mossy fibers which project from DGGCs to the hilus and CA3 regions. Endogenous Zn2+ is able to mediate GABAergic inhibition in mossy fiber regions (Ruiz et al., 2004). This leads to a rational assertion that Zn2+ may be homeostatically controlled in the hippocampus where neurosteroid levels are tightly regulated as well. Negative modulation by endogenous Zn2+ can differentially effect GABAergic inhibition and can be used to further understand neurosteroid modulation.
Electrophysiological basis of GABAergic tonic inhibition
GABAergic channel kinetics
The pharmacological modulation of GABAa receptors is measured by inhibitory current and channel kinetics, which provide important details to understand the differences between phasic and tonic receptors. Neurons containing highly expressed levels of receptors involved in tonic current have distinct, unique properties from phasic receptors (Stell and Mody, 2002). Single channel patches of neuronal membrane receptors possess channel gating properties ascribed to sensitivity and comparatively fast recovery of inward current. Synaptic inhibition is characterized by biphasic (fast followed by slow) desensitization and delayed deactivation of the channel’s inward current in response to GABA, and this is typified by the αβγ population of receptors which are prominent at the postsynaptic membrane. In contrast, extrasynaptic receptors are exemplified by a single phase of decay, more rapid deactivation, and a much lesser degree of desensitization to GABA-induced current (Bianchi et al., 2001; Brown et al., 2002). Specific residues of the δ subunit M1 domain and N-terminus are necessary for the observed extrasynaptic desensitization kinetics, and mutations in the same region of γ2L result in increased fast deactivation (Bianchi et al., 2001). Endogenous neurosteroids modify neuronal GABAa current by increasing the channel opening duration, prolonging slow deactivation and delayed recovery from desensitization (Zhu et al., 1997; Wohlfarth et al., 2002).
GABA transport and reuptake
Since extracellular GABA is responsible for setting the inhibitory tone, GABA transport and its maintenance within brain has large bearing on the control of tonic current. There are four GABA transporters, GAT-1, GAT-2, GAT-3, and BGT-1. GAT-1 is the most well studied in conjunction with rodent models. Knockout of GAT-1 in mouse cerebellum produces adverse motor disability and tremors as well as increased tonic conductance in cerebellar neurons (Chiu et al., 2005). Depletion or inhibition of GAT-1 raises levels of GABA, and this has been explored as a therapeutic possibility for anxiolytic, depressive, and epileptic disorders (Kristensen et al., 2011). Tiagabine is a GAT-1 selective inhibitor which does not alter the anticonvulsant or behavioral effects of gaboxadol, but EF1502, which has broadened selectivity for inhibiting GAT-1 and BGT-1, results in a decreased anticonvulsant effect in mice (Madsen et al., 2011). The GABA transporters may serve roles beyond recycling GABA into the axon terminal for vesicular release. Transporters also display a significant role in modulating the level of baseline tonic current in the dendritic regions of the neuron. Furthermore, neurosteroids and allosteric modulators of tonic current undergo regulation of receptor binding sensitivity due to GABA transporters (Fleming et al., 2010). Altered sodium electrochemical gradients may contribute to ambient extracellular GABA by reversing the direction of GAT-1 transport (Attwell et al., 1993; Wu et al., 2006, 2007). In addition, GABA synthesis itself is significant in extrasynaptic function, as lack of GAD65 reduces tonic inhibition (Walls et al., 2010).
Neurosteroid affinity/efficacy for extrasynaptic receptors
GABAa receptor-mediated tonic currents rely on a mechanism of control derived from channel properties and other exogenous stimuli (McCartney et al., 2007; Ransom et al., 2010). Certain neuronal regions exhibit high degrees of tonic inhibition determined by channel opening kinetics, even though tonic inhibition is observed throughout the brain. Neurosteroids have been shown to selectively potentiate GABAa receptor inhibition in a fashion independent to subunit composition, as revealed by switching the polarity of efficacy in αβγ and αβδ populations (Bianchi and Macdonald, 2003). The intrinsic low-efficacy activity of physiological δ-containing receptors is exemplified by brief channel openings and low levels of desensitization. The low-efficacy characteristics enable neurosteroids to increase the mean channel open time in their potent modulation of the receptor.
Neurosteroids acting on α4β3δ receptors displays a greater efficacy than on α4β3γ2 receptors (Brown et al., 2002). Mutation of residue L286S of the δ subunit modifies GABAa receptors to higher efficacy and channel characteristics similar to that of γ-containing, synaptic receptors (Zheleznova et al., 2008). Furthermore, differences in gating are expressed through altering α subunit isoforms. With a saturating concentration of GABA (1mM), α1β3δ channels desensitize slowly, deactivate rapidly, and exhibit peak-amplitude voltage-dependence. In contrast, α6β3δ channels have voltage-dependent desensitization and slower deactivation but do not show rectification (Bianchi et al., 2002). Therefore, it is not necessarily the subunit composition per se that confers the neurosteroid ability to modulate tonic current, but it is the channel efficacy properties of subunit pharmacore that confers preferential targeting by endogenous neurosteroids to potentiate GABA inhibitory function. As neurosteroids interact with extrasynaptic GABAa receptors, the high-affinity binding increases gating efficacy and is manifested as potent inhibitory function.
Tonic current inhibition in the brain
Tonic current is typically quantified through whole-cell, voltage-clamp recording of a brain slice preparation. Inhibitory activity is represented in terms of conductance which is normalized by cell membrane capacitance (pS/pF). This allows for control of variability based on the membrane surface area contributing to overall capacitance. Baseline currents are initially measured and averaged from epoch increments to signify GABAergic mediated current. Saturating concentrations of GABAa receptor antagonist bicuculline or gabazine (SR-95531) are then applied to the recording chamber to identify the loss of current due to blocking of the ion channels. Extrasynaptic channels have been previously classified as relatively insensitive or resistant to gabazine due to inability to inhibit GABAa receptors that are active in the absence of GABA (Bai et al., 2001; Yeung et al., 2003; McCartney et al., 2007). However, this data was based on pyramidal cell tonic currents, which display lower levels of chloride conductance than δ-containing DGGCs or CGCs, and gabazine antagonism is largely concentration-dependent (Stell and Mody, 2002; Stell et al., 2003; Houston et al., 2012). Upon pharmacological antagonism, the change in current from active to blocked condition is quantified to calculate the tonic conductance for the cell. The mean tonic inhibition detected is typically several-fold greater than the mean spontaneous IPSC mediated current (Farrant and Nusser, 2005). GABA transporter and uptake blockers, such as the GAT-1 inhibitor NO-711 may maintain or increase exogenous levels of GABA and may even enhance tonic inhibition without altering phasic inhibition (Nusser and Mody, 2002). Ionotropic glutamate channel antagonists are necessary in the external perfusion solution to block glutamateric receptor currents from obfuscating the chloride current recordings.
Extracellular concentrations of extrasynaptic GABA have been estimated to be approximately 160 nM in slice preparations (Santhakumar et al., 2006), which is significantly lower than in vivo determinations (Lerma et al., 1986). Most slice recording preparations incorporate a low effective concentration of GABA (1-3 μM) into the perfusion solution to establish GABAergic holding current for the cell, reflecting a physiological level of ambient GABA around the neuron but not so great a concentration that would elicit receptor desensitization. However, establishing a baseline current before applying GABA and subsequent antagonist introduces a more eloquent method for observing a shift in the holding current for deciphering tonic inhibitory activity. Sanna and colleagues demonstrate this method by establishing a baseline for 5 minutes, applying GABA to induce a shift in current, and thereafter the GABAergic current is blocked by adding bicuculline (Sanna et al., 2009). Therefore, in comparing ambient GABA-activated current and neurosteroid potentiation of the receptors, the foundational baseline current may be ascertained before the inclusion of modulatory agents (Fig.6). In effect, this allows for channel conductance and kinetics to be compared across experimental conditions before GABA or allosteric binding and before channel receptor antagonism.
There are many variables to consider during the process of slice electrophysiology that may have influential effects on the recorded neurons. Specific GABA concentrations employed, temperature, age of the neurons, chloride gradients, and oxygenation conditions of the slice preparation can all have substantial effects on recordings (Glykys and Mody, 2007; Succol et al., 2012). Hypoxia can significantly affect the health of the neuron being patched. In hypoxic conditions within the tissue, the extracellular volume is reduced, and this increases concentrations of neuroactive chemicals within the fluid, altering functionality of receptors and regulatory molecules alike. GABA transporter and reuptake molecules also contribute to fluctuations within the slice, as their activity or attenuation can significantly change the inhibitory faculty of GABAergic neurons (Chiu et al., 2005). The range of physiologically observed tonic inhibition is highly sensitive as well. The degree of GABAa receptor tonic modulation is centrally affected by the concentration of neuroactive agent applied and the GABAa receptor profile from the neuron being recorded. Membrane depolarization has recently been shown to influence tonic current GABAa receptor potentiation. Rapid increase in tonic conductance was observed following depolarization of cultured, hippocampal neurons, exhibiting voltage-dependent properties of GABAa receptors and current independent of ambient GABA or inward anion accumulation (Ransom et al., 2010). Postsynaptic, metabotropic GABAB receptors enhance extrasynaptic currents of δ-containing GABAa receptors in DGGCs (Tao et al., 2013).
While the pharmacological properties governing tonic and phasic current are distinct, the precise attributes that confer their divergence are not well understood. The pharmacological tools that are able to selectively modulate GABAa receptors are limited by their degree of sensitivity to the receptor subunit binding. Receptor conformation may be assessed based on molecular assays and known agonist pharmacology for a receptor profile, but as of yet there is no devised way to isolate extrasynaptic receptors (primarily of the δ subunit) by pharmacology alone. AA29504 is a novel positive modulator with strong δ subunit specificity that may possess some utility in further understanding modulation of synaptic versus extrasynaptic currents (Vardya et al., 2012). The heterogeneity of GABAa receptors remains an obstacle to accurate delineation of receptor physiology in vivo.
Functional characteristics of δ-containing receptors
δ Knockout physiology
The δ subunit knockout mouse model allows for the disambiguation of properties influencing GABAergic tonic current. Not only do δ-containing GABAa receptors possess distinctive pharmacology, they possess unique current-gating properties that play a role in controlling neuronal excitability through transduction of the inhibitory chloride channels. The global δ knockout mouse was first created and described by Mihalek and colleagues in 1999, but at that time little was known about the extent of neurosteroid interaction with δ-containing GABAa receptor isoforms. The construction and classification of the δ knockout was seminal in discerning the true functionality of neurosteroids at GABAa receptors. The initial knockout demonstrated that removal of the subunit from receptors substantially diminishes neuronal sensitivity to neurosteroids (Mihalek et al., 1999). Alphaxolone potentiation is lessened in δ knockouts, accompanying reduction in hippocampal levels of the α4 subunit (Spigelman et al., 2003). Allopregnanolone exhibits diminished dose-dependent modulation of GABAa receptor currents in DGGCs of δ knockout mice (Fig.7). The hypnotic activity of gaboxadol is reduced when administered to knockout mice as quantified by behavioral tests, signifying high affinity for gaboxadol at δ-containing receptors (Boehm et al., 2006; Meera et al., 2011). The δ knockout mice display lower behavioral impairment and reduced binding sensitivity to muscimol, the high-affinity agonist for GABAa receptors (Chandra et al., 2010). These findings provide pharmacological and behavioral evidence of GABAa receptor sensitivity based on discrete subunit composition profiles. The δ specific profiles confirm shifts in channel gating due to endogenous neurosteroid modulation (Wohlfarth et al., 2002), which is attenuated in the δ-deficient model (Stell et al., 2003).
Knockout constructs and electrophysiology have revealed that DGGCs and CGCs have high levels of GABAa receptors incorporating δ subunit into configuration (Pirker et al., 2000; Peng et al., 2002). The combination of knockout studies, electrophysiological parsing of current properties, and immunochemistry specific to DGGCs has shown that α4βδ extrasynaptic receptors comprise a majority of the tonic current observed in these cells (Sun et al., 2004; Chandra et al., 2006; Herd et al., 2008). While the α4βδ receptor composition accounts for a large portion of the observed tonic current, other compositions including α5γ2 or α6δ combinations in CA1 or CGCs, respectively, are located extrasynaptically as well and likely contribute to tonic current (Semyanov et al., 2004; Glykys and Mody, 2007). The α5-containing receptors are responsible for a minor residual tonic current in DGGCs of δ knockout animals (Glykys et al., 2008). In addition, dentate gyrus molecular layer interneurons possess α1βδ receptors (Glykys et al., 2007). Concomitant with δ knockout characteristics, α4 knockout mice display decreased GABAergic potentiation by alphaxolone in DGGCs (Liang et al., 2008). This mirrors physiological deficits of neurosteroid potentiation in mice receptors lacking δ subunits and provides evidence for neurosteroid selectivity for certain subunit compositions to modulate the receptor function.
Mice deficient in δ subunit also exhibit pathologically relevant changes in conjunction with reduced neurosteroid sensitivity. The GABA antagonist pentylenetetrazol administered to mice elicited a significantly greater number of clonic seizures in knockouts compared to control animals (Mihalek et al., 1999; Spigelman et al., 2002). This indicates a higher degree of acute seizure susceptibility marked by loss of δ-subunit-mediated tonic inhibition. Hippocampus kindling has not yet been implemented with the δ knockout model to further typify brain excitation due to limbic epileptiform activity. Furthermore, DGGCs from knockouts demonstrate faster current decay rates of mIPSCs and evoked IPSPs, but there are no observed changes in frequency, rise time, or amplitude (Spigelman et al., 2002). DGGCs from δ knockout mice have highly attenuated tonic current (Fig.8), and α5/δ double knockout mice effectively lack any tonic inhibitory current within hippocampal neurons (Stell et al., 2003; Glykys et al., 2008, Fig.9). CA1 Pyramidal cells possess GABAa receptors with a lower amount of δ subunit compared to DGGCs, (Peng et al., 2004) and the relatively low tonic conductances are not changed between wild-type and δ knockout mice (Stell et al. 2003; Maguire et al., 2005). The δ-containing GABAa receptors have high sensitivity to Zn2+, and wild-type mice DGGCs experience reduced spontaneous IPSC decay time in response to ZnCl2 application. In contrast, spontaneous IPSC decay time is not affected by 10 μM ZnCl2 in δ knockout neurons (Saxena and Macdonald, 1996; Wei et al., 2003). Knockout mice have been reported to develop generalized, spontaneous seizures (Olsen et al., 1997). These findings hint at a reduced ability of the available GABAa receptors in the brain to control excitability, owing to an important niche role for δ-containing receptors.
Fig. 8. Comparative analysis of allolpregnanolone potentiation of tonic currents in wildtype and δ-subunit knockout mouse hippocampus slices.
Tonic current recordings were made from DGGCs in 300 μM slices at —65 mV voltage-clamp recording. ACSF perfusion contained tetrodotoxin, AP5 (2R-amino-5-phosphonopentanoate), and DNQX (6,7-dinitroquinoxaline-2,3-dione) to block voltage-gated Na+ channels and glutamatergic currents to isolate GABAergic signal. No exogenous GABA or GABA transport blocker was included in this perfusion preparation. After holding current level was established in the presence of no endogenous GABAergic agent or 100 nM allopregnanolone, 50 μM gabazine (SR-95531) was applied to block all GABAa receptor currents. Root mean square noise is reduced and phasic IPSCs are blocked in the presence of gabazine in DGGCs. A noticeable shift in tonic conductance is observed in wildtype but not in δ knockout DGGCs. The gray dotted line indicates the mean holding level upon gabazine blockade of tonic current.
Fig 9. Relative contribution of δ and α5 subunits to tonic current in various hippocampus subfields as demonstrated using GABAa receptor subunit knockout mouse models.
Graphical representation of relative contributions of δ and α5 subunits to tonic current in CA1 pyramidal cells (CA1PC), CA3 pyramidal cells (CA3PC), dentate gyrus granule cells (DGGC), and molecular layer interneurons (ML interneuron). Values are average percent of tonic current (pA) recorded in wildtype and knockout mouse models (Glykys et al., 2008).
δ Subunit expression in the hippocampus and modulation by neurosteroids
The expressional differences of δ subunit between DGGCs and CA1 pyramidal cells may reveal an important key to understanding extrasynaptic receptor activity and function. The estrous cycle serves as a useful model because tonic inhibition changes are dependent on cell-type and receptor-type specificity. Due to changes in steroid hormone levels during the estrous cycle, allopregnanolone levels and their interaction with GABAa receptors undergo plasticity, albeit differently in DGGC and CA1. This modulation is exhibited by alterations in neuron electrophysiology between estrus and diestrus. DGGC tonic conductance is increased in diestrous phase, but no cycle-dependent changes in conductance are observed in CA1 pyramidal cells (Maguire et al., 2005, Wu et al., 2013). Whole-cell, GABAergic currents from native, adult rat DGGCs are highly sensitive to positive modulation by allopregnanolone and negative modulation by PS (Mtchedlishvili et al., 2003). Tonic conductance in DGGCs is mediated by δ-containing GABAa receptors and is sensitive to neurosteroid, however tonic conductance in CA1 pyramidal cells is not mediated by δ-containing GABAa receptors and is not sensitive to neurosteroid (Stell et al., 2003). While pathophysiology dependent on δ-subunit receptors has yet to be confirmed, there is compelling evidence that heightened excitability and increased seizure susceptibility are prominent in conditions where δ-containing receptors are not present or down-regulated (Mihalek et al., 1999; Spigelman et al., 2002; Stell et al., 2003). Mutations to the δ subunit structure found within human cases of epilepsy recapitulate functional changes that may increase excitability (Dibbens et al., 2004; Feng et al., 2006).There is ongoing work to understand how differences within δ-subunit expression modify neuroactive effects of neurosteroids.
The δ knockout model remains a useful tool to develop current knowledge of how GABAa receptors interact with neurosteroids to modulate brain network functions. Moreover, neurosteroid effects on the brain may be tightly controlled through temporal mechanisms of development in addition to discrete neuronal cell types. Within the dentate gyrus of adult mice, GABAa receptors with α4 but not δ subunits play a role in neurogenesis and early axonal/dendrite growth and migration, while α2 mediates late maturation of dendrites and pruning (Duveau et al., 2011). The tone of GABAergic current could therefore rely on spatiotemporal control of receptors since different isoforms influentially control neuronal maturation. A conditional knockout has yet to be devised for the δ subunit and would aid in elucidating spatial and temporal receptor changes to regulate inhibitory functions.
Regulation and plasticity of subunit expression and function
Subunit expression differences within GABAa receptors are reflected by conditional changes such as development, stress, pregnancy, and ovarian/hormonal cycling and may be contextually relevant to further elucidating the function of neurosteroids on GABAergic neurons (Smith et al., 2007; Belelli et al., 2009). Studies in pathophysiology corroborate evidence that irregularly functioning states of neural networks like epilepsy involve changes in subunit expression. Many studies report compensational changes of subunit expression due to genetic, functional, or pharmacological changes to the control of GABAergic inhibition (Peng et al., 2002; Liang et al., 2008; Gangisetty and Reddy, 2010; Uusi-Oukari and Korpi, 2010; Suryanarayanan, et al., 2011; Kuver et al., 2012). Evidence for neurosteroid-dependent modulation of receptor plasticity is beginning to emerge. Therefore, in conditions where there is a substantial change in endogenous neurosteroid levels, receptor subunit reconfigurations could occur.
Initial electrophysiology studies on DGGCs in δ knockout mice reported that there was no overarching change in synaptic inhibition, but an observed faster rate of decay for mIPSCs, suggesting that structural receptor modifications might occur (Mihalek et al., 1999). Molecular analysis of mRNA from δ knockouts reveals a localized shift in which expression of γ2 subunit increases and α4 subunit expression levels decrease in the forebrain, implicating functional compensation in response to the loss of δ (Korpi et al., 2002; Peng et al., 2002). Hippocampal peptide levels of δ knockouts also demonstrate that with a global loss of δ subunit, α4 levels are diminished, coupled to observed decreases in inhibition (Spigelman et al., 2003). In α4 knockout mice, γ2 subunit levels increase within the hippocampus, further signifying a modulatory mechanism for balancing inhibition within this brain region (Liang et al, 2008).
While receptor expression data from knockout mice show signs of compensatory adaptation, the regulatory processes involved in subunit assembly and incorporation of receptors into the membrane remain to be discovered. It is possible that neurons with high extrasynaptic function depend on endogenous neurosteroids to adjust baseline inhibition. These neurons cannot rely on synaptic GABAergic transmission for inhibitory set point control due to the transient availability of synaptic GABA and the considerable distance from the synapse. However, extrasynaptic receptors are fine-tuned to allow modulation by secondary ligands that adjust channel gating. It appears that certain subunit isoforms are preferentially assembled together, and their location on neuronal membrane reflects the regulatory needs of the neuron (Bogdanov et al., 2006). Conditional changes that augment expressional regulation provide a larger understanding of functional maintenance of GABAa receptors and how neurosteroids influence this plasticity.
Stress
Stress has profound effects on the neuroendocrine release of steroids into the blood; elevations of corticosteroid promote increase in plasma and brain levels of THDOC and allopregnanolone (Purdy et al., 1991; Reddy and Kulkarni, 1996; Reddy and Rogawski, 2002; Reddy, 2006). Glucocorticoid metabolites can enhance allopregnanolone potentiation of GABAa receptors (Strömberg et al., 2005). Acute stress in animals has been shown to increase δ subunit expression in conjunction with a rise in baseline levels of tonic inhibition (Maguire and Mody, 2007). This aspect of δ plasticity is compounded by the fact that treatment of THDOC, released in conditions of high stress, can also increase δ expression in a rapid manner (Smith et al., 2007). Following stress, THDOC induces a depolarizing shift in the GABA equilibrium potential which causes excitatory GABAergic transmission in corticotropin-releasing hormone neurons; acting on δ-containing receptors, THDOC provides positive feedback for the hypothalamic-pituitary-adrenal axis response to stress (Sarkar et al., 2011). Acute swim stress in rats significantly increases plasma THDOC levels as well as the threshold of pentylenetetrazol to induce seizures (Reddy and Rogawski, 2002). Compensational adjustments have been proposed to serve as a protective mechanism for neurons to subsequent stress insults for brief, confined periods of time. In contrast, chronic stress has the effect of decreasing overall GABA receptor function which could be related to significant changes in the receptor expression profile (Serra et al., 2000). The introduction of chronic stress results in decreased global levels of neurosteroid in the system. Desensitization of neurons to neurosteroid over prolonged or constant releases of stress-activated biochemical may also possibly be reflected by GABAa receptor plasticity. Interestingly, social isolation-induced stress in rats has been found to increase extrasynaptic α4,δ-containing receptors in the hippocampus (Serra et al., 2006). Isolation stress altered allopregnanlone sensitivity to GABAa receptor function and increased tonic inhibitory current the dentate gyrus.
Development
Formation of neural networks in the hippocampus has provided insight into receptor plasticity in the developing brain. In CA1 pyramidal neurons of embryonic and postnatal rats, a form of tonic inhibition is observed even before synapses are formed (Demarque et al., 2002). Interestingly, this mechanism of inhibition precludes the requirement of transient, synaptic GABA release to be governed by Ca2+ influx and GABA reuptake controlled by GAT-1. Tonic inhibition is also active at early developmental stages of the dentate gyrus and is important in regulating the excitation/inhibition balance in the maturing hippocampus (Holter et al., 2010). Within rat hippocampus, α4 and δ subunit proliferation of expression occurs late in postnatal development and into adulthood (Laurie et al., 1992). By the time adult age is reached, synaptic α and γ2 containing receptors are already highly expressed and established within the brain. Both α2 and α5 subunits increase in the mouse hippocampus in the initial postnatal months of brain development but steady out during adulthood, whereas γ2 -subunit expression declines continuously in the adult and aging brain (Yu et al., 2006). This study implies that gene expression of α2 and α5 are induced and γ gene expression is down regulated due to the large changes across neural development in multiple brain regions. In DGGCs, the GABAa receptor-mediated currents have been found to increase in sensitivity to allopregnanolone as rats mature, wherein α1 expression also increases from birth to adulthood (Brooks-Kayal et al., 2001; Mtchedlishvili et al., 2003). There are also developmental changes from α5 subunits to α1, α2, and γ2 in DGGC, linked to high zinc sensitivity of receptors, which subsequently declines and becomes channel-blocking as neurons mature (Galanopoulou, 2008b). As the brain establishes the structural and chemical foundations critical for neuronal activities, neurosteroid synthesis and modulation likely play a role in shaping function.
Traumatic brain injury
Post-traumatic epileptogenesis results from ischemic injury or mechanical forces that lead to the development of seizures. There are direct and indirect cellular pathways that are involved in controlling tissue damage triggered by traumatic brain injury (TBI), and the hippocampus is vulnerable to its adverse effects. Excitotoxicity is a well-studied change resultant from TBI, yet there are modulatory changes to brain inhibition as well. Consequent of traumatic insult, there are declining numbers of GABAergic interneurons and decreases in GABAa receptor-mediated phasic IPSC frequency, but preservation of tonic function (Pavlov et al., 2011). Within a rat model of TBI, GABAa subunits α1, α3, β3, and γ2 undergo time-dependent expressional changes (Gibson et al., 2010). Another study reported a decrease in γ2 expression to TBI, but examined a strong increase in δ subunit expression (Kharlamov et al., 2011). Perforant pathway lesion studies divulge a short-term decrease in γ-containing GABAa receptors followed by an increase in γ subunit and glutamate receptors 30 days post-lesion (Iwakiri et al., 2006). Functional studies reveal that TBI induces greater potentiation of DGGC tonic current through δ-specific application of gabaxodol, as well as decrease in mIPSC frequency (Mtchedlishvili et al., 2010). These studies suggest plasticity within hippocampal tissue for the maintenance of excitatory/inhibitory homeostatic balance.
Given that TBI results in excitotoxicity, cell death, and cognitive deficits within the hippocampus, neurosteroid modulation of GABA transmission may provide an important role in regulating the effects of trauma. Preclinical and clinical studies suggest that in the acute stage of injury, progesterone administration may limit or reduce tissue damage and can be neuroprotective of brain function in neuronal injury, in addition to reducing excitotoxicity and seizures (Sayeed and Stein, 2009). Within the dentate gyrus, treatment with progesterone after TBI reduces the increase of cell death due to injury to stabilize levels to that of control animals (Barha et al., 2011). Progesterone may influence neuronal injury directly or indirectly through metabolites (Reddy and Ramanathan, 2012). Allopregnanolone treatment has also been shown to aid in improving cognitive abilities and preventing neuronal loss post-trauma, suggesting a role for GABAergic transmission in neuroprotection (He et al., 2004). The progesterone’s ability to modify injury and epileptogenesis are attributed partly to neurosteroids (Reddy et al., 2010; Reddy and Ramanathan, 2012).
Estrous cycle
Exploration into the fluctuations in steroid synthesis and circulation during the ovarian cycle has yielded critical information as to the function of neurosteroids on GABAa receptors. The ovarian cycle progresses with consequent endocrine changes in estradiol and progesterone levels. In the rodent estrous phase, progesterone levels are typically low and estrogen levels are high. During the diestrous phase, heightened levels of progesterone induce its metabolite, allopregnanolone, to increase during luteal surges within the ovary and plasma (Reddy and Kulkarni, 1999; Reddy, 2009a). GABAa receptor subunit gene expression is affected by oscillations in the ovarian cycle due to this rise in neurosteroid concentration not only in peripheral tissue, but also in the brain. This expressional response promotes gene activation and results in a change in amount of specific receptors that are functional on the neuronal membrane. During the diestrous phase, receptor protein levels of δ subunit increase and γ2 subunit decrease in the hippocampus compared to the estrous phase, and this is coupled to significant increases in GABAergic conductance in DGGCs (Maguire et al., 2005; Wu et al., 2013). Levels of α4 do not change significantly between these two phases of the cycle. The mirrored compensation of specific subunit isoform up-regulation in response to neurosteroid deficit in the CNS implicates a substantial system of neuromodulatory control of GABAergic inhibition. Over the course of the cycle, neither progesterone receptor antagonist RU846 nor estrogen receptor antagonist tamoxifen affect regulation of GABAa receptors in the brain, suggesting neurosteroid-dependent plasticity (Maguire and Mody, 2007). Control of GABAa receptor populations in puberty and into adulthood may be maintained by an overarching feedback mechanism involving the neuroendocrine axis balance of hormones. Therefore, developmental defects or pathophysiological onset of disorder within this system could greatly alter the excitability of the brain, such as in catamenial epilepsy (Reddy, 2013a).
Pregnancy and parturition
The subunit compositions of GABAa receptors undergo drastic changes during pregnancy. Pregnancy-related fluctuations in neurosteroids in the plasma and the brain vary with respect to subunit expression and GABAergic function. Both progesterone and deoxycorticosterone brain and plasma levels are elevated during pregnancy, which in turn increase allopregnanolone, THDOC, DHEAS, and cortisol synthesis in circulation (Biggio et al., 2001; Paoletti et al., 2006).
Expression of γ2 subunit mRNA is decreased within the hippocampus during rat pregnancy; finasteride treatment of pregnant rats prevents a significant reduction of γ2 expression (Concas et al., 1998). Pregnancy in rats drastically increases the DGGC and CA1 expression of δ subunits, coupled with the decrease in expression of γ2, but α4 expression remains static during pregnancy (Sanna et al., 2009). A postpartum expressional upregulation of α4 and γ2, but decrease in δ is observed in the hippocampus. Subunit α4 increases only following delivery in parturition; this precise adjustment to subunit composition is corroborated by a notably intensified DGGC tonic inhibition in pregnancy which is subsequently reduced after delivery. The control animals used in these experiments by Sanna and colleagues were female rats in estrus. Furthermore, the allopregnanolone-induced changes to tonic current of DGGCs in pregnancy reflect the increase in δ subunit-containing receptors. A large downward shift in the holding current occurs with an application of 1 μM allopregnanolone, which is well above the physiological levels found within the brain, but it is reasonable that hormonal levels during pregnancy may drive an increase in neurosteroid concentration above normal levels. These functional and structural observations on GABAa receptors during pregnancy coincide with neurosteroid mediation of the receptors, as treatment with finasteride ablates the aforementioned effects.
In contrast, a separate study involving pregnant mice observed decreases to both δ and γ2 expression and decreases in tonic and phasic inhibition as a result of pregnancy, however this study used females in diestrus as controls (Maguire and Mody, 2008). The two studies employ as their controls differing estrous cycle conditions, which, given the differing expressional levels of δ expression, offer varying reference points for plasticity changes that occur to GABAa receptor subunits during pregnancy. Receptor plasticity remains a complex issue in identification of changes that influence excitability. Slice neurons examined from pregnant mice experience region-specific decreases in δ subunit expression owing to a compensational shift of the brain to control neuronal excitability, but δ knockout, pregnant mice do not present any changes to network excitability (Maguire et al., 2009). In the presence of allopregnanolone, both pregnant and nonovulatory, virgin wildtype mice show reductions in network excitability in dentate gyrus neurons. The dysregulation of δ-containing receptor function within the hippocampus can therefore be selectively altered by the levels of available neurosteroid (Belelli et al., 2009). These studies confirm that neurosteroids have an active role in modulating tonic current inhibition, preferential to δ-containing, extrasynaptic receptors.
Neurosteroid treatment
Treatment of animals with steroid exemplifies subunit expression compensation and plasticity. Administration of progesterone or pregnenolone selectively and substantially increases 3α,5α-reduced neurosteroid levels in both rodents and humans and can be measured in blood serum by high throughput methods (Porcu et al., 2011). Gonadotropin treatment increases allopregnanolone levels (Reddy et al., 2012). Progesterone and allopregnanolone treatment can up-regulate α4 and δ in the hippocampus, documented in both in vitro and in vivo functional studies (Biggio et al., 2006; Uusi-Oukari and Korpi, 2010). Up-regulation of δ subunit in hippocampus after allopregnanolone treatment is significant for channel kinetics regarding potentiation; both efficacy and potency to gaboxadol increase following neurosteroid administration. This direct alteration of GABAa receptor plasticity reflects subunit targeting specificity in which δ is involved, but it also modifies the functionality of receptors where α4 and γ2 are coexpressed.
In response to neurosteroid treatment, CA1 synaptic current decay time is reduced (Hsu et al., 2003). A brief 48-hour treatment with allopregnanolone highly increases the expression profiles of the α4 and δ composition and decreases expression of the typically synaptic α1 and γ2 subunits within rat CA1 (Shen et al., 2005). However, δ-containing receptors are not as functionally significant at CA1 as compared to the dentate gyrus subfield, where expressional and functional changes to GABAa receptors have a higher impact on network excitability. Neonatal exposure to estradiol in female rats decreases brain levels of progesterone and allopregnanolone in addition to increasing the quantity of synaptic α1, α2, and γ2 subunits with no change to α3, α4, α5, or δ subunits (Calza et al., 2010). An increase in anxiolytic behavior results when diazepam is applied to estradiol-treated animals, but there is little change by allopregnanolone. This estradiol-modulated, increased sensitivity exhibits subunit plasticity-directed specificity to allosteric ligands concerning broader GABAa receptor gating.
Neurosteroid withdrawal
Withdrawal from neurosteroid in the body and brain has striking impact on subunit plasticity within the hippocampus. Neurosteroid withdrawal, which occurs during the perimenstrual period, is linked to catamenial epilepsy and seizure susceptibility in women (Reddy et al., 2001; Reddy, 2013). In rat hippocampal cultures, withdrawal following chronic exposure to either progesterone or allopregnanolone causes a transient increase in δ subunit expression (Mostallino et al., 2006). Induced neurosteroid withdrawal following chronic doses of progesterone or allopregnanolone in rodents has shown to increase the expression of α4 subunit approximately 3-fold (Smith et al., 1998; Gangisetty and Reddy, 2009a; 2010). Progesterone and neurosteroid withdrawal models exhibit an up-regulation in α4 and δ subunits with effects on current, pharmacology, and seizure susceptibility (Sundstrom-Poromaa et al., 2002; Reddy et al., 2012). Due to neurosteroid withdrawal-dependent increases of α4 and δ subunits, fully kindled, epileptogenic mice experience enhanced sensitivity to the antiseizure effects of allopregnanolone (Reddy et al., 2012). There is no observed compensational shift in α1, α2, β2, or γ2 subunits in response to withdrawal. This may represent a divergent mechanism of expression for synaptic and extrasynaptic receptors.
Increase in seizure activity and excitation of the brain has differential effects on GABAa receptor physiology of CA1 and DGGCs (Gibbs et al., 1997). Progesterone withdrawal has been linked to α4-dependent increase in CA1 pyramidal neuron excitability (Hsu and Smith, 2003). In CA1 subfield, GABAergic inhibition is primarily mediated by other subunits (Caraiscos et al., 2004; Prenosil et al., 2006).The α4 subunit is more prevalent in dentate gyrus than in the CA1 pyramidal region (Pirker et al., 2000) and serves as a more crucial pharmacological target for tonic current modulation in the dentate gyrus. Alterations to receptor function within the dentate gyrus have severe effects on the control of epileptiform spread of hyperactivity (Coulter and Carlson, 2007). Extrasynaptic profiles of δ-containing receptors reflect considerable plasticity to neurosteroid withdrawal that influences their channel opening probability (Bianchi and Macdonald, 2003). Phasic and tonic GABAa receptors may rely on disparate mechanisms that control expression based on neuronal requirements to maintain network inhibition. Due to the high neurosteroid sensitivity to GABAa receptors mediating tonic current within the dentate gyrus, it is probable that neurosteroids drive expressional changes to regulate baseline inhibition.
Epilepsy
Epilepsy is a disease characterized by recurrent seizures resultant from aberrant hyperexcitability within the brain. While there are many types of epileptic seizures, certain types may be studied to elucidate the nature of the disease and formulate appropriate treatments in control of seizures. Receptor plasticity has been reported in several models of epilepsy, implicated to play a role in the molecular changes that promote seizure susceptibility. Alterations to the function of GABAa receptors through a pilocarpine-induced model of temporal lobe epilepsy (TLE) reveal that CA1 neurons from epileptic animals undergo a reduced maximal efficacy (decrease of 45%) to GABA with an enhanced potency, but DGGC neurons experience an increased efficacy (increase of 89%) to GABA without a change in potency, and an increased sensitivity to zinc blockade of current (Gibbs et al., 1997). Epileptiform bursting activity promotes cellular internalization of GABAa receptors and has consequent effects on synaptic GABA transmission in reducing inhibition (Goodkin et al., 2005). Both mature and newborn dentate cells undergo reductions in spine density and number in response to induced status epilepticus (Santos et al., 2011). Hippocampal neurons possess varying GABAa receptor compositional changes in response to hyperexcitability of the brain. The foundation for greater seizure susceptibility is maintained by the markedly large decrease in available neurosteroid in the brain, altered receptor expression levels on the neuronal surface, and reduced overall GABAergic inhibition.
Catamenial epilepsy is characterized by increased seizure frequency due to menstrual cycle-related hormonal fluctuations (Reddy, 2007; 2009a; 2012). Deficits in cyclical fluctuations in steroid hormones and receptor plasticity play a central role this condition. The seizures produced by the disease are believed to result from cyclic, temporal withdrawal during a deficit of circulating neurosteroid, which in turn increases seizure susceptibility in conjunction with a dearth of GABAergic inhibitory control over excitability (Reddy, 2004, 2009a; 2013a,b). The δ-containing receptors are crucial mediators of tonic inhibition and limbic epileptogenesis and are regulated within the estrous cycle (Reddy et al., 2012; Wu et al., 2013). In women with perimenstrual catamenial epilepsy, overall THDOC levels and DHEAS/cortisol ratio are reduced, but the pregnane steroids progesterone, pregnenolone, and allopregnanolone are not different compared to control groups (Tuveri et al., 2008). However, in the perimenstrual part of the cycle, the luteinizing surge produces a rapid flux in progesterone and its metabolites that afterward sharply decrease (Reddy, 2009a). Parturition is also associated with a substantial decrease in progesterone and allopregnanolone levels, and there is a postpartum increase in α4 and γ2 subunit expression, but decrease in δ within the hippocampus (Sanna et al., 2009). Thus, epilepsy may cause a different compensational mechanism of GABAa receptor plasticity within the brain compared to the physiological condition of progesterone withdrawal in order to allow for parturition to proceed.
Treatment with neurosteroids has shown to reduce seizure activity in several epilepsy models (Lawrence et al., 2010; Reddy and Jian, 2010; Reddy et al., 2010). Using a rat model for TLE, Kapur and colleagues reported that allopregnanolone sensitivity was reduced, δ subunit was down-regulated in expression, but tonic inhibition remained intact in tissue isolated from epileptic rats (Sun et al., 2007; Rajasekaran et al., 2010). This is an interesting result and clinically relevant, but these findings have yet to be confirmed by an independent group. Noda model epileptic rats have decreased δ subunit expression in DGGCs along with decreased tonic current, smaller tonic response to allopregnanolone (Pandit et al., 2013). Furthermore, in Noda rat slices, allopregnanolone prolonged synaptic receptor decay in DGGC similarly to the normal Wistar strain counterparts. Pilot studies from our lab show that allopregnanolone retains its antiepileptic efficacy in this animal model of TLE and is successful in suppressing the occurrence of spontaneous seizures in chronically epileptic rats (Reddy et al., 2005c). Better discernment of the role of neurosteroid-receptor interactions in the context of epilepsy necessitate that in vitro studies are corroborated with in vivo studies that typify the whole network response to GABAa modulation.
The physiological, intrinsic low excitability of the dentate gyrus has an important role in the filtering of excitatory activity from the entorrhinal cortex and regulating propagation of current that passes through to other hippocampal neuronal tissue (Heinemann et al., 1992, Lothman et al., 1992, Coulter and Carlson, 2007). DGGCs from TLE rats have increased synaptic efficacy, exemplified by increase in number of postsynaptic channels, but not channel conductance (Otis et al., 1994). However, these changes to miniature IPSC amplitude do not take into account possible alterations in extrasynaptic GABAa receptors. Kainate treatment to induce seizures decreases frequency of miniature IPSCs, but does not change the frequency of action potential-dependent, spontaneous IPSCs (Shao and Dudek, 2005). While IPSC recordings do not provide a comprehensive view of how epilepsy modifies GABAergic inhibition, these findings denote compensatory effects within the hippocampus to maintain inhibitory current. Observed reductions in phasic inhibition in epileptic animals agree with evidence that γ2-containing receptors may increasingly shift to extrasynaptic sites and assemble with α4 subunits. DGGC receptor plasticity in epileptic mice is reported to have static α4 expression, δ down-regulation, and γ2 increase in dendritic areas of neurons (Zhang et al, 2007).
In contrast, epileptiform DGGCs have been found to display increased inhibition of mIPSCs and altered GABAa receptor sensitivity to zinc and benzodiazepine pharmacology, also associated with increased membrane receptors (Cohen et al., 2003). There are various contrasting findings regarding changes in dentate gyrus inhibition that could be interpreted by the location of the synaptic inhibition recording (Zhang et al., 2007). Pharmacological and electrophysiological evidence of pilocarpine-induced status epilepticus also suggests enhanced tonic, GABAergic inhibition, largely modulated by L-655,708-sensitive α5-containing receptors in lieu of δ-containing receptors (Zhan and Nadler, 2009). There is still considerable uncertainty regarding how receptor physiology influences GABAergic inhibition in epileptic animals, however the rodent model of TLE provides indication of compensatory shifts in subunit composition that influences tonic current (Mody, 2012). Nevertheless, pharmacological shifts in receptor sensitivity are concomitant with changes in receptor subunit composition.
In epileptic CA1 pyramidal cells, α5 subunit was down-regulated (Houser and Esclapez, 2003), but tonic current was increased, providing evidence that compensation of other subunit types mediates tonic current in epileptic tissue instead of α5-containing receptors (Scimemi et al., 2005). The α5 subunit has been implicated in modulating hippocampal excitability (Bonin et al., 2007), but the extent and degree of their inhibitory function in the context of epileptogenesis have yet to be fully explored. The down-regulation of key subunits involved in tonic inhibition demonstrates a mechanism of receptor plasticity, but it is not known why increasing excitation within the hippocampus results in dampened expression of receptors that control the excitability. Status epilepticus rats have a more positive reversal potential for IPSC than control counterparts due to an increase in NKCC1 chloride ion cotransporters (Barmashenko et al., 2011). Maintaining inhibitory tonic current through extrasynaptic receptors appears to be integral in preventing hyperexcitable states. The retention of neurosteroid ability to counteract epileptic effects within the brain illustrates the capability of neurosteroids beyond their typified GABAa receptor subunit specificity. Further insight into neurosteroid treatment of epileptic animals will yield a more precise understanding of the efficacy of neurosteroids to control seizures. Additional investigation into the bioavailability and efficacy of neurosteroids in conditions that reflect seizure susceptibility holds potential in devising therapies for epilepsy.
Mechanisms of plasticity
There is strong support that the potentiating effects of neurosteroids are incongruent with classical steroid hormone receptor interaction. Nuclear receptors do not play a role in GABAa subunit plasticity or inhibitory current properties observed during ovarian-cycle related fluctuations. Therefore, the presence of neurosteroid to drive subunit expression may act through a mechanism disparate from classical steroid gene transcription, but this mechanism is largely unknown. Conversely, it remains a possibility that neurosteroid biotransformation into steroidal precursors could genomically alter expression of subunits. While various different conditions can convey an underlying ability of neurosteroids to modulate GABAa receptor inhibition, regulation of the receptor structures and subunit assembly remains elusive. The early growth response factor-3 (Egr3) pathway has been implicated in mediating α4 subunit up-regulation induced by neurosteroid withdrawal, and this has found to occur independently of any progesterone receptor interaction (Gangisetty and Reddy, 2010). The extent of transcription factor-based mediation of subunit regulation remains to be clarified. Expressional changes in transcriptional control of mRNA, based on Egr3 activation data, shed some light on the uncertainty of subunit regulation. Protein kinases or cellular machinery involved in protein and receptor trafficking may have an important role in the mechanistic changes observed in subunit composition as well (Jacob et al., 2008).
Sex-specific differences
Since endocrine fluctuations in plasma levels of progesterone and other steroids can mediate neurosteroid availability, there are apparent differences between males and females concerning concentrations of neurosteroids in the brain. In addition, brain development greatly differs between genders and likely contributes to neurosteroid function (Reddy, 2009b). While neurosteroids are able to shape inhibition and produce behavioral effects in both genders, regulation of neurosteroid activity may be sex-specific (Gulinello and Smith, 2003). Differences in maximal GABAa receptor potentiation are observed between male and female rats for THDOC, but not for allopregnanolone or androgenic neurosteroids (Wilson and Biscardi, 1997). Gender differences in expression of 3α-hydroxysteroid dehydrogenase are evident during puberty, but these differences subside in the brain as it matures into adulthood; sex-specific gonadal and adrenal endocrine activity have a significant effect on the ability of allopregnanolone to modify anxiolytic actions based on variations in biosynthesis of steroid hormones (Mitev et al., 2003).
In early stages of nervous system development, GABAa receptors possess excitatory functions on neurons by chloride channel-mediated depolarization, and this switches to inhibitory function as the brain matures (Cherubini et al., 1991; Rivera et al., 1999). Developmental disparities in neurosteroid activity may be exemplified by evidence that the hippocampus in newborn males undergoes a longer period of this GABA-directed excitatory function than in females (Nuñez and McCarthy, 2007; Galanopoulou 2008a). Fluctuations in intracellular chloride concentrations have been shown to modulate α1, α3, and δ subunit expression as well as influence tonic inhibition (Succol et al., 2012). Such modifications within the brain have implications on seizure susceptibility within the developing hippocampus and changing neuronal network environment. In addition, allopregnanolone and other neurosteroids could have differential neuroprotective effects in male and females experiencing potential excitotoxic or ischemic events that could damage neurons (Kelley et al., 2011).
Post-translational modification of δ-containing receptors
Phosphorylation
Post-translational modifications to GABA-gated receptors may possess a significant role in the allocation of functioning receptors in the membrane. GABAa receptor trafficking, assembly, and recycling often occur in concert with intracellular phosphorylation. The phosphorylation state of the receptors within the membrane could be influential in adjusting their interaction with neurosteroids. Inhibition of either protein kinase A or protein kinase C greatly reduces sensitivity of CA1 synaptic GABAa receptors to pregnenolone, but not to pentobarbitone, whereas stimulation of PKC has no effect on steroid sensitivity of CA1, but increases steroid sensitivity on DGGCs (Harney et al., 2003). Specific subunit targets of phosphorylation may have selective influence over neurosteroid sensitivity in conjunction with preferential modification of receptor conformations. PKA-directed activation produced greater spontaneous currents in α4β3δ than in α4β3γ2L receptors due to greater channel open frequency, however it did not alter total tonic current (Tang et al., 2010). PKA may possibly aid in regulation of tonic currents in a GABA concentration-dependent manner when other expressional changes occur. This phosphorylation of extrasynaptic receptors reiterates the low-efficacy properties that may be adjusted to produce tonic levels of chloride ion conductance.
Furthermore, homeostatic stability of endogenous phosphatase and PKC activity may exert an influence over the sensitivity of the GABAa receptor to allopregnanolone (Brussaard and Koksma, 2003). PKC has been found to have an active role in phosphorylating residues on α4 and β3 subunits, but not δ. In addition, PKC-dependent phosphorylation of α4 has a post-translational task in increasing the availability and rate of membrane insertion of α4-containing receptors (Abramian et al., 2010). Different PKC isoforms exist for phosphorylation of GABAa receptors responsible for phasic and tonic currents, however this recent finding is not yet comprehensively confirmed across various neuronal types known to be involved in tonic inhibition (Kia et al., 2011).
Receptor trafficking, scaffolding, and clustering
GABAa receptor associated protein (GABARAP) is involved in receptor trafficking to the plasma membrane. It associates with γ subunits as well as cytoskeletal microtubules. GABARAP and other similar proteins are highly available at Golgi, but are not present at synapses (Luscher et al., 2011). The over-expression of GABARAP results in transport of GABAa receptors in hippocampal neurons to the neuronal membrane (Leil et al., 2004). In addition, GABARAP mediated exocytosis of receptors onto the membrane has significance in regulating inhibitory, activity-dependent function of GABAa receptors (Vithlani et al., 2011). A recent studied has detailed α subunit types to have a direct role in targeting of receptors to extrasynaptic or synaptic sites (Wu et al., 2012).
Gephyrin is an important postsynaptic clustering protein, closely associated with α2- and γ2-containing receptors. Clustering of gephyrin is essential for synaptic formation and maintenance of GABAergic receptors (Yu et al., 2007). Hippocampal immunohistochemistry reveals that colocalization of gephyrin and GAD65 with α1 or γ2 is significantly higher than with α4 and δ subunits (Sun et al., 2004). The fact that α4 and δ subunits are not closely associated with synaptic markers suggests that there may be separate coordinated expression of structural proteins located on the dendritic spines of neurons. It is unclear what scaffolding or structural proteins are necessary in directing non-synaptic receptor associations with the membrane. The interaction of gephyrin and the α1 subunit modulates the accumulation of receptors at the synapse and influences amplitude of mIPSCs (Mukherjee et al., 2011). Immunostaining confirms gephyrin colocalization in high levels with α1-containing receptors. In α1 knockout mice, cerebellar gephyrin clusters are decreased, but gephyrin clusters that are colocalized with α3-containing receptors increase (Kralic et al., 2006). Gephyrin, in conjunction with phosphorylated residues on GABAa receptors, is important for the localization of receptors at synaptic regions and the modulation of GABAergic inhibition. Collybistin is coexpressed with gephyrin, and is important for clustering at inhibitory synapses (Luscher et al., 2011). It is unclear how neurosteroid modulation affects the trafficking and targeting of receptors to synaptic or extrasynaptic loci within neurons.
Conclusions and future directions
New questions raised by recent research on the interaction between neurosteroids and GABAa receptors provide opportunities to further explore inhibitory function in the brain. The specific physiology of extrasynaptic receptors remains to be clarified, although neurosteroid specificity provides indication of an important modulatory role in network inhibition. This modulation is observed to be highly plastic under a variety of physiological and pathophysiological conditions. More precise experimental and conditional manipulation of δ subunit expression would aid in explaining compensational functions of receptor plasticity. Thus far, global knockout mice have implicated expressional compensation of receptor subunits in response to lack of a particular isoform present in neuronal membrane. Regional knockout of these receptor profiles would serve to clarify the role that the δ subunit plays in tonic inhibition and compensation adjustment could be mapped to specific neural tissue. The true nature of hippocampal receptor plasticity could be attributed to specific changes in the hippocampus physiology. Temporal control of δ subunit gene expression in animal models would also substantiate claims that δ incorporation within GABAa receptors is a developmentally regulated function. In addition, precise changes in subunit expression at an investigator-determined instance could establish functional significance of this particular receptor isoform in epilepsy, ethanol dependence, stress, and other behavioral paradigms in which neurosteroid interaction plays a significant role.
As exploration of neurosteroid pharmacology continues, new agents that are more specific in binding to extrasynaptic GABAa receptors could help to further identify the allosteric and direct binding of neurosteroids. The current perception is that δ subunit incorporation involves indirect modulation by pharmacological agents at altogether separate binding sites on the GABAa receptor. The lipophilic nature of neurosteroids denotes more complex lateral binding on the receptor structure. Various physiological and pathological conditions cause plastic changes in GABAa receptors that reflect modulatory control of inhibition. Studies in epilepsy provide distinctive perspectives that suggest fine-adjustment to excitability within the hippocampus and other limbic regions. While there are a myriad of agents that operate in modulation of brain inhibition, neurosteroids possess a unique ability to alter receptors with a specificity and efficacy unlike any other endogenous compounds synthesized within the brain. The diversity of their function in various physiological conditions and diseases indicates a wide influence on regulation of neural network homeostasis.
Extrasynaptic GABAa receptors that mediate tonic inhibition are emerging as important targets for novel neurological drugs (Houston et al., 2012). During the past two decades, a number of synthetic neurosteroid analogs have been developed that target GABAa receptors. In animal models, neurosteroid analogs display pharmacological profile that is much superior to benzodiazepines. However, the success in identifying a clinically viable analog is limited. Typical limitations of synthetic analogs include short half-life, water-insolubility, and non-selective activation of GABAa receptors. The example of ganaxolone a steroid that has been undergoing clinical evaluation for epilepsy indications demonstrates that more efforts are needed to identify compounds with improved pharmacokinetics, subtype-specificity and efficacy. Further studies are required to determine if neurosteroid therapy is a useful therapeutic approach for seizure exacerbations related to endogenous neurosteroid fluctuations, such as in catamenial epilepsy and stress (Reddy and Rogawski, 2009). In the future, agents that influence the endogenous synthesis of neurosteroids, such as TSPO ligands, may find utility as an alternative to neurosteroids themselves in the treatment of insomnia, anxiety, epilepsy and other neurological conditions.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke Grants NS051398, NS071597 and NS076426 (to D.S.R.). We thank Drs. William Griffith and Gerald Frye for comments on the manuscript. The authors would like to thank Xin Wu for technical help with illustrations.
Abbreviations
- 3α-HSOR
3α-hydroxysteroid oxidoreductase
- 17PA
(3α,5α)-17-phenylandrost-16-en-3-ol
- AP
allopregnanolone (3α-hydroxy-5α-pregnan-20-one)
- CGC
cerebellar granule cell
- CNS
central nervous system
- DGGC
dentate gyrus granule cell
- DHEAS
dehydroepiandrosterone sulfate
- GABARAP
GABAa receptor associated protein
- IPSC
inhibitory postsynaptic current
- NMDA
N-methyl-D-Aspartate
- P
progesterone
- PS
pregnenolone sulfate
- TBI
traumatic brain injury
- TBPS
tert-butylbicyclophosphorothionate
- THDOC
allotetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one)
- THIP
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol
- TLE
temporal lobe epilepsy
- TSPO
translocator protein
References
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAa receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAa receptors: mechanism and involvement of a residue in the M2 region of the α-subunit. J Physiol. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covery DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. The influence of the membrane on neurosteroid actions at GABAa receptors. Psychoneuroendocrinology. 2009;34S:S59–S66. doi: 10.1016/j.psyneuen.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu H, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAa receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAa receptors. Neuropharmacol. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acida receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Baker C, Sturt BL, Bamber BA. Multiple roles for the first transmembrane domain of GABAa receptor subunits in neurosteroid modulation and spontaneous channel activity. Neurosci Lett. 2010;473:242–247. doi: 10.1016/j.neulet.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurobiol. 2011;231:72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmashenko G, Hefft S, Aertsen A, Kirschstein T, Kohling R. Positive shifts of the GABAa receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia. 2011;52:1570–1578. doi: 10.1111/j.1528-1167.2011.03247.x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Steroid hormones in the brain: several mechanisms? In: Fuxe F, Gustafsson JA, Wetterberg L, editors. Steroid Hormone Regulation of the Brain. Pergamon Press; Oxford: 1981. pp. 3–14. [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAa receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional anognist sites in GABAa receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JA, Löw K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAa receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAa receptors: form, pharmacology, and function. J Neurosci. 2009;29:12763–12757. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JL. Neurosteroids: endogenous regulators of the GABAa receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Benke D, Michel C, Mohler H. GABAa receptors containing the α4 subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAa receptors containing the δ subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAa receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G, Follesa P, Sanna E, Purdy RH, Concas A. GABAa-receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol. 2001;46:207–241. doi: 10.1016/s0074-7742(01)46064-8. [DOI] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Mostallino MC, Sanna E, Follesa P. Plastic neuronal changes in GABAa receptor gene expression induced by progesterone metabolites- In vitro molecular and functional studies. Pharm Biochem Behav. 2006;84:545–554. doi: 10.1016/j.pbb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Homanics GE, Blednov YA, Harris RA. δ-Subunit containing GABAa receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol. Eur J Pharmacol. 2006;541:158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAa receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. α5GABAa receptors regulate the intrinsic excitability of hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussard AB. Neonatal development of the rat visual cortex: synaptic function of GABAa receptor α subunits. J Physiol. 2002;(545):169–81. doi: 10.1113/jphysiol.2002.026534. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracamontes J, McCollum M, Esch C, Li P, Ann J, Steinbach JH, Akk G. Occupation of either site for the neurosteroid allopregnanolone potentiates the opening of the GABAa receptor induced from either transmitter binding site. Mol Pharmacol. 2011;80:79–86. doi: 10.1124/mol.111.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKinzie G, Hosie AM, Farrant M, Brickley SG. Profound desensitization by ambient GABA limits activation of δ-containing GABAa receptors during spillover. J Neurosci. 2011;31:753–763. doi: 10.1523/JNEUROSCI.2996-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briyal S, Reddy DS. Antiepileptic efficacy of the delta-subunit-preferring GABAa receptor agonist gaboxedol on spontaneous recurrent seizures in a rat model of temporal lobe epilepsy. Society for Neuroscience Abstracts. 2007;24(375.22):36. [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA. γ-Aminobutyric acida receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem. 2001;77:1266–1278. doi: 10.1046/j.1471-4159.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAa receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicnell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAa receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Koksma JJ. Conditional regulation of neurosteroid sensitivity of GABAa receptors. Ann N Y Acad Sci. 2003;1007:29–36. doi: 10.1196/annals.1286.003. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAa receptor function in the adult brain: insights from oxytocin neurone. Trends Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- Buhr A, Wagner , Fuchs K, Sieghart W, Sigel E. Two novel residues in M2 of the γ-aminobutyric acid type A receptor affecting gating by GABA and picrotoxin affinity. J Biol Chem. 2001;276:7775–7781. doi: 10.1074/jbc.M008907200. [DOI] [PubMed] [Google Scholar]
- Calza A, Sogliano C, Santoru F, Marra C, Angioni MM, Mostallino MC, Biggio G, Concas A. Neonatal exposure to estradiol in rats influences neuroactive steroid concentrations, GABAa receptor expression, and behavioral sensitivity to anxiolytic drugs. J Neurochem. 2010;113:1285–1295. doi: 10.1111/j.1471-4159.2010.06696.x. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newll JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3 -hydroxy-3 -methyl-5 -pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biohphys Mol Biol. 2005;87:3–16. doi: 10.1016/j.pbiomolbio.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Halonen LM, Linden AM, Procaccini C, Hellsten K, Homanics GE, Korpi ER. Prototypic GABAa receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology. 2010;35:999–1007. doi: 10.1038/npp.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, et al. GABAa receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABAa receptor. Mol Pharmacol. 2012;82:408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAa receptor modulation: evidence for a low0affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA-A receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CS, Brickley S, Jensen K, Southwell A, Mckinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro R, Russo E, Di Paola ED, Ibbadu GF, Gratteri S, Marra R, De Sarro G. Effects of some neurosteroids injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy. Neuropharmacology. 2006;50:1059–1071. doi: 10.1016/j.neuropharm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Clarke RS, Dundee JW, Carson IW. Proceedings: a new steroid anaesthetic-althesin. Proc R Soc Med. 1973;66:1027–1030. doi: 10.1177/003591577306601023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAa receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumsk CF, Evers AS. Enantioselectivity of pregnanolone-induced γ-aminobutyric acida receptor modulation and anesthesia. J Pharm Exp Ther. 2000;293:1009–1116. [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejin L. Paracrine intracellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Derry JMC, Dunn SMJ, Davies M. Identification of a residue in the γ-aminobutyric acid type A receptor α subunit that differentially affects diazepam-sensitive and -insensitve benzodiazepine site binding. J Neurochem. 2004;88:1431–1438. doi: 10.1046/j.1471-4159.2003.02264.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acida receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharm Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF. Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Front Neurosci. 2012;5:148. doi: 10.3389/fnins.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, et al. GABRD encoding a protein for extra- or peri-synaptic GABAa receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Duveau V, Laustela S, Barth L, Gianolini F, Vogt KE, Keist R, Chandra D, Homanics GE, Rudolph U, Fritschy JM. Spatiotemporal specificity of GABAa receptor-mediated regulation of adult hippocampal neurogenesis. Eur J Neurosci. 2011;34:362–373. doi: 10.1111/j.1460-9568.2011.07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- Ernst M, Brauchart D, Boresch S, Sieghart W. Comparative modeling of GABAa receptors: limits, insights, future developments. Neuroscience. 2003;119:933–43. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory them: phasic and tonic activation of GABAa receptors. Nat Rev. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL. δ subunit susceptibility variants E177A R220H associated with complex epilepsy alter channel gating and surface expression of α4β2δ GABAa receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. GABA transport modulates the ethanol sensitivity of tonic inhibition in the rat dentate gyrus. Alcohol. 2011;45:577–583. doi: 10.1016/j.alcohol.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiss E, Schiffelholz T, Steckler T, Steiger A. Dehydroepiandorsterone--a neurosteroid. Eur J Clin Invest Suppl. 2000;3:46–50. doi: 10.1046/j.1365-2362.2000.0300s3046.x. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neuroactive steroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABAa receptors. J Neurosci. 2008a;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. GABAa receptors in normal development and seizures: friends or foes. Curr Neuropharmacol. 2008b;6:1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABAa receptor subunit plasticity. J Neurosci Methods. 2009;181:58–66. doi: 10.1016/j.jneumeth.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABAa receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Griffith NC, Kaura V, Ingram CD. The neurosteroid dehydroepiandrosterone (DHEA) and its metabolites alter 5-HT neuronal activity via modulation of GABAa receptors. J Psychopharmacol. 2010;24:1717–1724. doi: 10.1177/0269881109105836. [DOI] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure–activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAa receptor function in dentate granule and CA1 neurons. J Physiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Gibson CJ, Meyer RC, Hamm RJ. Traumatic brain injury and the effects of diazepam, diltiazem, and MK-801 on GABA-A receptor subunit expression in rat hippocampus. J Biomed Sci. 2010;17:38. doi: 10.1186/1423-0127-17-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAa receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAa receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAa receptor subunit with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAa receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAa receptor pharmacology in adult rats. J Pharm Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat α6β2δ GABAa receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAa receptor occupancy. Eur J Neurosci. 2000;12:810–818. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Han JW, Nakamura M, Choi IS, Cho JH, Park HM, Lee MG, Choi BJ, Jang HJ, Jang IS. Differential pharmacological properties of GABAa receptors in axon terminals and soma of dentate gyrus granule cells. J Neurochem. 2009;109:995–1007. doi: 10.1111/j.1471-4159.2009.06018.x. [DOI] [PubMed] [Google Scholar]
- Hancar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAa receptors. Proc Natl Acad Sci USA. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Frenquelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAa receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19–31. [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. 1992;7(Suppl):273–280. [PubMed] [Google Scholar]
- Herd MB, Belelli, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABAa receptors. Pharamcol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herd MB, Haythornwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAa β subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG. Psychoneuroendocrine aspects of temporolimbic epilepsy: part II: epilepsy and reproductive steroids. Psychosomatics. 1999;40:102–108. doi: 10.1016/S0033-3182(99)71255-7. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Hormonal therapies: progesterone. Neurotherapeutics. 2009;6:383–391. doi: 10.1016/j.nurt.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci. 2010;32:1300–1309. doi: 10.1111/j.1460-9568.2010.07331.x. [DOI] [PubMed] [Google Scholar]
- Horak M, Vlcek K, Petrovic M, Chodounska H, Vyklicky L., Jr Molecular mechanism of pregnenolone sulfate action at NR1/NR2B receptors. J Neurosci. 2004;24:10318–10325. doi: 10.1523/JNEUROSCI.2099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAa receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAa receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAa receptors through two discrete transmembrane sites. Nat Lett. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAa receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the α5 subunit of the GABAa receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–645. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Houston CM, McGee TP, Mackenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG. Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? J Neurosci. 2012;32:3887–3897. doi: 10.1523/JNEUROSCI.5406-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Smith SS. Progesterone withdrawal reduces paired-pulse inhibition in rat hippocampus: dependence on GABAa receptor α4 subunit upregulation. J Neurophysiol. 2003;89:186–198. doi: 10.1152/jn.00195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–1940. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing T, Poulter MO. Diversity of GABAa receptor synaptic currents on individual pyramidal cortical neurons. Eur J Neurosci. 2007;25:723–734. doi: 10.1111/j.1460-9568.2007.05331.x. [DOI] [PubMed] [Google Scholar]
- Iwakiri M, Mizukami K, Ishikawa M, Asada T. GABAa receptor γ subunits in the hippocampus of the rat after perforant pathway lesion. Neurosci Lett. 2006;394:88–91. doi: 10.1016/j.neulet.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAa receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABAa receptors in the thalamus. J Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate C. High-fl ux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002;110:881–890. doi: 10.1172/JCI16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jursky F, Fuchs K, Buhr A, Tretter V, Sigel E, Sieghart W. Identification of amino acid residues of GABAa receptor subunits contributing to the formation and activity of the tertbutylbicyclophosphorothionate binding site. J Neurochem. 2000;74:1310–1316. doi: 10.1046/j.1471-4159.2000.741310.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Fu Z, Venkatesan K, Mazzuferi M, Leclerq K, Seutin V, Vicini S. 11-Deoxycortisol impedes GABAergic neurotransmission and induces drug-resistant status epilepticus in mice. Neuropharmacology. 2011;60:1098–1108. doi: 10.1016/j.neuropharm.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Ortinski PI, Vicini S, Rogawski MA. The pheromone androstenol (5α-androst-16-en-3α-ol) is a neurosteroid positive modulator of GABAa receptors. J Pharmacol Exp Ther. 2006;317:694–703. doi: 10.1124/jpet.105.098319. [DOI] [PubMed] [Google Scholar]
- Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of δ-subunit-containing GABAa receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MH, Kuroiwa M, Taguchi N, Herson PS. Sex difference in sensitivity to allopregnanolone neuroprotection in mice correlates with effect on spontaneous inhibitory post synaptic currents. Neuropharmacology. 2011;61:724–729. doi: 10.1016/j.neuropharm.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SP, Alan JK, O’Buckley TK, Mennerick S, Krishnan K, Covey DF, Morrow AL. Antagonism of neurosteroid modulation of native gamma-aminobutyric acid receptors by (3α,5α)-17-phenylandrost-16-en-3-ol. Eur J Pharmacol. 2007;572:94–101. doi: 10.1016/j.ejphar.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna M, Qin KN, Cheng KC. Distribution of 3α-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J Steroid Biochem Mol Biol. 1995;53:41–46. doi: 10.1016/0960-0760(95)00019-v. [DOI] [PubMed] [Google Scholar]
- Kharlamov EA, Lepsveridze E, Meparishvili M, Solomonia RO, Lu B, Miller ER, Kelly KM, Mtchedlishvili Z. Alterations of GABAa and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95:20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Kia A, Ribiero F, Nelson R, Gavriolovici C, Ferguson SSG, Poulter MO. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAa receptor –mediated currents: role of phosphorylation. J Neurochem. 2011;116:1043–1056. doi: 10.1111/j.1471-4159.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- Kim BG, Cho JH, Choi IS, Lee MG, Jang IS. Modulation of presynaptic GABAa receptors by endogenous neurosteroids. Br J Pharmacol. 2011;164:1698–1710. doi: 10.1111/j.1476-5381.2011.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita A, Furukawa K. Involvement of neurosteroids in the anxiolytic-like effects of AC-5216 in mice. Pharmacol Biochem Behav. 2008;89:171–178. doi: 10.1016/j.pbb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke Dietmar, Wang Yu, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acida receptors α4β2γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neuroactive steroid pregnenolone sulfate in mice. Brain Res. 1999;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Korneyev A, Pan BS, Polo A, Romeo E, Guidotti A, Costa E. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J Neurochem. 1993;61:1515–1524. doi: 10.1111/j.1471-4159.1993.tb13647.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Lüddens H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Korpi ER, Lüddens H. Furosemide interaction with brain GABAa receptors. Br J Pharmacol. 1997;120:741–748. doi: 10.1038/sj.bjp.0700922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Lüddens H. Altered receptor subtypes in the forebrain of GABAa receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic current in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Lol CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Reddy DS. Neuroactive steroids: a new class of neuromodulators. Drugs Today. 1995;31:433–455. [Google Scholar]
- Kuver A, Shen H, Smith SS. Regulation of the surface expression of α4β2δ GABAa receptors by high efficacy states. Brain Res. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAa receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neuroactive steroids and GABAa receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAa receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RDJ, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAa receptors. Psychoneuroendocrinology. 2009;34S:S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Lan NC, Gee KW, Bolger MB, Chen JS. Differential responses of expressed recombinant human γ-aminobutyric acidA receptors to neurosteroids. J Neurochem. 1991;57:1818–1821. doi: 10.1111/j.1471-4159.1991.tb06388.x. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAa receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAa receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Leil TA, Chen ZW, Chang CS, Olsen RW. GABAa receptor-associated protein traffics GABAa receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Río R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Cohen JB, Olsen RW. Neurosteroids allosterically modulate binding of the anesthetic etomidate to γ-aminobutyric acid type A receptors. J Biol Chem. 2009;284:11771–11775. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAa receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAa receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAa receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Madsen KK, Ebert B, Clausen RP, Krogsgaard-Larsen Povl, Schousboe A, White HS. Selective GABA reuptake inhibitors tiagabine and EF1502 exhibit mechanistic differences to modulate the ataxia and anticonvulsant action of the extrasynaptic GABAa receptor agonist gaboxadol. J Pharm Exp Ther. 2011;338:214–219. doi: 10.1124/jpet.111.179671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAa receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAa receptors: relevance to ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABAaR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAa receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maitra R, Reynolds JN. Subunit depedent modulation of GABAa receptor function by neuroactive steroids. Brain Res. 1999;819:75–82. doi: 10.1016/s0006-8993(98)01316-x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAa receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABAa receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol. 2007;71:539–548. doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAa receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAa receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5α-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Mol Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. Selective antagonism of 5α-reduced neurosteroid effects at GABAa receptors. Mol Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Midzak A, Akula N, Lecanu L, Papadopoulos V. Novel androstanediol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem. 2011;286:9875–9887. doi: 10.1074/jbc.M110.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papdopoulos V. Mitochondrial protein import and the steroidogenic mitochondria. Mol Cell Endocrinol. 2011;336:70–79. doi: 10.1016/j.mce.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Gentet LJ, Dempster J, Belelli D. GABAa and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol. 2007;583:1021–1040. doi: 10.1113/jphysiol.2007.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAa receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mitev YA, Darwish M, Wolf SS, Holsboer F, Almedia OFX, Patchev VK. Gender differences in the regulation of 3α-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–549. doi: 10.1016/s0306-4522(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAa receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I. Jasper’s Basic Mechanisms of the Epilepsies. 4th. NCBI; Bethesda: 2012. Plasticity of GABAa receptors relevant to neurosteroid actions. [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAa receptors. J Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Patel B, Smart TG. GABA potency at GABAa receptors found in synaptic and extrasynaptic zones. Front Neurosci. 2012;6:1–10. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAa receptors on rat hippocampal pyramidal neurons. J Physiol. 2010;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the δ subunit of the GABAa receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, Kelly KM. Increase of GABAa receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol Dis. 2010;38:464–475. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Sun CS, Harrison MB, Kapur J. Increased neurosteroid sensitivity of hippocampal GABAa receptors during postnatal development. Neuroscience. 2003;118:655–666. doi: 10.1016/s0306-4522(03)00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, et al. The residence time of GABAaRs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Macdonald RL. Two γ2L subunit domains confer low Zn2+ sensitivity to ternary GABAa receptors. J Physiol. 2001;532:17–30. doi: 10.1111/j.1469-7793.2001.0017g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohria V, Tsai J, Shaw K, Rogawski MA, Pieribone VA, Farfel G, et al. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92:104–107. doi: 10.1016/j.eplepsyres.2010.09.001. Ganaxolone, in Bialer M. [DOI] [PubMed] [Google Scholar]
- Nothdurfter C, Rammes G, Baghai TC, Schüle C, Schumacher M, Papadopoulos V, Rupprecht R. TSPO (18 kDa) as a target for novel anxiolytics with a favourable side-effect profile. J Neuroendocrinol. 2012;24(1):82–92. doi: 10.1111/j.1365-2826.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation during the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, DeLorey TM, Handfort A, Ferguson C, Mihalek RM, Homanics GE. Epilepsy in mice lacking GABAa receptor delta (δ) subunits. Epilepsia. 1997;38(Suppl. 8):123. [Google Scholar]
- Olsen RW, Sieghart W. GABAa receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Takagagi K, Fu Z, Vicini S. Expression of distinct α subunits of GABAa receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92:1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- Otis TS, Koninck YD, Mody I. Lasting potentiation of inhibition is associated with an increased number of γ-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit S, Jeong JA, Jo JY, Cho HS, Kim DW, Kim JM, Ryu PD, Lee SY, Kim HW, Jeon BH, Park JB. Dual mechanisms diminishing tonic GABAa inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol. 2013;110:95–102. doi: 10.1152/jn.00727.2012. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Romagnino S, Contu R, Orru MM, Marotto MF, Zedda P, Lello S, Biggio G, Concas A, Melis GB. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABAa receptor agonist activity. Psychoneuroendocrinology. 2006;31:485–492. doi: 10.1016/j.psyneuen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère J, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang M, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pavlov I, Huusko N, Drexel M, Kirchmair E, Sperk G, Pitkanen A, Walker MC. Progressive loss of phasic, but not tonic, GABAa receptor-mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience. 2011;194:208–219. doi: 10.1016/j.neuroscience.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAa receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAa receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richard JG. Comparative molecular neuroanatomy of cloned GABAa receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- Petratos S, Hirst JJ, Mendis S, Anikijenko P, Walker DW. Localization of p450scc and 5α-reductase type-2 in the cerebellum of fetal and newborn sheep. Dev Brain Res. 2000;123:81–86. doi: 10.1016/s0165-3806(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Petrovic M, Sedlacek M, Cais O, Horak M, Chodounska H, Vyklicky L., Jr Pregnenolone sulfate modulation of N-methyl-D-aspartate receptors is phosphorylation dependent. Neuroscience. 2009;160:616–628. doi: 10.1016/j.neuroscience.2009.02.052. [DOI] [PubMed] [Google Scholar]
- Pillai GV, Smith AJ, Hunt PA, Simpson PB. Multiple structural features of steroids mediate subtype-selective effects on human α4β3δ GABAa receptors. Biochem Pharmacol. 2004;68:819–831. doi: 10.1016/j.bcp.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAa receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAa receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Prince RJ, Simmonds MA. 5 beta-pregnan-3 beta-ol-20-one, a specific antagonist at the neurosteroid site of the GABAa receptor-complex. Neurosci Lett. 1992;135:273–275. doi: 10.1016/0304-3940(92)90454-f. [DOI] [PubMed] [Google Scholar]
- Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAa receptor subunit composition? Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neuroactive steroids act on recombinant human GABAa receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAa receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Joshi S, Sun C, Mtchhedlishvilli Z, Kapur J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L, Hess GP. Mechanism of potentiation of a dysfunctional epilepsy-linked mutated GABAa receptor by a neurosteroid (3 ,21-dihydroxy-5 -pregnan-20-one): transient kinetic investigations. Biochemistry. 2010;49:7892–7901. doi: 10.1021/bi901241g. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Wu Y, Richerson GB. Postdepolarization potentiation of GABAa receptors: a novel mechanism regulating tonic conductance in hippocampal neurons. J Neurosci. 2010;30:7672–7684. doi: 10.1523/JNEUROSCI.0290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003a;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003b;24(3):103–6. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17β-estradiol. Neuroscience. 2004a;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Anticonvulsant activity of the testosteronederived neurosteroid 3α-androstanediol. Neuroreport. 2004b;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–920. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Premenstrual catamenial epilepsy. Women’s Health. 2007;3:195–206. doi: 10.2217/17455057.3.2.195. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Mass spectrometric quantification and physiological-pharmacological activity of androgenic neurosteroids. Neurochem International. 2008;52:541–553. doi: 10.1016/j.neuint.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009a;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Steroid hormones and sex differences in seizure susceptibility. In: Schwartzkroin Philip., editor. Book chapter In: Encyclopedia of Basic Epilepsy Research. Vol. 1. Academic Press; Oxford: 2009b. pp. 526–533. [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol. 2011;2:1–11. doi: 10.3389/fendo.2011.00038. article 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Hormones & Behavior. 2013a;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of hormones and neurosteroids in epileptogenesis. Frontiers in Cellular Neuroscience. 2013b;7:1–20. doi: 10.3389/fncel.2013.00115. article 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Apanites LA. Anesthetic effects of progesterone are undiminished in progesterone receptor knockout mice. Brain Res. 2005;1033(1):96–101. doi: 10.1016/j.brainres.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Chien B, Ramu K. A high-performance liquid chromatography-tandem mass spectrometry assay of the androgenic neurosteroid 3α-androstanediol (5α-androstan-3δ,17β-diol) in plasma. Steroids. 2005a;70:879–885. doi: 10.1016/j.steroids.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005b;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Shetty AK, Apanites LA. Antiepileptic effects of the neurosteroid allopregnanolone on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2005c;46(Suppl.8):298. [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Ramanathan G. Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy Behav. 2012;25(1):92–7. doi: 10.1016/j.yebeh.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testerone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAa receptors. J Pharmacol Exp Ther. 2010;334:1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Role of GABAa and mitochondrial diazepam binding inhibitor receptors in the antistress activity of neurosteroids in mice. Psychopharmacology. 1996;128:280–292. doi: 10.1007/s002130050136. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Res. 1998a;791:108–116. doi: 10.1016/s0006-8993(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Proconvulsant effects of neurosteroid pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. Eur J Pharmacol. 1998b;345:55–59. doi: 10.1016/s0014-2999(98)00034-x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behavior in rats. Pharmacol Biochem Behav. 1999;62:53–60. doi: 10.1016/s0091-3057(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Development of neurosteroid-based novel psychotropic drugs. Progress in Medicinal Chemistry. 2000;37:135–175. doi: 10.1016/s0079-6468(08)70059-6. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000a;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000b;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Kim Y-H, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAa receptor function and seizure suscebtibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010;89:254–260. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Woodward R. Ganaxolone: a prospective overview. Drugs Future. 2004;29:227–242. [Google Scholar]
- Reddy DS, Zeng YC. Differential anesthetic activity of ketamine and the GABAergic neurosteroid allopregnanolone in mice lacking progesterone receptor A and B subtypes. Meth Find Exp Clin Pharmacol. 2007;29:659–664. doi: 10.1358/mf.2007.29.10.1147766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nature Rev Drug Discovery. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAa receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. Endogenous zinc inhibits GABAa receptors in a hippocampal pathway. J Neurphysiol. 2004;91:1091–1096. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325(5939):490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kirkcaldie MTK, Waldron S, Calford MB. Cellular distribution of the GABAa receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Saarelainen KS, Ranna M, Rabe H, Sinkkonen ST, Möykkynen T, Uusi-Oukari M, Linden AM, Lüdens H, Korpi ER. Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic α6β GABAa receptors. J Neurochem. 2008;105:338–350. doi: 10.1111/j.1471-4159.2007.05136.x. [DOI] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. Allosteric modulators induce distinct movements at the GABA-binding site interface of the GABAa receptor. Neuropharmacology. 2011;60:520–528. doi: 10.1016/j.neuropharm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABAa receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAa receptor α6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the α6 gene. J Neurosci. 2006;26:3357–3364. doi: 10.1523/JNEUROSCI.4799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos VR, De Castro OW, Pun RY, Hester MS, Murphy BL, Loepke AW, Garcia-Cairasco N, Danzer SC. Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience. 2011;197:348–357. doi: 10.1016/j.neuroscience.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAa receptors. J Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAa receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullman DM, Walker M. Multiple and plastic receptors mediate tonic GABAa receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield CN. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980;383:249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Selye H. Anesthetics of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. [Google Scholar]
- Sedelnikova A, Erkkila BE, Harris H, Zakharkin SO, Weiss DS. Stoichiometry of a pore mutation that abolishes picrotoxin-mediated antagonism of the GABAa receptor. J Physiol. 2006;577:569–577. doi: 10.1113/jphysiol.2006.120287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAa receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, Floris I, Maciocco E, Sanna E, Biggio G. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAa receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Changes in mIPSCs and sIPSCs after kainite treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol. 2005;94:952–960. doi: 10.1152/jn.01342.2004. [DOI] [PubMed] [Google Scholar]
- Shen H, Gon QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAa receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HJ, Bracamontes J, Taylor A, Wu K, Eaton MM, Gustav Akk, Manion B, Evers AS, Krishnan K, Covey DF, et al. Characteristics of concatemeric GABAa receptors containing α4/δ subunits expressed in Xenopus oocytes. Br J Pharmacol. 2012;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABAa receptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAa receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAa receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, et al. Effect of α subunit on allosteric modulation of ion channel function in stably expressed human recombinant γ-aminobutyric acida receptors determined using 36Cl ion flux. Mol Pharmacol. 2001;59:1108–1118. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Neurosteroid administration and withdrawal alter GABAa receptor kinetics in CA1 hippocampus of female rats. J Physiol. 2005;564:421–436. doi: 10.1113/jphysiol.2004.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAa receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAa receptors: Focus on the α4 and δ subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC., 3rd Ganaxolone, a selective, high-affinity steroid modulator of the γ-aminobutyric acid-A receptor, exacerbates seizures in animal models of absence. Ann Neurol. 1998;44:688–691. doi: 10.1002/ana.410440417. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAa receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAareceptor δ subunit. Epilepsia. 2002;43(Suppl. 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABAa receptor δ subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAa receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAa conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neuroactive steroid metabolism in the human brain. Eur J Endocrinol. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Beyenburg S, Watzka MS, Blumcke I, Bauer J, Schramm J, Bidlingmaier F, Elger CE. Expression of 5α-reductase and 3α-hydroxysteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 2000;41:140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Ludwig M, Clusmann H, Bidlingmaier F, Casarosa E, Luisi S, Elger CE, Beyenburg S. Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 2003;54:11–19. doi: 10.1016/s0920-1211(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Stórustovu S, Ebert B. Gaboxadol: in vitro interaction studies with benzodiazepines and ethanol suggest functional selectivity. Eur J Pharmacol. 2003;467:49–56. doi: 10.1016/s0014-2999(03)01603-0. [DOI] [PubMed] [Google Scholar]
- Stórustovu S, Ebert B. Pharmacological characterization of agonists at δ-containing GABAa receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharm Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Strömberg J, Bäckström T, Lungren P. Rapid non-genomic effects of glucocorticoid metabolites and neurosteroids on the γ-aminobutyric acid-A receptor. Eur J Neurosci. 2005;21:2083–2088. doi: 10.1111/j.1460-9568.2005.04047.x. [DOI] [PubMed] [Google Scholar]
- Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. Intracellular chloride concentration influences the GABAa receptor subunit composition. Nat Commun. 2012;3:738. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of α1, γ2, and δ subunits of GABAa receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAa receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAa receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I. Subunit compensation and plasticity of synaptic GABAa receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci. 2011;5:110. doi: 10.3389/fnins.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyndler J, Maciejak P, Turzynska D, Sobolewska A, Lehner M, Taracha E, Walkowiak J, Skorzewska A, Wislowska-Stanek A, Hamed A, et al. Changes in the concentration of amino acids in the hippocampus of pentylenetetrazole-kindled rats. Neurosci Lett. 2008;439:245–249. doi: 10.1016/j.neulet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Tang X, Hernandez CC, Macdonald RL. Modulation of spontaneous and GABA-evoked tonic α4β3δ and α4β3γ2l GABAa receptor currents by protein kinase A. J Neurophysiol. 2010;103:1007–1019. doi: 10.1152/jn.00801.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Higgs MH, Spain WJ, Ransom CB. Postsynaptic GABAb receptors enhance GABAa receptor function in dentate gyrus granule cells. J Neurosci. 2013;33:3738–3743. doi: 10.1523/JNEUROSCI.4829-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschemacher A, Kasparov S, Kravitz EA, Rahamimoff R. Presynaptic action of the neurosteroid pregnenolone sulfate on inhibitory transmitter release in cultured hippocampal neurons. Brain Res. 1997;772:226–232. doi: 10.1016/s0006-8993(97)00872-x. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAa receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Suzuki T, Misawa M. Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam-withdrawn mice by the neuroactive steroid 5α-pregnan-3α,21-diol-20-one (alloTHDOC) Addiction Biol. 1997;2:455–460. doi: 10.1080/13556219772516. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orru M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAa receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Regulation of GABAa receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- Vardya I, Hoestgaard-Jensen K, Nieto-Gonzalez JL, Dósa Z, Boddum K, Holm MM, Wolinsky TD, Jones KA, Dalby NO, Ebert B, Jensen K. Neuropharmacology. 2012;63:469–479. doi: 10.1016/j.neuropharm.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABAa receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol – a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acida receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Walls AB, Nilsen LH, Eyjolffson EM, Vestergaard HT, Hansen ST, Schousboe A, Sonnewald U, Waagepetersen HS. GAD65 is essential for synthesis of GABA destined for tonic inhibition regulating epileptiform activity. J Neurochem. 2010;115:1398–1408. doi: 10.1111/j.1471-4159.2010.07043.x. [DOI] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, et al. 3beta-Hydroxypregnane steroids are pregnenolone sulfate-like GABAa receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MD, Rahman M, Zhu D, Johansson IM, Bäckström T. 3 -hydroxsteroids and pregnenolone sulfate inhibit recombinant rat GABAa receptor through different channel property. Eur J Pharmacol. 2007;557:124–131. doi: 10.1016/j.ejphar.2006.11.071. [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5alpha-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAa receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Belluzzi JD, Stein L, Lan NC. Comparative behavioral characterization of the neuroactive steroids 3α-OH, 5α-pregnan-20-one and 3α-OH,5β-pregnan-20-one in rodents. Psychopharmacology. 1995;118:65–71. doi: 10.1007/BF02245251. [DOI] [PubMed] [Google Scholar]
- Williams CA, Bell SV, Jenkins A. A residue in loop 9 of the β2-subunit stabilizes the closed state of the GABAa receptor. J Biol Chem. 2010;285:7281–7287. doi: 10.1074/jbc.M109.050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J, Mtchedlishvili Z, Kapur J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology. 2004;46:856–864. doi: 10.1016/j.neuropharm.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rat. Life Sci. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Herb A, Wieland H, Keinä nen, Lüddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAa receptor α4 subunit. FEBS. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAa receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the Nmethyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABAa receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, Luscher B, Chen G. GABAa receptor alpha subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem. 2012;287:27417–27430. doi: 10.1074/jbc.M112.360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reveral of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol. 2006;96:2425–2436. doi: 10.1152/jn.00545.2006. [DOI] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppä E, Linden A-M, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAa receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JYT, Canning KJ, Zhu G, Pennefather P, Macdonald JF, Orser BA. Tonically activated GABAa receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- You H, Dunn SM. Identification of a domain in the δ subunit (S238-V264) of the α4β3δ GABAa receptor that confers high agonist sensitivity. J Neurochem. 2007;103:1092–1101. doi: 10.1111/j.1471-4159.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAa receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotropic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102:670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAa receptor subunits on dentate granule cell dendrites influence tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova NN, Sedelnikova A, Weiss DS. α1β2δ, a silent GABAa receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova NN, Sedelnikova A, Weiss DS. Function and modulation of δ-containing GABAa receptors. Psychoneuroendocrinology. 2009;34S:S67–S73. doi: 10.1016/j.psyneuen.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]