Abstract
The evolutionarily conserved JAK/STAT pathway plays important roles in development and disease processes in humans. Although the signaling process has been well established, we know relatively little about what the relevant target genes are that mediate JAK/STAT activation during development. Here, we have used genome-wide microarrays to identify JAK/STAT targets in the optic lobes of the Drosophila brain and identified 47 genes that are positively regulated by JAK/STAT. About two-thirds of the genes encode proteins that have orthologs in humans. The STAT targets in the optic lobe appear to be different from the targets identified in other tissues, suggesting that JAK/STAT signaling may regulate different target genes in a tissue-specific manner. Functional analysis of Nop56, a cell-autonomous STAT target, revealed an essential role for this gene in the growth and proliferation of neuroepithelial stem cells in the optic lobe and an inhibitory role in lamina neurogenesis.
Keywords: Drosophila, JAK/STAT, optic lobe, neuroepithelial stem cell, neurogenesis
THE Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is highly conserved from invertebrates to vertebrates and plays important roles in a number of developmental processes, particularly in innate immune response, hematopoiesis, and growth control. JAK kinase hyperactivation is causally linked with leukemia and myeloproliferative disorders in humans (Lacronique et al. 1997; Constantinescu et al. 2008), while loss of JAK3 function leads to immunodeficiency (O’Shea et al. 2002). Originally identified in studies of interferon responses in mammalian cells, this pathway is activated by a large number of cytokines and growth factors (Levy and Darnell 2002). In the canonical model, the binding of a cytokine to its receptor causes dimerization of the receptor chains, which brings about close apposition of two associated JAK molecules leading to their activation through trans-phosphorylation. Activated JAKs phosphorylate STAT proteins on specific tyrosine residues, which then dimerize and translocate to the nucleus where they bind to specific DNA sequences and activate transcription of target genes. Although the signaling processes have been well studied, relatively little is known about how activation of this pathway is effected through the expression of target genes.
In Drosophila, this pathway consists of a complete but simpler set of components including three cytokine-like ligands, Unpaired (Upd), Upd2 and Upd3, a single receptor Domeless (Dome), a single JAK kinase Hopscotch (Hop), and a single STAT protein STAT92E (Arbouzova and Zeidler 2006). The JAK/STAT pathway plays a wide range of roles in Drosophila development, including embryonic segmentation, blood cell proliferation and differentiation, eye growth and polarity determination, heart development, border cell migration and stalk cell specification in the ovary, stem cell self-renewal in the testis and intestine, long-term memory formation, and circadian behavior (Arbouzova and Zeidler 2006; Jiang et al. 2009; Liu et al. 2010; Copf et al. 2011; Johnson et al. 2011; Xu et al. 2011; Luo and Sehgal 2012). Recent studies also indicated that the JAK/STAT pathway is required for the development of the larval optic lobe (Yasugi et al. 2008; Ngo et al. 2010; Wang et al. 2011a).
The optic lobe is the visual processing center of the Drosophila brain that contains several neuropils: the lamina, the medulla, the lobula, and the lobula plate (Figure 1, A and B; Meinertzhagen and Hanson 1993). The optic lobe derives from an embryonic optic placode (Green et al. 1993; Hofbauer and Campos-Ortega 1990). During early larval stages, the optic neuroepithelium undergoes extensive proliferation as two distinct populations: the outer proliferation center (OPC) and the inner proliferation center (IPC) (Figure 1A; Hofbauer and Campos-Ortega 1990). Lamina neurogenesis begins in the mid-third instar, on the lateral side of the OPC where retinal axon-delivered signals induce lamina precursor cells (LPCs) to divide once, generating postmitotic cells that differentiate into lamina neurons (Figure 1C; Kunes 2000). Medulla neurogenesis, on the other hand, starts in the late-second instar when neuroepithelial cells (NEs) on the medial edge of the OPC differentiate into neuroblasts (NBs), which undergo asymmetric division producing a daughter neuroblast that self-renews, and a smaller ganglion mother cell (GMC) that divides once producing two medulla neurons (Figure 1C; Nassif et al. 2003; Egger et al. 2007; Hayden et al. 2007). The maintenance of neuroepithelial stem cells and their differentiation into asymmetrically dividing progenitors must be tightly regulated. In JAK mutant brains, the NEs cannot be maintained and prematurely differentiate into medulla NBs, resulting in severe defects in lamina and medulla development (Yasugi et al. 2008; Ngo et al. 2010; Wang et al. 2011a). It is not well understood how the JAK/STAT pathway regulates neuroepithelial maintenance/expansion and the differentiation from NE to NB. To gain further insight into the roles of JAK/STAT in Drosophila brain development, we have attempted to identify JAK/STAT targets in the larval brain. We performed microarray analyses of genes that are differentially expressed in JAK/STAT mutant brains as compared with wild type and identified 47 targets positively regulated by JAK/STAT. More than 60% of these genes encode proteins highly conserved in humans. We further demonstrate that Nop56 is a cell-autonomous STAT92E target and is required for neuroepithelial growth and negatively regulates lamina neurogenesis.
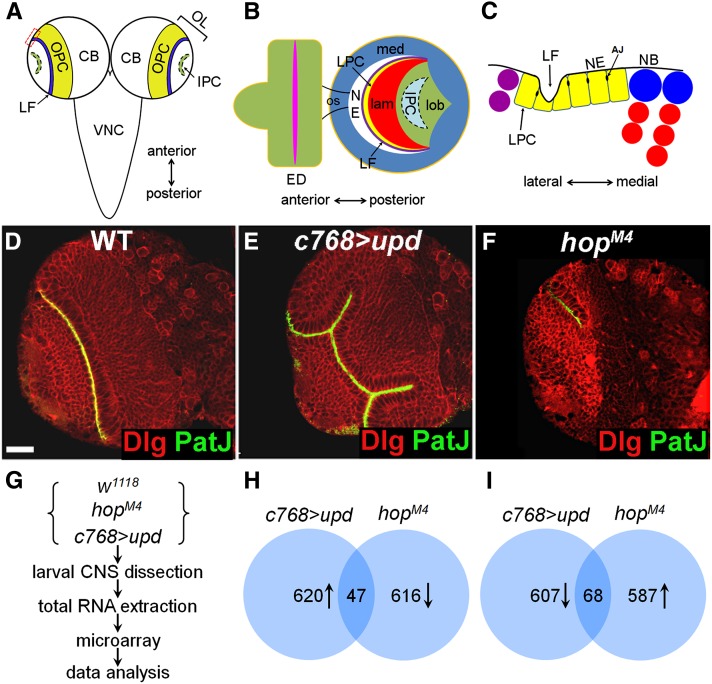
Figure 1.
Identifying JAK/STAT target genes by microarrays. (A) The CNS consists of a pair of brain lobes and a ventral nerve cord (VNC). Each brain lobe is divided into central brain (CB) and laterally located optic lobe (OL) in which reside the outer proliferation center (OPC) and the inner proliferation center (IPC). (B) Lateral view of optic lobe showing the medulla (med), the lamina (lam), and the lobula complex (the lobula and the lobula plate) (lob). The eye disc (ED) is connected with the optic lobe through the optic stalk (os). (C) Magnified view of boxed region in A showing lamina and medulla neurogenesis on the lateral and the medial side of the OPC, respectively. LF, lamina furrow; LPC, lamina precursor cell; NE, neuroepithelial cell; NB, medulla neuroblast; AJ, adherens junction. (D–F) Brains dissected from late-third-instar larvae were stained with PatJ (green) and Dlg (red). (D) Wild-type brain; (E) upd-overexpressing brain; and (F) hopM4 brain. Frontal view, lateral is to the left and medial to the right. Scale bar: 20 μm for D–F. (G–I) The microarray assays. (G) Flowchart of microarray experiments. (H) Genes that are upregulated in upd-overexpressing brains but down regulated in hopM4 brains. (I) Genes that are downregulated in upd-overexpresing brains but upregulated in hopM4 brains.
Materials and Methods
Fly strains and genetic crosses
Flies were reared on standard cornmeal food at 25° unless otherwise indicated. w1118 was used as a wild-type strain. The following alleles and transgenic lines used are described in FlyBase: hopM4, Nop56G4900, UAS-upd, UAS-hopTum-l, UAS-domeDN, c768-Gal4, c855a-Gal4, and GMR-Gal4. We also used UAS-Nop56 (Murata et al. 2008), UAS-Nop56RNAi lines [Vienna Drosophila RNAi Center (VDRC) stocks 51775 and 52165, and National Institute of Genetics (NIG) stocks 13849R2 and 13849R3], UAS-stat92ERNAi (Tsinghua Stock Center, THU 0573), and UAS-FibrillarinRNAi (VDRC 104372). The 10XSTAT92E-GFP line, carrying five tandem repeats of a genomic fragment in the SOCS36E gene that contains two potential STAT92E binding sites, is a reporter of JAK/STAT activity (Bach et al. 2007).
For overexpression and RNAi experiments, UAS flies were crossed with c768-Gal4, c855a-Gal4, or GMR-Gal4 flies, and the progeny were cultured at 25° or 31° until various larval stages before analyses. To induce flip-out clones (Struhl and Basler 1993), UAS-Nop56RNAi or UAS-stat92ERNAi females were crossed to y w hsFlp1/Y; actin < y+< Gal4, UAS-nGFP males; larval progeny were heat-shocked at 38° for 30 min at 48 hr after larval hatching (ALH) and then cultured at 25° until mid-late third (about 84 hr ALH) to late-third instar stages (96 hr ALH) before dissection.
To generate Nop56 mosaic clones, FRT82B Nop56G4900/TM6B Tb females were crossed with y w hs Flp1/Y; FRT82B Ub-GFP/TM2 males. Larval progeny were subjected to a 1-hr heat shock at 38° at about 48 hr ALH to induce somatic recombination. Mid-late third and late-third instar larvae were dissected for analyses.
RNA preparations and microarray analyses
The central nervous system (CNS) consisting of the brain lobes and ventral nerve cord (VNC) was dissected from late-third instar larvae in PBS and stored in RNAlater (Ambion) at 4° until 250–300 CNS specimens were collected. Total RNA was then extracted using Trizol (Invitrogen) and further purified using RNAeasy (Qiagen). Biotin-labeled cRNAs were hybridized to the Affymetrix Drosophila genome 2.0 arrays according to manufacturer’s recommendations. After hybridization, the arrays were scanned with Affymetrix Gene Array Scanner 3000, and the data sets were analyzed using Microarray Suite 5.0 (MAS 5.0). Two independent array assays were performed for each genotype to reduce false-positive signals. A cutoff of a 1.3-fold increase or decrease in expression was used to identify genes that had differential expression in the mutant brains as compared with wild type.
In situ hybridization
Larval CNS was dissected from late-third instar larvae in PBS and fixed overnight at 4° with 8% paraformaldehyde in PBS containing 0.3% Triton X-100. Standard protocols for in situ hybridization were used (Tautz and Pfeifle 1989). Digoxigenin-labeled antisense probes were made for the following 25 genes: CG1732, CG8772, CG8965, CG10376, CG14434, CG15220, CG32634, CG33275, CG33494, CR42862, Chromatin accessibility complex 16kD protein (Chrac-16), Enhancer of Polycomb [E(Pc)], E(spl)mδ, E(spl)m7, Female sterile (2) Ketel [Fs(2)Ket], glial cells missing (gcm), Hairless (H), Juvenile hormone epoxide hydrolase 2 (Jheh2), Nop56, O/E-associated zinc finger protein (Oaz), Replication factor C subunit 4 (RfC4), sulfateless (sfl), Twin of m4 (Tom), Trehalase (Treh), and vielfaltig (vfl).
Antibody production
The entire coding sequence of Nop56 was amplified by PCR using Drosophila genomic DNA as template and the following primer set 5′-TTGCGGCCGCTCGGCATATATTCGGCATAG-3′ and 5′-CCCTCGAGATCAGGGGGTCAAAGGAAAT-3′, with the underlying sequences added to facilitate cloning. The amplified PCR product was cloned into the bacterial expression vector pET28a. The protein was expressed by induction with 0.1 mM IPTG for 16 hr at 18°. Soluble protein fractions containing Nop56 were purified using a nickel column and used to immunize guinea pig. The anti-sera obtained were preadsorbed with fixed embryos and larval CNS specimens and then used to detect Nop56 protein expression.
Immunohistochemistry, BrdU labeling, and TUNEL assays
Larval brain staining was performed as previously described (Wang et al. 2011b). The following primary antibodies were used: guinea pig anti-Deadpan (1:1000, gift from J. Skeath), guinea pig anti-Miranda (1:500, gift from C. Doe), rabbit anti-PatJ (1:1000, gift from H. Bellen), guinea pig anti-Nop56 (1:100, this study), rabbit anti-SOCS36E (1:1000, gift from S. Hou), rabbit anti-PTP61F (1:100, gift from L. Rabinow), rabbit anti-Zfh-1 (1:100, gift from R. Lehmann), mouse anti-Discs large [1:100, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Dachshund (1:100, DSHB), rat anti-Elav (1:100, DSHB), mouse anti-Prospero (1:100, DSHB), rabbit anti-DE-Cadherin (sc-33743, 1:100, Santa Cruz Biotechnology), mouse anti-Fibrillarin (ab4566-250, 1:200, Abcam), and rat anti-BrdU (1:100, Abcam). Secondary antibodies used were: Alexa Fluo-488 goat anti-rabbit (1:200) and Alexa Fluo-488 goat anti-rat (1:200) (Molecular Probes); Cy3-conjugated goat anti-mouse (1:200), Cy3-conjugated goat anti-rat (1:200), Cy5-conjugated goat anti-rat (1:200), and Cy5-conjugated donkey anti-guinea pig (1:200) (Jackson ImmunoResearch Lab).
For BrdU labeling, brains were dissected from mid-third instar larvae in Schneider Drosophila medium, incubated in 200 μg/ml BrdU in the medium for 1 hr at room temperature and then fixed as described above. Prior to incubation with anti-BrdU antibody, the brains were incubated in 2 N HCl for 30 min and neutralized by washing with 0.1 M boric acid. A TUNEL assay kit (Millipore no. S7165) was used to detect apoptotic cells in larval brains. Confocal images were acquired by an Olympus FV500 confocal microscope (60× objective, N.A.1.4) and a Nikon A1R MP confocal microscope [60× (WI) objective, N.A.1.27], and processed using Imaris (Bitplane) and Adobe Photoshop 7.0 software.
Results
Whole-genome analyses of JAK/STAT targets in the Drosophila brain
The JAK/STAT pathway is active in the optic lobe (Yasugi et al. 2008; Ngo et al. 2010; Wang et al. 2011a). Overexpression of upd or hopTum-l, which encodes an activated JAK kinase (Harrison et al. 1995; Luo et al. 1995), results in neuroepithelial overgrowth whereas loss of hop activity leads to an early depletion of NEs and a small brain lobe (Figure 1, E and F; Wang et al. 2011a). The JAK/STAT pathway appears to be specifically required in the optic lobe, as loss of JAK/STAT function has no effect on central brain or ventral nerve cord development (Wang et al. 2011a). To obtain a genome-wide survey of JAK/STAT targets in the brain, we isolated total RNA from the CNS of wild-type, hemizygous hopM4, and upd-overexpressing late-third instar larvae cultured at 25° and conducted microarray assays using Affymetrix oligonucleotide array chips representing about 13,600 annotated Drosophila genes.
Compared with wild type, 620 genes were upregulated in JAK activated (upd-overexpressing) brains (Figure 1H and Supporting Information, Table S1), and an almost equal number of genes (607) were downregulated (Figure 1I and Table S1). By contrast, in JAK-inactivated (hopM4) brains, 616 genes were downregulated, and 587 genes were upregulated (Figure 1, H and I, and Table S2). Thus, it appears that JAK/STAT signaling not only activates gene expression but also represses a large number of genes.
We expected that positively regulated genes would have increased, decreased expression in JAK activated, and JAK inactivated brains, respectively, whereas negatively regulated genes would have opposite changes in expression. Using these criteria, we identified 47 positively regulated (Figure 1H and Table 1) and 68 negatively regulated genes (Figure 1I and Table 2). In the studies presented below, we focused on the positively regulated genes.
Table 1. Genes positively regulated by JAK/STAT.
| Probe Set ID | Gene Name | FC oe/wt | FC lof/wt | Function | Human Homolog | SB Sites | Conserved SBS | Reported Target |
|---|---|---|---|---|---|---|---|---|
| 1628052_at | Cyp6a17 | 3.5 | −6.3 | Cytochrome P450, electron transport | CYP5A1 | |||
| 1638568_s_at | H | 3.3 | −1.5 | Transcription corepressor | 2/434 | Yes | ||
| 1628238_at | Tektin-C | 3.3 | −7 | Microtubule cytoskeleton organization | TEKT1 | |||
| 1640821_at | ftz-f1 | 2.6 | −1.3 | Zinc finger transcription factor | NR5A2 | 2/359 | Yes | |
| 1628689_at | CG17211 | 2.5 | −2.6 | Unknown | ||||
| 1635692_s_at | Treh | 2.4 | −1.3 | Trehalase activity | TREH | 3/228 | Yes | |
| 1635766_at | Fs(2)Ket | 2.4 | −1.7 | Protein import into nucleus | KPNB1 | 2/342 | Yes | |
| 1633334_at | CG15822 | 2.3 | −1.7 | Unknown | KALRN | 4/981 | Yes | |
| 1637631_at | tutl | 2.2 | −1.9 | Axon guidance, signal transduction | IGSF9B | 2/379 | Yes | |
| 1633033_s_at | CG1732 | 2.2 | −1.5 | Neurotransmitter transport | SLC6A1 | 3/186 | ||
| 1628411_at | Oaz | 2.1 | −2.6 | Zinc finger transcription factor | ZNF423 | 4/993 | Yes | |
| 1623256_at | GstE1 | 2.1 | −7.2 | Glutathione transferase activity | ||||
| 1629181_at | CG33494 | 2.1 | −1.8 | Unknown | 3/414 | |||
| 1639074_at | CG33275 | 2 | −1.8 | Guanyl-nucleotide exchange factor | PLEKHG4 | 3/773 | ||
| 1633109_at | CG33229 | 2 | −29.9 | Unknown | ||||
| 1626553_at | CG10376 | 2 | −1.5 | Protein serine/threonine phosphatase | PPM1F | 2/670 | ||
| 1637900_at | CG11852 | 1.9 | −1.6 | Hormone binding, odorant binding protein | 2/411 | |||
| 1633591_at | gcm2 | 1.9 | −2.6 | Transcription factor | GCM2 | |||
| 1634658_a_at | CG8772 | 1.8 | −1.3 | Glutaminase activity | GLS | 2/961 | Yes | |
| 1628110_s_at | E(Pc) | 1.8 | −1.5 | Maintenance of chromatin architecture | EPC1 | |||
| 1626233_at | CG8965 | 1.8 | −2.2 | Signal transduction | 2/428 | Upa | ||
| 1625530_at | CG10462 | 1.8 | −1.7 | Zinc finger transcription factor | Upb | |||
| 1624345_a_at | Nop56 | 1.8 | −1.3 | rRNA metabolism | NOP56 | 2/564 | ||
| 1641174_at | RFeSP | 1.8 | −3.9 | Ubiquinol-cytochrome-c reductase activity | UQCRF1 | |||
| 1637610_at | Chrac-16 | 1.7 | −1.7 | Chromatin accessibility complex | CHRAC-1 | 2/444 | Yes | Downb |
| 1638370_s_at | vfl | 1.7 | −1.6 | Zinc finger transcription factor | 2/208 | Yes | ||
| 1635089_at | tun | 1.7 | −1.6 | Hydrolase activity | WDYHV1 | Upb | ||
| 1633451_at | CG32634 | 1.7 | −2.7 | Unknown | ||||
| 1629940_at | CG13895 | 1.7 | −1.6 | Centromere protein Cenp-B | TIGD3 | |||
| 1631126_at | gcm | 1.6 | −2.2 | Transcription factor | GCM1 | |||
| 1625116_at | trol | 1.6 | −1.4 | Extracellular matrix protein | HSPG2 | 2/165 | Upa, b | |
| 1629523_at | lola | 1.6 | −1.6 | Zinc finger transcription factor | 2/188 | Upb | ||
| 1631534_at | sfl | 1.6 | −1.5 | Sulfotransferase activity | NDST2 | 2/522 | Downb | |
| 1624881_at | CG11448 | 1.6 | −28.3 | Protein binding | RILPL1 | 2/937 | ||
| 1624476_at | Tom | 1.5 | −1.4 | Target of Notch signaling | 3/71 | Yes | ||
| 1625493_at | E(spl)m7 | 1.5 | −1.6 | Target of Notch signaling | HES1 | 2/503 | ||
| 1631048_at | CG14434 | 1.5 | −1.4 | Unknown | ||||
| 1631363_at | Nuf2 | 1.5 | −1.5 | Kinetochore protein | 2/147 | |||
| 1635936_at | CG13822 | 1.5 | −3.8 | Protein binding | GILT | 2/510 | Downb | |
| 1634011_at | E(spl)mδ | 1.5 | −1.4 | Target of Notch signaling | ||||
| 1634199_at | CG15220 | 1.5 | −1.4 | DNA replication factor A complex | RPA3 | |||
| 1626996_s_at | br | 1.5 | −1.5 | Zinc finger transcription factor | 2/604 | Upa | ||
| 1627773_a_at | Jheh2 | 1.4 | −1.3 | Juvenile hormone epoxide hydrolase | EPHX1 | 2/257 | Yes | |
| 1634943_at | RfC4 | 1.4 | −1.5 | DNA replication factor C complex | RFC2 | |||
| 1641102_at | CG14141 | 1.4 | −1.5 | Unknown | PTPRD | |||
| 1628318_at | Spc105R | 1.4 | −1.4 | Kinetochore assembly | Spc105 | 2/515 | ||
| 1625672_s_at | CR42862 | 1.4 | −1.6 | Noncoding RNA |
FC, fold change; wt, wild type; oe, upd overexpression; lof, hopM4; SB Sites, STAT92E binding sites, the numbers refer to the number of STAT92E binding sites in the no. of base pairs of DNA sequence; Conserved SBS, STAT92E binding sites found in other Drosophila species, indicated by “Yes.” Up, positive regulation by JAK/STAT; Down, negative regulation by JAK/STAT.
Table 2. Genes negatively regulated by JAK/STAT.
| Probe Set ID | Gene Name | FC oe/wt | FC lof/wt | Function | Human Homolog | SB Sites | Conserved SBS | Reported Target |
|---|---|---|---|---|---|---|---|---|
| 1640035_at | Fbp2 | −57.7 | 2.9 | lipid metabolism | FBP2 | |||
| 1638338_a_at | Fbp1 | −22.2 | 2.7 | protein transporter | FBP1 | |||
| 1635689_at | Lcp2 | −21.1 | 1.9 | structural constituent of larval cuticle | LCP2 | |||
| 1623675_at | Obp99b | −20.7 | 1.7 | odorant binding protein | ||||
| 1626830_at | Lcp1 | −8 | 3.5 | structural constituent of larval cuticle | LCP1 | |||
| 1626429_at | Lsp1 | −7 | 2.7 | oxygen transporter | LSP1 | |||
| 1628699_at | Lsp1 | −7 | 2.1 | oxygen transporter | LSP1 | |||
| 1638108_at | Sgs8 | −6.7 | 13.9 | structural molecule activity, puparial adhesion | 4/993 | Yes | ||
| 1634409_at | CG31775 | −6 | 1.7 | unknown | ||||
| 1641070_at | CG9021 | −5.9 | 1.5 | protein binding | 4/629 | |||
| 1626088_at | CG2177 | −5.5 | 3.1 | metal ion transporter | SLC39A9 | Upa | ||
| 1626910_at | CG15282 | −5.2 | 2.7 | unknown | 2/705 | |||
| 1634997_at | Sgs5 | −5 | 15.5 | puparial adhesion | ||||
| 1632529_at | tinc | −4.6 | 1.5 | eye photoreceptor cell development | 2/278 | |||
| 1629106_at | CG2233 | −4.5 | 2 | unknown | ||||
| 1627946_at | CG12068 | −4 | 2.1 | visual perception | HSD17B6 | |||
| 1635549_at | TotA | −3.8 | 2.7 | regulation of translation | 3/146 | |||
| 1633607_at | CG2444 | −3.7 | 1.8 | unknown | ||||
| 1630238_at | Lcp4 | −3.5 | 1.7 | unknown | 4/596 | |||
| 1635548_s_at | CG15281 | −3.5 | 2.1 | unknown | ||||
| 1631701_a_at | CG8502 | −3.4 | 1.6 | structural constituent of larval cuticle | ATP1B2 | |||
| 1637552_s_at | CG32423 | −3 | 1.7 | mRNA processing | RBMS1 | 2/102 | ||
| 1627219_at | nAcR64B | −3 | 1.5 | cation transport | CHRNA4 | |||
| 1630593_at | NLaz | −2.9 | 1.9 | lipid transport | APOD | Downa | ||
| 1641634_at | Lsp2 | −2.8 | 2.1 | transport | ||||
| 1630635_s_at | CG7720 | −2.8 | 2 | cation transporter activity | SLC5A8 | 3/511 | Downa | |
| 1631059_at | Pkc98E | −2.7 | 1.8 | protein serine/threonine kinase activity | PRKCE | 2/183 | ||
| 1634525_at | CG7738 | −2.6 | 3.6 | protein binding | 2/735 | |||
| 1638789_at | fau | −2.6 | 1.8 | protein binding | FAU | |||
| 1638314_at | CG12418 | −2.6 | 1.7 | unknown | ||||
| 1625880_at | CG17618 | −2.6 | 1.4 | maintenance of cell polarity | ||||
| 1629229_a_at | srp | −2.4 | 1.9 | transcription factor | GATA6 | 2/542 | ||
| 1632121_a_at | CG6416 | −2.4 | 1.6 | mesoderm development | 3/479 | |||
| 1624211_at | CG9005 | −2.4 | 1.4 | unknown | FAM214 | |||
| 1634012_at | CG5002 | −2.3 | 1.9 | anion transport | SLC26A11 | 3/254 | Yes | |
| 1625236_s_at | Prosap | −2.3 | 1.4 | protein binding | 3/354 | Yes | Downa | |
| 1634447_at | CG31140 | −2.2 | 1.4 | diacylglycerol kinase activity | DGKH | |||
| 1636835_at | CG16700 | −2.2 | 1.3 | amino acid transport | SLC36A4 | Upa | ||
| 1632431_s_at | Ance | −2.1 | 2.1 | proteolysis,peptidyl-dipeptidase A activity | ACE | Downa | ||
| 1628005_at | CG31666/chinmo | −2.1 | 1.4 | zinc finger transcription factor | BTBD10 | 2/219 | Upa,b | |
| 1633048_at | CG8193 | −2.1 | 1.4 | transport | ||||
| 1638899_s_at | CG15312 | −2.1 | 1.3 | unknown | CNTN2 | 2/520 | ||
| 1639079_at | Adgf-D | −2 | 1.6 | adenosine deaminase activity, cell proliferation | CECR1 | 2/910 | Yes | |
| 1624686_a_at | dnc | −2 | 1.4 | 3′,5′-cyclic-AMP phosphodiesterase | PDE4D | 4/985 | Yes | |
| 1634552_at | TepIV | −2 | 1.3 | endopeptidase inhibitor activity | 4/785 | |||
| 1630095_a_at | Drl-2 | −1.9 | 1.8 | transmembrane receptor protein tyrosine kinase | RYK | 4/764 | ||
| 1625967_s_at | l(3)10615 | −1.9 | 1.5 | unknown | ||||
| 1629889_s_at | regucalcin | −1.9 | 1.4 | calcium-mediated signaling | 2/829 | Yes | ||
| 1631554_at | CG11473 | −1.9 | 1.4 | unknown | ||||
| 1624363_at | CG15201 | −1.8 | 2.7 | unknown | RGN | |||
| 1627242_at | l(2)efl | −1.8 | 2.7 | protein folding | CRYAB | |||
| 1626842_a_at | l(3)82Fd | −1.8 | 1.6 | cell wall catabolism | NCOA7 | 2/427 | Yes | |
| 1638820_at | CG12870 | −1.8 | 1.6 | apoptosis | 2/236 | |||
| 1628757_at | Glut1 | −1.7 | 6 | glucose transporter activity | SLC2A1 | |||
| 1626113_at | CG4080 | −1.7 | 2.3 | ubiquitin cycle, zinc finger | C3HC4 | 2/454 | ||
| 1636313_at | CG4914 | −1.7 | 2.1 | transcription factor | TMPRSS11B | Downa | ||
| 1639320_a_at | Ddc | −1.7 | 1.9 | catecholamine metabolism | DDC | |||
| 1631215_at | CG32132 | −1.7 | 1.8 | unknown | ||||
| 1636103_a_at | nocturnin | −1.7 | 1.7 | nucleic acid binding | CCRN4L | 2/322 | ||
| 1625462_s_at | CG31221 | −1.7 | 1.4 | lipoprotein receptor activity | BRCA2 | 2/98 | ||
| 1639826_at | CG32662 | −1.7 | 1.4 | unknown | GOLGA6L10 | 2/64 | ||
| 1640729_s_at | nrv3 | −1.7 | 1.4 | potassium ion transport | 2/620 | |||
| 1633592_a_at | CREG | −1.6 | 2.2 | transcriptional repressor activity | CREG1 | 2/310 | ||
| 1628632_at | Paip2 | −1.6 | 1.4 | negative regulation of translation | PAIP2 | 2/275 | ||
| 1627151_at | CG4612 | −1.6 | 1.3 | mRNA binding | PABPN1 | |||
| 1626417_at | Cht3 | −1.4 | 1.5 | chitinase activity | CHIA | 3/621 | ||
| 1626272_s_at | CG3066 | −1.4 | 1.3 | serine carboxypeptidase | ||||
| 1636122_at | Socs16D | −1.4 | 1.3 | signal transduction | SOCS7 |
FC, fold change; wt, wild type; oe, upd overexpression; lof, hopM4; SB Sites, STAT92E binding sites, the numbers refer to the number of STAT92E binding sites in the number of base pairs of DNA sequence; Conserved SBS, STAT92E binding sites found in other Drosophila species, indicated by “Yes.” Up, positive regulation by JAK/STAT; Down, negative regulation by JAK/STAT.
Verification of target gene expression in the brain
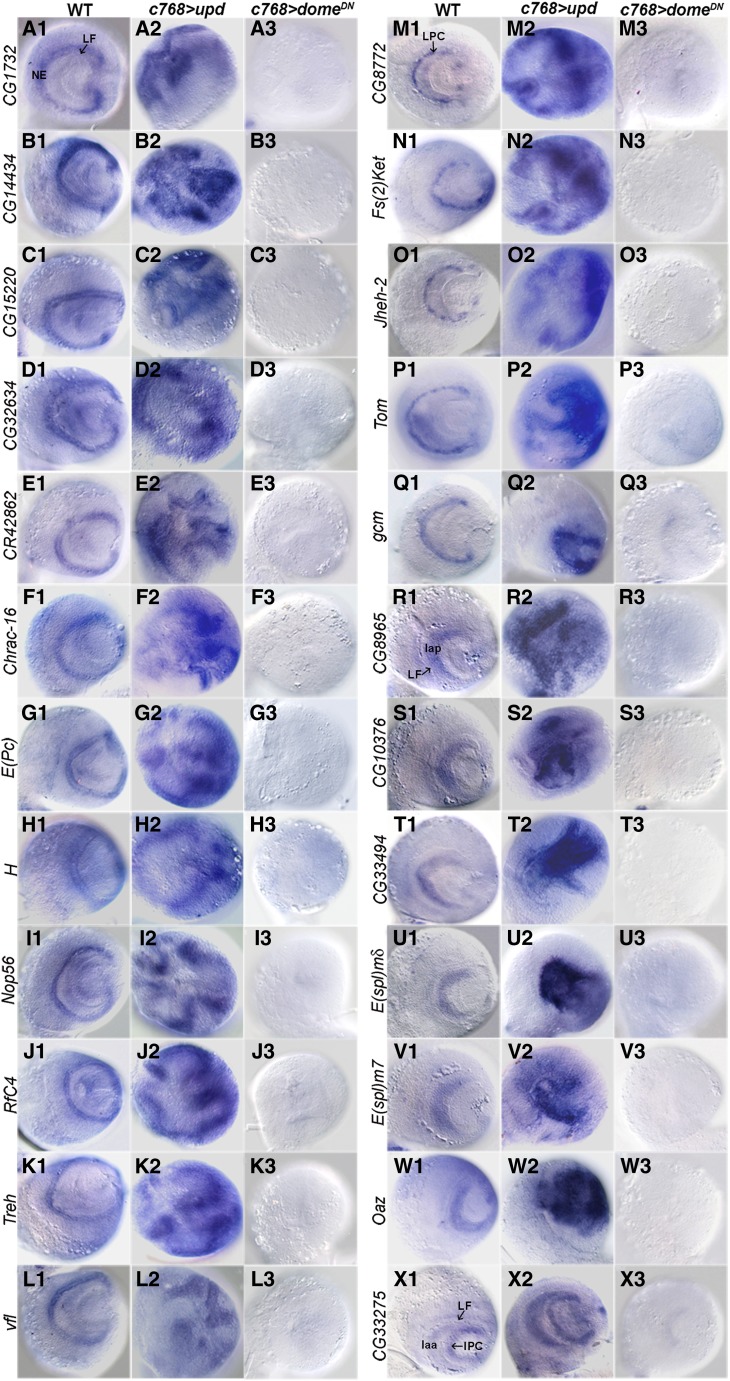
In situ hybridization assays were performed for 25 positively regulated genes. We found that 24 of them are expressed in the optic lobe with specific patterns (Figure 2), while sfl has a ubiquitous, low level of expression in the optic lobe (not shown). These 24 genes can be grouped into four classes: (1) class I genes are expressed in the NEs, including CG1732, CG14434, CG15220, CG32634, CR42862, Chrac-16, E(Pc), H, Nop56, RfC4, Treh, and vfl (Figure 2, A–L); (2) class II genes are expressed in LPCs (Figure 2, M–Q), including CG8772, Fs(2)Ket, Jheh-2, and Tom, which are expressed in discrete groups of cells, and gcm, which is expressed in a continuous stretch of cells; (3) class III genes are expressed in mature lamina neurons, including CG8965, CG10376, CG33494, E(spl)mδ, E(spl)m7, and Oaz (Figure 2, R–W); and (4) class IV gene, CG33275, is expressed in developing lamina neurons and the IPC (Figure 2X). Each of these genes was upregulated in JAK-activated brains, but was strongly reduced or undetectable in JAK-inactivated brains (Figure 2). These results are consistent with the microarray data (Table 1) and validate our approach to identifying JAK/STAT target genes.
Figure 2.
Verification of JAK/STAT targets by in situ hybridization. (A–L) Targets expressed in the NEs. (M–Q) Targets expressed in LPCs. (R–W) Targets expressed in mature lamina neurons. (X) CG33275 expressed in developing lamina neurons and the IPC. Each gene was upregulated in brains overexpressing upd and downregulated in brains expressing a dominant-negative Dome receptor (domeDN). NE, neuroepithelial cells; LF, lamina furrow; LPC, lamina precursor cell; laa, anterior lamina cells (developing lamina neurons); lap, posterior lamina cells (mature lamina neurons). Lateral view, anterior is to the left and dorsal is up.
To examine which genes might be direct STAT92E targets, we searched for putative enhancers with STAT92E binding site clusters from 5 kb upstream to 5 kb downstream of each gene. In vitro studies showed that STAT92E binds to the consensus sequence TTCNNNGAA (Yan et al. 1996), which is similar to mammalian STAT binding sites. Twenty-eight genes (60%) that have at least two STAT92E binding sites in a short noncoding genomic sequence were identified (Table 1). Further, the STAT92E binding site clusters in 12 genes are conserved in diverse Drosophila species (Table 1), increasing the possibility that these sites are activated by STAT92E. In comparison, a similar search for 40 randomly selected genes identified only four genes (10%) with STAT92E binding site clusters (data not shown).
Interestingly, nearly half of the negatively regulated genes (31/68) also contain clusters of STAT92E binding sites (Table 2), suggesting that these genes may be directly repressed by activated STAT92E. While not extensively studied, the repressor function of STAT protein has been reported in the slime mold Dictyostelium (Mohanty et al. 1999).
Nop56 is a functional target of JAK/STAT signaling
We examined in more detail the relationship between JAK/STAT signaling and Nop56, a potential direct target gene. Nop56 encodes a protein highly conserved from yeast to humans. In the yeast, it is involved in ribosome biogenesis and cell growth (Gautier et al. 1997). The role of Nop56 in animal development has not been well studied. Previous studies showed that Nop56 gene expression is activated by dMyc, implicating Nop56 in cellular growth in Drosophila (Orian et al. 2003; Hulf et al. 2005; Pierce et al. 2008; Furrer et al. 2010). Neumüller et al. (2011), through RNA interference (RNAi) screens, identified Nop56 as a regulator of neuroblast proliferation in the central brain.
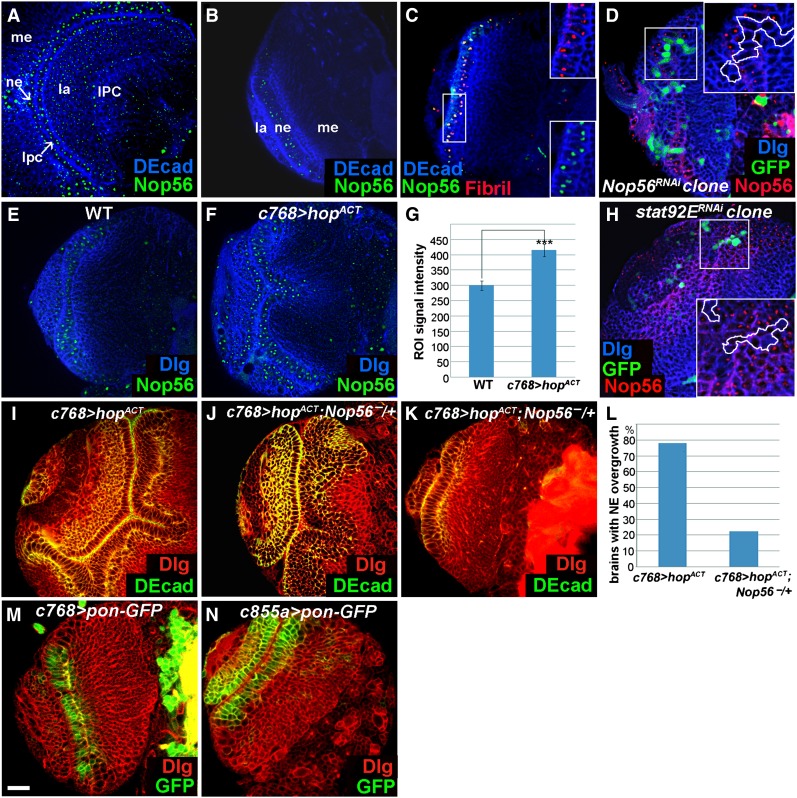
Nop56 RNA is detected in the NEs (Figure 2I1), and its expression positively responds to JAK signaling activity (Figure 2I2, I3). We raised an antibody to study Nop56 protein expression and found that the protein is strongly expressed in the NEs, medulla neuroblasts, LPCs, and the IPC (Figure 3, A and B) and in neuroblasts of the central brain and VNC (not shown). Nop56 expression is much reduced in lamina and medulla neurons (Figure 3A). Costaining with Fibrillarin, a marker for the nucleolus, showed that Nop56 is localized in the nucleolus (Figure 3C). The specificity of this antibody can be demonstrated by the strong reduction or loss of Nop56 protein in NE clones expressing Nop56RNAi (Figure 3D). Importantly, Nop56 protein expression increased in the NEs overexpressing hopTum-l (Figure 3, E–G, compare F with E; quantification is shown in G), and was strongly reduced or eliminated cell autonomously in clones of NEs expressing stat92ERNAi (Figure 3H). A reduction of Nop56 gene dosage to half (Nop56G4900/+) significantly suppressed neuroepithelial overgrowth of JAK-activated brains (Figure 3, I–L), indicating that Nop56 is an important mediator of JAK/STAT activation in the optic lobe.
Figure 3.
Nop56 is a functional target of the JAK/STAT pathway. Brains dissected from mid-late third- and late-third-instar larvae cultured at 25° were stained with the makers indicated. (A and B) Nop56 protein is strongly expressed in the NEs, medulla neuroblasts, LPCs, and the IPC, but is weakly expressed in lamina and medulla neurons. ne, neuroepithelial cells; lpc, lamina precursor cell; me, medulla; la, lamina; IPC, inner proliferation center. (C) Nop56 protein colocalizes with Fibrillarin (Fibril). (D) Nop56 expression is reduced or eliminated in clones of cells expressing Nop56RNAi. (E–G) Nop56 level increases in the NEs overexpressing hopACT (c768–Gal4/UAS–hopTum-l) (F), as compared with wild type (E); (G) quantification of Nop56 staining intensity, n = 17 ROIs (region of interests) for wild-type and for c768–Gal4/UAS–hopTum-l brains, each ROI contains 50 nucleoli. ***, P < 0.01. (H) Nop56 expression is reduced or eliminated in clones of cells expressing stat92ERNAi. (I–L) Overexpression of hopTum-l causes neuroepithelial overgrowth (I), a reduction of Nop56 gene dosage to half (Nop56G4900/+), partially (J), or almost completely (K) suppressed neuroepithelial overgrowth; (L) quantification of suppression: 78% of c768–Gal4/UAS-hopTum-l brains (n = 23) had neuroepithelial overgrowth; only 22% of c768–Gal4/UAS-hopTum-l; Nop56G4900/+ brains (n = 36) still had significant neuroepithelial overgrowth. (M and N) Expression patterns of c768–Gal4 and c855a–Gal4 as revealed by UAS–pon–GFP. (A) Lateral view, anterior is to the left, dorsal is up; (B–F, H–K, M, and N) frontal view, lateral is to the left, medial to the right. Scale bar, 20 μm.
Nop56 is required for neuroepithelial growth in the optic lobe
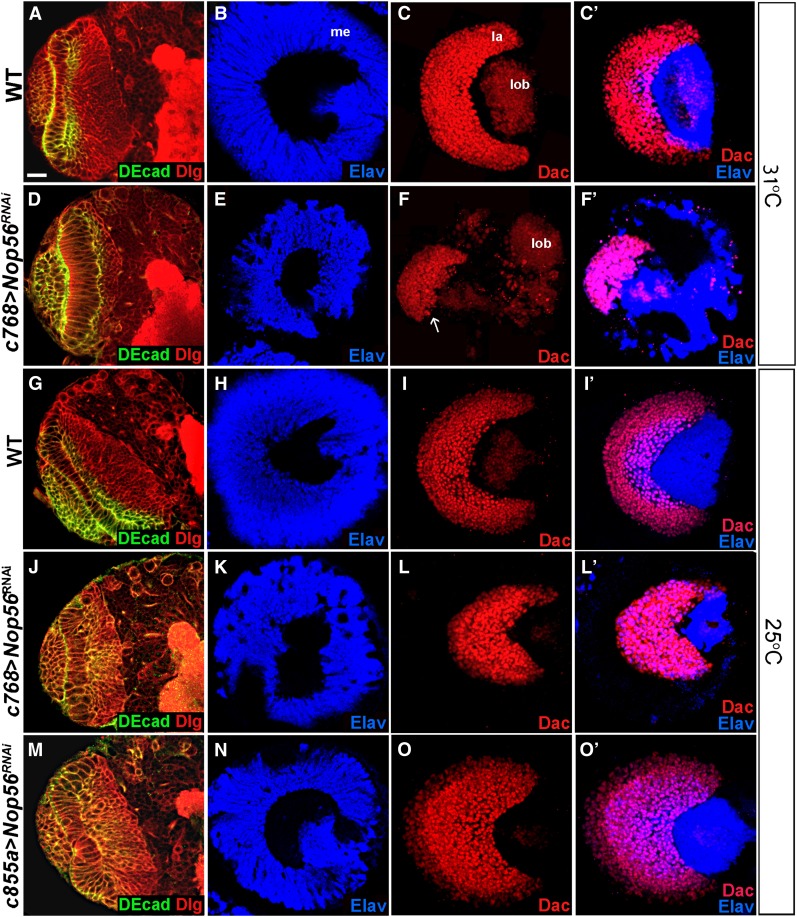
To study the role of Nop56 in optic lobe development, we used RNAi to knock down Nop56 expression, using c768–Gal4, a driver active in the NEs from the first-instar stage onward (Figure 3M; Wang et al. 2011b). Knockdown of Nop56 RNA at 31° caused severe optic lobe defects. The medulla was much reduced in size (Figure 4E; 97%, n = 36), and the lamina had only small patches of cells (Figure 4F; 100%, n = 36). Knockdown of Nop56 RNA at 25° led to similar but less severe defects in medulla and lamina development (Figure 4, K and L), which is expected because RNAi knockdown would be less efficient at lower temperature due to reduced Gal4 activity. To ascertain that the Nop56 RNAi did not cause nonspecific effects, we expressed several additional Nop56RNAi constructs, which together targeted two different regions of Nop56 RNA; expression of these constructs led to similar optic lobe defects (Figure S1). The eye imaginal discs were not affected by Nop56 RNAi under c768–Gal4 control (Figure S2B), and knockdown of Nop56 RNA using GMR–Gal4, which is active only in eye imaginal disc cells behind the morphogenetic furrow, did not cause defects in eye or lamina development (Figure S2D). Thus, the lamina defect did not arise from abnormal eye development. We conclude that Nop56 activity is required for both lamina and medulla development.
Figure 4.
Nop56 is essential for optic lobe development. (A–F′) Brains dissected from late-third-instar larvae cultured at 31° were stained with the makers indicated. (A–C′) Wild-type optic lobes having columnar NEs (A), a dome shaped medulla cortex (B), and a crescent lamina (C, C′). me, medulla; la, lamina; lob, lobula complex. (D–F′) c768–Gal4/UAS-Nop56RNAi optic lobes having elongated NEs (D), a small medulla cortex (E), and a small, truncated lamina (F, F′; arrow indicates lamina). Note that all lamina cells differentiated into mature neurons (F′). (G–O′) Brains dissected from late-third-instar larvae cultured at 25° were stained with the markers indicated. (G–I′) Wild-type optic lobes having columnar NEs (G), a dome-shaped medulla cortex (H), and a crescent lamina (I and I′). (J–L′) c768–Gal4/UAS–Nop56RNAi optic lobes having elongated NEs (J), a smaller medulla (K), and a smaller lamina (L) in which all cells differentiated into mature lamina neurons (L′). (M–O′) c855a–Gal4/UAS–Nop56RNAi optic lobes having elongated NEs (M), a smaller medulla (N), but an enlarged lamina (O) in which more lamina cells differentiated into mature neurons (O′, compare with I′). (A, D, G, J, M) Frontal view, lateral is to the left, medial to the right; (B–C′, E–F′, H–I′, K–L′, N–O′) lateral view, anterior is to the left, dorsal is up. Scale bar, 20 μm.
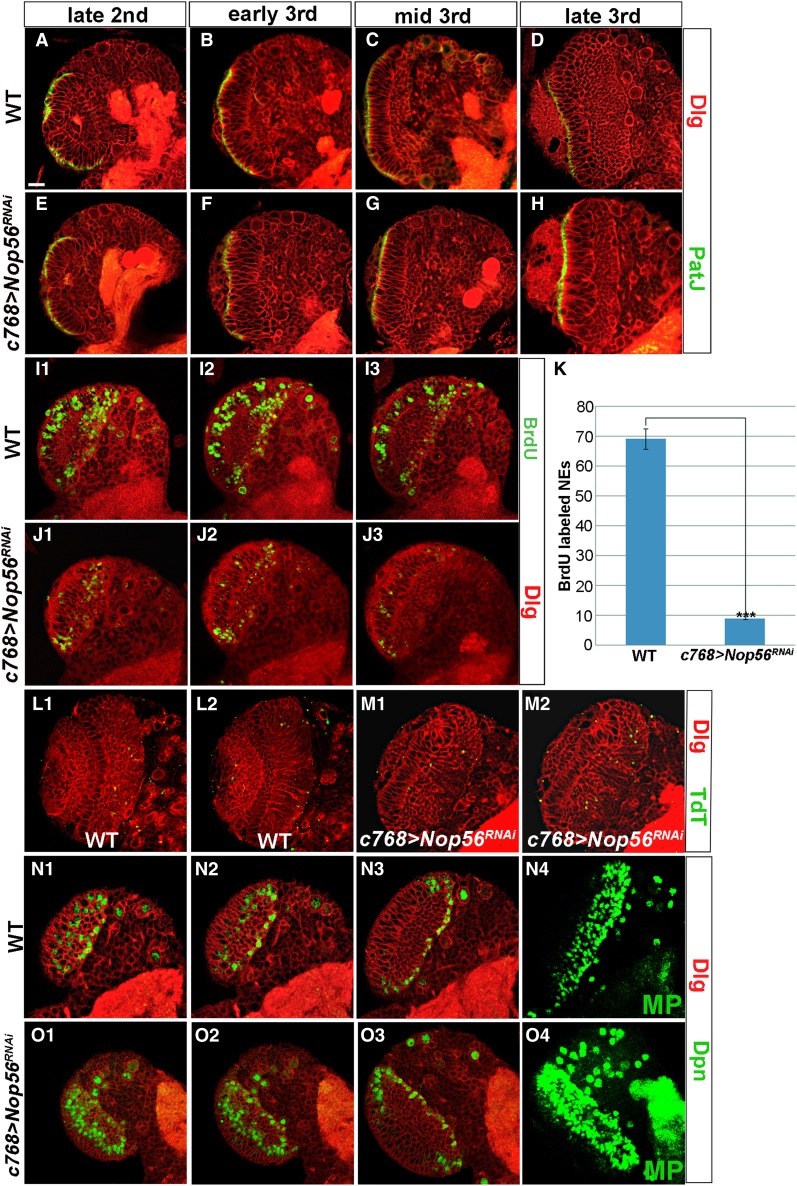
Since the OPC generates precursor cells for lamina and medulla neurons, the defects observed in Nop56 RNAi brains might arise from a deficiency in neuroepithelial maintenance and expansion in earlier larval stages. We thus performed a time-course study to examine whether neuroepithelial growth was affected by the loss of Nop56 activity (Figure 5, A–H). The NEs can be recognized by staining for apical epithelial markers PatJ and atypical PKC (aPKC), adherens junction protein DE-cadherin, and baso-lateral marker Discs large (Dlg). In wild-type brains, the optic neuroepithelium grows during early larval stages and reaches a maximal size by mid-third instar; the NEs then gradually reduce in number as neurogenesis takes place on both the lateral and the medial side of the OPC (Figure 5, A–D). In brains expressing Nop56RNAi, early neuroepithelial proliferation did not seem to be affected since the mutant brains had similar numbers of NEs to wild type till the late-second-instar stage (Figure 5E). However, the mutant NEs proliferated poorly subsequently and did not expand from the early third-instar stage onward (Figure 5, F–H); instead, the NEs became elongated, perhaps reflecting an increase in cell mass and volume (Figure 5, F–H; compare with C and D and see Figure 4D). To further examine the effect of Nop56 loss on neuroepithelial proliferation, we performed BrdU labeling to monitor DNA replication in wild-type and Nop56RNAi brains dissected from mid-third-instar larvae. We found that Nop56 mutant NEs had much reduced BrdU labeling (Figure 5, J and K), suggesting that loss of Nop56 inhibited cell-cycle progression. No significant cell death was detected in the mutant brains (Figure 5M). We conclude that Nop56 activity is required for neuroepithelial growth, probably by affecting cell-cycle progression.
Figure 5.
Nop56 is essential for neuroepithelial growth. (A–H) Brains dissected from larvae cultured at 25° were stained with PatJ (green) and Dlg (red). In wild-type brains (A–D), NE numbers per brain section increased from 42 at early third-instar to 71 at mid-third instar and then dropped to 60 at late-third instar, whereas in c768–Gal4/UAS–Nop56RNAi brains (E–H), NE numbers did not change from early third to late-third-instar stages, averaging about 29 cells, but the mutant NEs became elongated in the third-instar stages (F, G, H, compare with C and D). (I–K) BrdU labeling of brains dissected from mid-third-instar larvae cultured at 25°. Three brain sections at comparable levels were shown for wild-type (I1, I2, I3) and c768–Gal4/UAS–Nop56RNAi brains (J1, J2, J3); (K) quantification of BrdU labeling, the total number of labeled NEs in three sections was used for calculation. Nop56 RNAi brains had a much reduced number of labeled NEs (n = 10 for wild-type and for Nop56 RNAi brains; ***, P < 0.01). (L and M) Apoptotic cells in brains dissected from late-third-instar larvae were labeled with TdT (green). No significant cell death occurred in c768–Gal4/UAS–Nop56RNAi brains (M). (N–O) Brains dissected from mid-third-instar larvae cultured at 25° were stained with Dpn and Dlg. (N1–N3) Three sections of a wild-type brain lobe, (N4) is a maximal projection (MP) image showing total Dpn+ cells in the entire optic lobe. (O1–O3) Three sections of a c768–Gal4/UAS–Nop56RNAi brain lobe; (O4) is a maximal projection image showing total Dpn+ cells in the entire optic lobe. Nop56 RNAi optic lobes had an increased number of Dpn+ cells compared to wild type. Frontal view, lateral is to the left, medial to the right. Scale bar, 20 μm.
Loss of Nop56 leads to premature neuroepithelial differentiation into neuroblast
Cell-cycle progression is tightly linked with neuroepithelial maintenance and the transition from NE to NB (Zhou and Luo 2013). We therefore tested whether loss of Nop56 might lead to premature neuroepithelial differentiation. Indeed, we found that while wild-type brains had a limited number of medulla NBs at mid-third instar (Figure 5, N1–N4), the number of NBs increased significantly in Nop56RNAi brains (Figure 5, O1–O4).
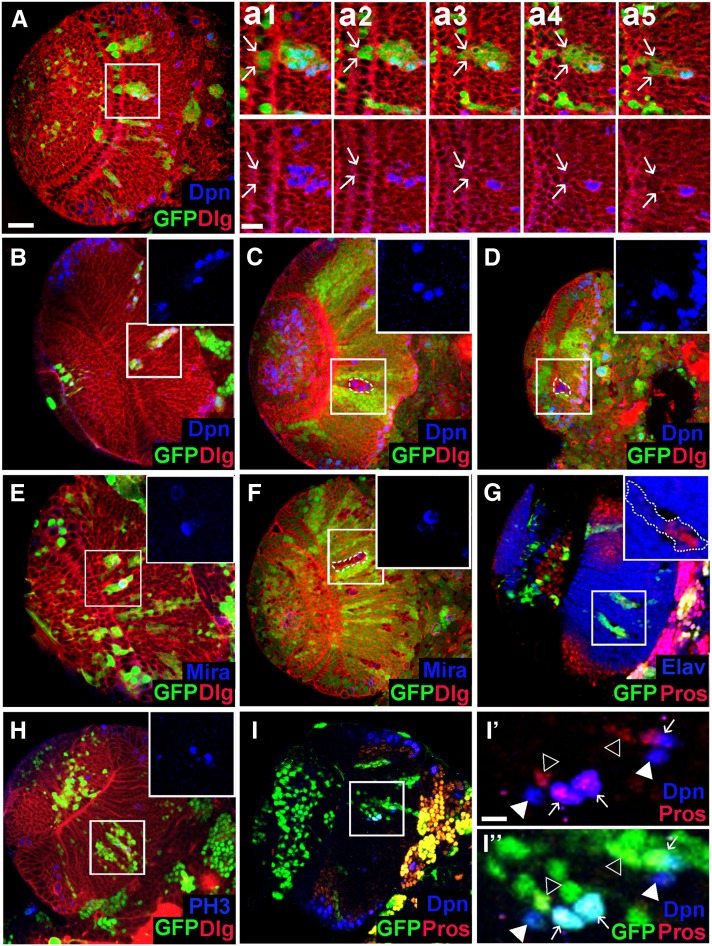
To further examine the cell-autonomous requirement of Nop56 in neuroepithelial maintenance, we removed Nop56 activity in cell clones. Clones of cells expressing Nop56RNAi were induced at late-second instar, and brains were examined at mid-late-third to late-third instar stages. NE clones were infrequently found, and most clones were entirely localized in the medulla cortex. The NE clones, when found, contained a few NEs and a number of cells in the medulla cortex (Figure 6A). Some mutant cells in the medulla expressed Dpn, indicating that they had prematurely differentiated into neuroblasts (Figure 6, A and B). The neuroblasts appeared to undergo asymmetric cell division as Miranda (Mira) was localized in a crescent at one pole during mitosis (Figure 6E). Prospero (Pros), which is weakly expressed in the GMCs but not in medulla neurons, was detected in a number of cells (Figure 6, G and I), suggesting that GMCs were generated. Mature neurons were also generated in the clone, as revealed by the expression of Elav, a neuron-specific marker (Figure 6G). Similarly, in mosaic clones mutant for a strong loss-of-function Nop56 allele, Nop56G4900, ectopic neuroblasts were generated; the clones were most frequently found in the medulla, presumably extruded from the neuroepithelium (Figure 6, C, D, and F). Taken together, these data indicate that Nop56 is required for neuroepithelial growth/expansion, and loss of Nop56 function in cell clones leads to premature differentiation of NEs into NBs, which can self-renew and generate medulla neurons.
Figure 6.
Loss of Nop56 activity in cell clones leads to premature neuroepithelial differentiation into neuroblast. (A) A Nop56RNAi flip-out clone containing two to three NEs (indicated by arrows in a1 and a2) and a number of cells located in the medulla. a1–a5 show consecutive confocal sections of the mutant clone; arrows indicate the lateral edge of the clone. Several mutant cells in the medulla express Deadpan (Dpn). (B–D) Ectopic Dpn+ cells seen in Nop56RNAi clones (B) and Nop56G4900 clones (C and D) entirely localized in the medulla. (E and F) Mutant cells in a Nop56RNAi clone (E) or a Nop56G4900 clone (F) in the medulla undergo mitotic division with asymmetric polar localization of Miranda (Mira). (G) Mutant cells in a Nop56RNAi clone in the medulla express Prospero (Pros) or Elav. (H) Mutant cells in Nop56RNAi clones in the medulla undergo mitosis as detected by anti-phospho histone 3 (PH3) staining. (I, I′, I′′) Some Dpn+ cells in Nop56RNAi clones in the medulla express Prospero while other Dpn+ cells do not. Arrows in I′ and I′′ indicate coexpression of Dpn and Pros; arrowheads indicate expression of either Dpn or Pros. (A–I) Frontal view, lateral is to the left, medial to the right. Scale bar in A, 20 μm for A–I; scale bar in a1, 10 μm for a1–a5; scale bar in I′, 10 μm for I′ and I′′.
Loss of Nop56 accelerates lamina neurogenesis
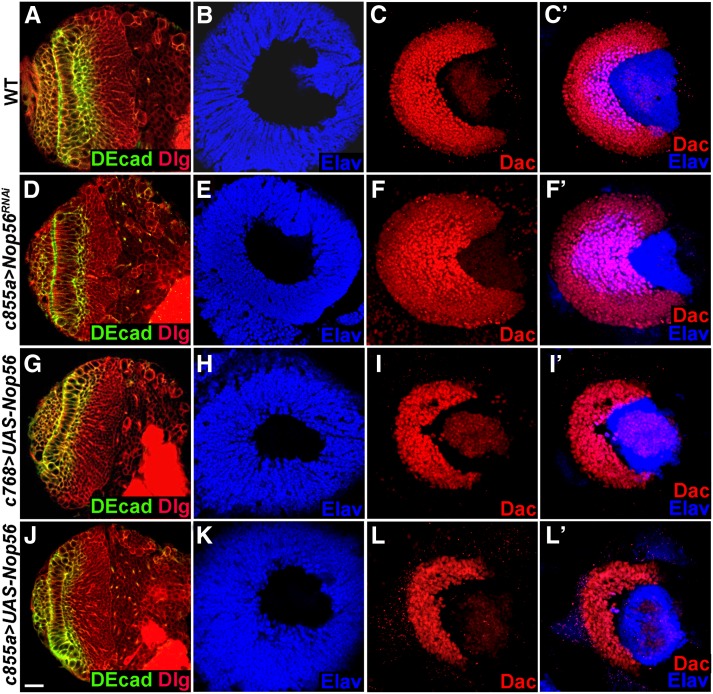
The expression of Nop56 RNAi using c768–Gal4 caused defects in both medulla and lamina development (Figure 4, E and F), which could be attributed to a deficiency in neuroepithelial growth during the third-instar larval stages (Figure 5, E–H). When Nop56 RNAi was induced by a different driver, c855a–Gal4, which is also expressed in the NEs from the first-instar stage onward (Figure 3N; Egger et al. 2007; Wang et al. 2011b), we found that although the medulla was somewhat smaller than wild type (Figure 7E; 92%, n = 39), the lamina was much enlarged (Figure 7F; 87%, n = 39); anti-Elav staining showed that more lamina cells had differentiated into neurons (Figure 7F′, 87%, n = 39). Several different Nop56RNAi constructs were tested; the expression of each construct caused an enlarged lamina (Figure S3). Enhanced lamina neurogenesis was also observed when Nop56 RNA was knocked down at 25° (Figure 4, O and O′; compare with wild-type lamina in Figure 4, I and I′). Nop56 RNAi using c855a–Gal4 did not affect eye development (Figure S2C), indicating that lamina enlargement did not arise from abnormalities in the eye. These data indicate that reducing Nop56 activity favors lamina neurogenesis. We noted that Nop56 RNAi using c855a–Gal4 consistently had a weaker effect on neuroepithelial growth than Nop56 RNAi using c768–Gal4 (compare Figure 4M with Figure 4J at 25°, and Figure 7D with Figure 4D at 31°), which may explain why we did not observe enlarged lamina in brains expressing Nop56RNAi using c768–Gal4; however, lamina cells that formed in such brains were all mature neurons (Figure 4F′ and L′), consistent with accelerated lamina neurogenesis seen in brains expressing Nop56RNAi under c855a–Gal4 control.
Figure 7.
Nop56 activity inhibits lamina neurogenesis. Brains dissected from late-third-instar larvae cultured at 31° were stained with the markers indicated. (A–C′) Wild-type optic lobes having columnar NEs (A), a dome-shaped medulla cortex (B), and a crescent lamina (C and C′). (D–F′) c855a–Gal4/UAS–Nop56RNAi optic lobes having elongated NEs (D), and a smaller medulla (E), but a much enlarged lamina (F) with more mature lamina neurons (F′, Elav staining). (G–I′) Overexpression of Nop56 using c768–Gal4 caused a small lamina (I) and inhibited lamina neuron differentiation (I′, Elav staining), but did not affect medulla development (G and H). (J–L′) Overexpression of Nop56 using c855a–Gal4 caused a small lamina (L), and inhibited lamina neuron differentiation (L′, Elav staining), but had no effect on medulla development (J and K). (A, D, G, J) Frontal view, lateral is to the left, medial to the right; (B, C, C′, E, F, F′, H, I, I′, K, L, L′) lateral view, anterior is to the left, dorsal is up. Scale bar, 20 μm.
Overexpression of Nop56 suppresses lamina neurogenesis
Since loss of Nop56 function resulted in accelerated lamina neurogenesis, we tested whether Nop56 overexpression may inhibit lamina neurogenesis. Indeed, overexpression of Nop56 using c855a–Gal4 suppressed lamina development. The lamina was reduced in size and there were very few mature lamina neurons (Figure 7, L and L′, 94%, n = 16). Nop56 overexpression did not appear to affect medulla development as judged by a normal size of the medulla cortex (Figure 7K, n = 16). Overexpression of Nop56 using c768–-Gal4 also inhibited lamina development as reflected by a much smaller lamina with virtually no mature lamina neurons (Figure 7, I and I′, 85%, n = 13), and again no effect on medulla development was observed (Figure 7H, n = 13). We conclude that high Nop56 activity suppresses lamina neuron differentiation.
Discussion
In this study, we have used genome-wide microarray assays to identify JAK/STAT target genes in the Drosophila brain. Forty-seven positively regulated genes have been identified. Many targets encode highly conserved proteins, which are excellent candidates for mediating JAK/STAT responses in mammalian brain development.
Several previous studies examined JAK/STAT targets in different tissues or cell types, including germ-line stem cells in the testis (Terry et al. 2006), eye imaginal discs (Flaherty et al. 2009), whole third-instar larvae (Kwon et al. 2008), and cultured Kc167 cells (Bina et al. 2010). Each of these studies compared differences between wild-type and JAK-activated mutants, and in each case, hundreds of potential targets were identified. Our study differs from the previous studies in that we examined not only JAK activated mutants but also, for the first time, JAK loss-of-function mutants. Thus, although we similarly identified hundreds of genes upregulated in JAK-activated brains (Figure 1H and Table S1), the JAK loss-of-function experiments allowed us to filter out a large number of genes that did not change expression in JAK-inactivated brains. Our approach was efficient since most of the candidate targets subsequently tested by in situ hybridization are indeed specifically expressed in the optic lobe, and all the targets positively responded to JAK signaling activity.
A comparison between our targets and those reported by Flaherty et al. (2009) and Terry et al. (2006) identified several common genes (Table 1 and Table 2). terribly reduced optic lobes (trol), which encodes a Perlecan, is the only positively regulated gene found in the eye disc, testis, and optic lobe, while several other targets in the optic lobe were found in either the eye disc or the testis. Notably, chronologically inappropriate morphogenesis (chinmo) is repressed by JAK/STAT in the optic lobe (Table 2), in contrast to it being activated in the eye disc and testis (Flaherty et al. 2009, 2010; Terry et al. 2006).
Conversely, several previously reported targets, namely, Suppressor of cytokine signaling at 36E (SOCS36E) (Karsten et al. 2002), zinc finger homeodomain 1 (Zfh-1) (Terry et al. 2006; Leatherman and Dinardo 2008), Protein tyrosine phosphatase 61F (PTP61F) (Baeg et al. 2005; Muller et al. 2005), and dome (Ghiglione et al. 2002; Hombria et al. 2005; Rivas et al. 2008; Flaherty et al. 2009) were not identified in the optic lobe. Examination of their expression in the brains using antibody staining and quantitative PCR assays showed that these genes were not positively regulated by JAK/STAT in the optic lobe, as their expression either did not increase cell autonomously in JAK-activated brains (in the case of SOCS36E, PTP61F, and Zfh-1) (Figure S4) or did not decrease in JAK-inactivated brains (in the case of dome) (Figure S5), although SOCS36E and dome total RNA did increase significantly in JAK-activated brains (Table S1 and Figure S5). Altogether, these results suggest that the JAK/STAT pathway may regulate different target genes in a tissue-specific manner.
JAK/STAT signaling and transcriptional control of gene expression
Our targets are enriched with transcription factors (Table 1). Strikingly, four of these genes are associated with Notch signaling. E(spl)mδ, E(spl)m7, and Tom are direct targets of the Notch pathway (Bray 2006), while H is a transcription corepressor involved in repression of Notch signaling activity (Bang et al. 1995). The Notch pathway is required for neuroepithelial maintenance and expansion (Egger et al. 2010; Ngo et al. 2010; Reddy et al. 2010; Yasugi et al. 2010; Orihara-Ono et al. 2011; Wang et al. 2011b; Weng et al. 2012). Our microarray data support a model in which JAK/STAT acts upstream of Notch signaling in the optic lobe (Wang et al. 2011a).
gcm and gcm2 are both expressed in LPCs (Figure 2Q1; Chotard et al. 2005), and promote lamina neuron differentiation (Chotard et al. 2005). Although gcm and gcm2 are activated by JAK/STAT (Figure 2Q2, Table 1), no STAT92E binding site clusters are identified in the noncoding genomic sequences of gcm or gcm. The JAK/STAT pathway may control their expression indirectly. Alternatively, STAT92E may utilize binding sites found somewhat further upstream of gcm2 or downstream of gcm (not shown).
broad (br), ftz transcription factor 1(ftz-f1), longitudinals lacking (lola), Oaz, vfl (also called Zelda), and CG10462 encode zinc finger transcription factors. br, ftz-f1, lola, Oaz, and vfl play diverse developmental roles in Drosophila; they each contain STAT92E binding site clusters in noncoding genomic sequences. It will be important to examine the roles of these zinc finger proteins in optic lobe development and their significance as effectors in mediating JAK/STAT activation.
Nop56 and optic lobe development
We have shown that Nop56 is a functional downstream effector of STAT92E in the larval brain. The nucleolar localization of Nop56 suggests that like yeast Nop56p, Nop56 may be involved in ribosome synthesis and growth in Drosophila. Support for this role of Nop56 comes from the observation that RNAi knockdown of Fibrillarin, another component in the ribosomal RNA processing complex, caused very similar defects in neuroepithelial growth and lamina neurogenesis (Figure S6). The severe proliferation defects of Nop56RNAi mutant NEs might arise from retarded cellular growth. Paradoxically, Nop56RNAi mutant NEs became much elongated, suggesting an accumulation of cell mass. Perhaps Nop56 RNAi did not entirely inhibit cellular growth while it blocked cell cycle progression. Nop56 activity is inhibitory to lamina neurogenesis at late larval stages. This effect is specific to the lamina, as the medulla was not affected by Nop56 overexpression. We envision that cell growth is incompatible with neuron differentiation, and the loss of Nop56 activity might have accelerated the transition from LPCs to neurons.
Supplementary Material
Acknowledgments
We thank Drs. Erika Bach, Hugo Bellen, Chris Doe, Doug Harrison, Steve Hou, James Hombria, Yuh Nung Jan, Shigeaki Kato, Juergen Knoblich, Ruth Lehmann, Leonard Rabinow, and James Skeath for antibodies and fly stocks; the Bloomington Stock Center, the Tsinghua Stock Center, the National Institute of Genetics Stock Center, and the Vienna Drosophila RNAi Center for fly stocks; and the Developmental Studies Hybridoma Bank for antibodies. This work was supported by grants from the National Science Foundation of China (grant no. 30671035) and National Basic Sciences Research Programs (grant no. 2007CB947203) to H.L.
Footnotes
Communicating editor: I. K. Hariharan
Literature Cited
- Arbouzova N. I., Zeidler M. P., 2006. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133: 2605–2616. [DOI] [PubMed] [Google Scholar]
- Bach E. A., Ekas L. A., Ayala-Camargo A., Flaherty M. S., Lee H., et al. , 2007. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7: 323–331. [DOI] [PubMed] [Google Scholar]
- Baeg G. H., Zhou R., Perrimon N., 2005. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 19: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang A. G., Bailey A. M., Posakony J. W., 1995. Hairless promotes stable commitment to the sensory organ precursor cell fate by negatively regulating the activity of the Notch signaling pathway. Dev. Biol. 172: 479–494. [DOI] [PubMed] [Google Scholar]
- Bina S., Wright V. M., Fisher K. H., Milo M., Zeidler M. P., 2010. Transcriptional targets of Drosophila JAK/STAT pathway signalling as effectors of haematopoietic tumour formation. EMBO Rep. 11: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. J., 2006. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7: 678–689. [DOI] [PubMed] [Google Scholar]
- Chotard C., Leung W., Salecker I., 2005. glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron 48: 237–251. [DOI] [PubMed] [Google Scholar]
- Constantinescu S. N., Girardot M., Pecquet C., 2008. Mining for JAK-STAT mutations in cancer. Trends Biochem. Sci. 33: 122–131. [DOI] [PubMed] [Google Scholar]
- Copf T., Goguel V., Lampin-Saint-Amaux A., Scaplehorn N., Preat T., 2011. Cytokine signaling through the JAK/STAT pathway is required for long-term memory in Drosophila. Proc. Natl. Acad. Sci. USA 108: 8059–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B., Boone J. Q., Stevens N. R., Brand A. H., Doe C. Q., 2007. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev. 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B., Gold K. S., Brand A. H., 2010. Notch regulates the switch from symmetric to asymmetric neural stem cell division in the Drosophila optic lobe. Development 137: 2981–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty M. S., Zavadil J., Ekas L. A., Bach E. A., 2009. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of Notch signaling by the JAK/STAT pathway. Dev. Dyn. 238: 2235–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty M. S., Salis P., Evans C. J., Ekas L. A., Marouf A., et al. , 2010. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell 18: 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer M., Balbi M., Albarca-Aguilera M., Gallant M., Herr W., et al. , 2010. Drosophila Myc interacts with host cell factor (dHCF) to activate transcription and control growth. J. Biol. Chem. 285: 39623–39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier T., Bergès T., Tollervey D., Hurt E., 1997. Nucleolar KKE/D repeat proteins Nop56p and Nop58p interact with Nop1p and are required for ribosome biogenesis. Mol. Cell. Biol. 17: 7088–7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C., Devergne O., Georgenthum E., Carballes F., Medioni C., et al. , 2002. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development 129: 5437–5447. [DOI] [PubMed] [Google Scholar]
- Green P., Hartenstein A. Y., Hartenstein V., 1993. The embryonic development of the Drosophila visual system. Cell Tissue Res. 273: 583–598. [DOI] [PubMed] [Google Scholar]
- Harrison D. A., Binari R., Nahreini T. S., Gilman M., Perrimon N., 1995. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14: 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. A., Akong K., Peifer M., 2007. Novel roles for APC family members and Wingless/Wnt signaling during Drosophila brain development. Dev. Biol. 305: 358–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer A., Campos-Ortega J. A., 1990. Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Rouxs Arch. Dev. Biol. 198: 264–274. [DOI] [PubMed] [Google Scholar]
- Hombría J. C., Brown S., Häder S., Zeidler M. P., 2005. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288: 420–433. [DOI] [PubMed] [Google Scholar]
- Hulf T., Bellosta P., Furrer M., Steiger D., Svensson D., et al. , 2005. Whole-genome analysis reveals a strong positional bias of conserved dMyc-dependent E-boxes. Mol. Cell. Biol. 25: 3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., et al. , 2009. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. N., Mokalled M. H., Haden T. N., Olson E. N., 2011. JAK/Stat signaling regulates heart precursor diversification in Drosophila. Development 138: 4627–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten P., Häder S., Zeidler M. P., 2002. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech. Dev. 117: 343–346. [DOI] [PubMed] [Google Scholar]
- Kunes S., 2000. Axonal signals in the assembly of neural circuitry. Curr. Opin. Neurobiol. 10: 58–62. [DOI] [PubMed] [Google Scholar]
- Kwon S. Y., Xiao H., Glover B. P., Tjian R., Wu C., et al. , 2008. The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev. Biol. 316: 538–547. [DOI] [PubMed] [Google Scholar]
- Lacronique V., Boureux A., Valle V. D., Poirel H., Quang C. T., et al. , 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278: 1309–1312. [DOI] [PubMed] [Google Scholar]
- Leatherman J. L., DiNardo S., 2008. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3: 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Darnell J. E., Jr., 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3: 651–662. [DOI] [PubMed] [Google Scholar]
- Liu W., Singh S. R., Hou S. X., 2010. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J. Cell. Biochem. 109: 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Sehgal A., 2012. Regulation of circadian behavioral output via a microRNA-JAK/STAT circuit. Cell 148: 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Hanratty W. P., Dearolf C. R., 1995. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 14: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen I. A., Hanson T. E., 1993. The development of the optic lobe, pp. 1363–1491 in The Development of Drosophila melanogaster, edited by Bate M., Martinez-Arias A. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Mohanty S., Jermyn K. A., Early A., Kawata T., Aubry L., et al. , 1999. Evidence that the Dictyostelium Dd-STATa protein is a repressor that regulates commitment to stalk cell differentiation and is also required for efficient chemotaxis. Development 126: 3391–3405. [DOI] [PubMed] [Google Scholar]
- Muller P., Kuttenkeuler D., Gesellchen V., Zeidler M. P., Boutros M., 2005. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436: 871–875. [DOI] [PubMed] [Google Scholar]
- Murata T., Suzuki E., Ito S., Sawatsubashi S., Zhao Y., et al. , 2008. RNA-binding protein hoip accelerates polyQ-induced neurodegeneration in Drosophila. Biosci. Biotechnol. Biochem. 72: 2255–2261. [DOI] [PubMed] [Google Scholar]
- Nassif C., Noveen A., Hartenstein V., 2003. Early development of the Drosophila brain: III. The pattern of neuropile founder tracts during the larval period. J. Comp. Neurol. 455: 417–434. [DOI] [PubMed] [Google Scholar]
- Neumüller R. A., Richter C., Fischer A., Novatchkova M., Neumüller K. G., et al. , 2011. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell 8: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo K. T., Wang J., Junker M., Kriz S., Vo G., et al. , 2010. Concomitant requirement for Notch and Jak/Stat signaling during neuro-epithelial differentiation in the Drosophila optic lobe. Dev. Biol. 346: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian A., van Steensel B., Delrow J., Bussemaker H. J., Li L., et al. , 2003. Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev. 17: 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihara-Ono M., Toriya M., Nakao K., Okano H., 2011. Downregulation of Notch mediates the seamless transition of individual Drosophila neuroepithelial progenitors into optic medullar neuroblasts during prolonged G1. Dev. Biol. 351: 163–175. [DOI] [PubMed] [Google Scholar]
- O’Shea J. J., Gadina M., Schreiber R. D., 2002. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109(Suppl.): S121–S131. [DOI] [PubMed] [Google Scholar]
- Pierce S. B., Yost C., Anderson S. A. R., Flynn E. M., Delrow J., et al. , 2008. Drosophila growth and development in the absence of dMyc and dMnt. Dev. Biol. 315: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. V., Rauskolb C., Irvine K. D., 2010. Influence of Fat-Hippo and Notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development 137: 2397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas M. L., Cobreros L., Zeidler M. P., Hombría J. C., 2008. Plasticity of Drosophila Stat DNA binding shows an evolutionary basis for Stat transcription factor preferences. EMBO Rep. 9: 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Basler K., 1993. Organizing activity of wingless protein in Drosophila. Cell 72: 527–540. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C., 1989. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85. [DOI] [PubMed] [Google Scholar]
- Terry N. A., Tulina N., Matunis E., DiNardo S., 2006. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev. Biol. 294: 246–257. [DOI] [PubMed] [Google Scholar]
- Wang W., Li Y., Zhou L., Yue H., Luo H., 2011a Role of JAK/STAT signaling in neuroepithelial stem cell maintenance and proliferation in the Drosophila optic lobe. Biochem. Biophys. Res. Commun. 410: 714–720. [DOI] [PubMed] [Google Scholar]
- Wang W., Liu W., Wang Y., Zhou L., Tang X., et al. , 2011b Notch signaling regulates neuroepithelial stem cell maintenance and neuroblast formation in Drosophila optic lobe development. Dev. Biol. 350: 414–428. [DOI] [PubMed] [Google Scholar]
- Weng M., Haenfler J. M., Lee C. Y., 2012. Changes in Notch signaling coordinates maintenance and differentiation of the Drosophila larval optic lobe neuroepithelia. Dev. Neurobiol. 72: 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang S. Q., Tan D., Gao Y., Lin G., et al. , 2011. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354: 31–43. [DOI] [PubMed] [Google Scholar]
- Yan R., Small S., Desplan C., Dearolf C. R., Darnell J. E., Jr., 1996. Identification of a Stat gene that functions in Drosophila development. Cell 84: 421–430. [DOI] [PubMed] [Google Scholar]
- Yasugi T., Umetsu D., Murakami S., Sato M., Tabata T., 2008. Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT. Development 135: 1471–1480. [DOI] [PubMed] [Google Scholar]
- Yasugi T., Sugie A., Umetsu D., Tabata T., 2010. Coordinated sequential action of EGFR and Notch signaling pathways regulates proneural wave progression in the Drosophila optic lobe. Development 137: 3193–3203. [DOI] [PubMed] [Google Scholar]
- Zhou L., Luo H., 2013. Replication protein A links cell cycle progression and the onset of neurogenesis in Drosophila optic lobe development. J. Neurosci. 33: 2873–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.