Abstract
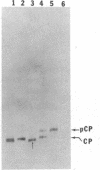
The coat protein (CP) of bacteriophage f1 is integrated into an Escherichia coli plasma membrane fraction consisting of inverted vesicles when it is synthesized in a cell-free, coupled transcription--translation system supplemented with the inverted vesicles. By using proteolytic enzymes as probes, we found by subsequent peptide mapping and determination of the sequence of the proteolytic products that CP was inserted into the inverted vesicles in an orientation indistinguishable from that in inverted vesicles prepared from infected E. coli: only a COOH-terminal portion of approximately 10 residues was accessible to proteolysis, whereas the remainder of CP (CP') was entirely protected. Protection of CP' was dependent on the integrity of the vesicle membrane, because it was abolished when proteolysis was done in the presence of nonionic detergents. Insertion was observed when the inverted vesicles were present during translation in the cell-free system, not when they were added after translation. Thus, the asymmetric insertion of this type of integral membrane protein is strictly coupled to translation. These findings are discussed with respect to prokaryotic membrane biogenesis and are related to bacteriophage f1 assembly and infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey G. S., Gillett D., Hill D. F., Petersen G. B. Automated sequencing of insoluble peptides using detergent. Bacteriophage fl coat protein. J Biol Chem. 1977 Apr 10;252(7):2218–2225. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. D. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J Cell Biol. 1970 Apr;45(1):130–145. doi: 10.1083/jcb.45.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Schwartz M., Silhavy T. J. Mutations altering the cellular localization of the phage lambda receptor, an Escherichia coli outer membrane protein. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5802–5806. doi: 10.1073/pnas.75.12.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Genetic evidence on the nature of the repressor for alkaline phosphatase in E. coli. J Mol Biol. 1963 May;6:433–438. doi: 10.1016/s0022-2836(63)80054-6. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- Marco R., Jazwinski S. M., Kornberg A. Binding, eclipse, and penetration of the filamentous bacteriophage M13 in intact and disrupted cells. Virology. 1974 Nov;62(1):209–223. doi: 10.1016/0042-6822(74)90316-x. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Wachtel E. J. Structure and assembly of filamentous bacterial viruses. Nature. 1975 Jan 3;253(5486):19–23. doi: 10.1038/253019a0. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Novotny C. P., Fives-Taylor P. Retraction of F pili. J Bacteriol. 1974 Mar;117(3):1306–1311. doi: 10.1128/jb.117.3.1306-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Casadaban M. J., Shuman H. A., Beckwith J. R. Conversion of beta-galactosidase to a membrane-bound state by gene fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3423–3427. doi: 10.1073/pnas.73.10.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Shuman H. A., Beckwith J., Schwartz M. Use of gene fusions to study outer membrane protein localization in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5411–5415. doi: 10.1073/pnas.74.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H. Bacteriophage f1 infection: fate of the parental major coat protein. J Virol. 1974 Jan;13(1):94–99. doi: 10.1128/jvi.13.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell D. T., Offord R. E. The amino acid sequence of the B-protein of bacteriophage ZJ-2. Biochem J. 1972 Mar;127(1):167–178. doi: 10.1042/bj1270167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. doi: 10.1016/s0022-2836(77)80065-x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975 Oct 10;250(19):7687–7692. [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. doi: 10.1073/pnas.72.12.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Mandel G., Zwizinski C., Bates M., Killick T. Synthesis of phage M13 coat protein and its assembly into membranes in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1754–1758. doi: 10.1073/pnas.75.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D., VALENTINE R. C., ROGER M., STOECKENIUS W. F1, A ROD-SHAPED MALE-SPECIFIC BACTERIOPHAGE THAT CONTAINS DNA. Virology. 1963 Aug;20:638–640. doi: 10.1016/0042-6822(63)90290-3. [DOI] [PubMed] [Google Scholar]