Abstract
Tetrahydrobiopterin (BH4) lowers blood phenylalanine (Phe) in individuals with PKU who are responders, but its effects on the brain and cognition have not been explored thoroughly. We examined blood Phe, microstructural white matter integrity, and executive abilities in 12 BH4 responders before (i.e., baseline) and after (i.e., follow-up) six months of treatment with BH4. Compared with baseline, Phe in these responders decreased by 51% during a 4 week screening period after initiation of treatment and remained lowered by 37% over the 6 month follow-up period. Significant improvements in white matter integrity, evaluated by mean diffusivity from diffusion tensor imaging, were also found following six months of treatment. Improvements in executive abilities were not identified, although six months may have been a period too brief for changes in cognition to follow changes in brain. To our knowledge, our study is the first to explore relationships among Phe, white matter integrity, executive abilities, and BH4 treatment within a single study.
Keywords: phenylketonuria, tetrahydrobiopterin, sapropterin, white matter, diffusion tensor imaging, executive
1. Introduction
Phenylketonuria (PKU) is a hereditary disorder characterized by disruption in the metabolism of the amino acid phenylalanine (Phe) due to an abnormality or deficiency of the phenylalanine hydroxylase (PAH) enzyme [1]. As a result, blood Phe is elevated in individuals with PKU (i.e., > 120 μmol/L) in comparison with individuals without PKU. Newborn screening and early implementation of dietary Phe restrictions have largely eliminated the gravest sequelae of PKU, such as profound intellectual disability [1, 2] and serious neurological abnormalities [3]. That said, early-treated PKU remains associated with significant changes in the white matter of the brain [4, 5], mild reductions in general intellect [6], and impairments in specific domains of cognition such as executive abilities [7, 8].
For decades, the primary treatment for lowering blood Phe levels in individuals with PKU has been dietary restriction of protein-rich foods [9]. In recent years, sapropterin dihydrochloride, a synthetic form of tetrahydrobiopterin (BH4), has been introduced as a pharmaceutical agent that reduces Phe in responsive individuals with PKU [9, 10]. This agent acts as a cofactor in the metabolic process by which PAH facilitates the conversion of Phe into tyrosine; in turn, tyrosine is synthesized to form dopamine and other important neurotransmitters. Some but not all individuals with PKU respond to treatment with BH4, because residual PAH activity is required for BH4 to be effective.
In the current study, blood Phe, brain white matter, and executive abilities were evaluated in individuals with early-treated PKU before (i.e., baseline) and after (i.e., follow-up) initiation of treatment with BH4. Diffusion tensor imaging (DTI) was used to assess microstructural white matter integrity, whereas working memory and strategic processing tasks were used to assess executive abilities (IQ was also assessed). Treatment with BH4 was implemented after baseline evaluation, and screening for response to BH4 occurred over the next four weeks by assessing changes in blood Phe. Individuals who responded to BH4 with a decrease in blood Phe of at least 20% during screening and continued BH4 treatment were asked to return six months later for follow-up DTI and executive abilities evaluations. DTI and executive abilities findings at baseline and follow-up were compared with those of healthy controls without PKU. More importantly, comparisons were made between baseline and follow-up findings within the subset of individuals with PKU who were BH4 responders.
Results from baseline evaluation are reported in detail elsewhere [4]. Briefly, in comparison with controls, DTI for individuals with PKU showed a widespread lowering of mean diffusivity (MD) in the white matter of the brain using both region of interest (ROI) analysis and voxel-wise Tract Based Spatial Statistics (TBSS) analysis. In addition, performance on tasks assessing executive abilities was significantly poorer for individuals with PKU than controls. Finally, lower MD and poorer executive abilities were significantly related to higher blood Phe levels.
In this report, we describe changes in blood Phe that occurred during four weeks of screening for response to BH4. In addition, we describe findings regarding blood Phe, DTI, and executive abilities following six months of treatment with BH4 in individuals with PKU who were BH4 responders. To our knowledge, this study is the first to describe relationships between treatment with BH4, microstructural white matter integrity, executive abilities, and Phe control.
2. Material and methods
2.1. Participants
2.1.1. Participants at baseline
The baseline sample included 31 (12 female, 19 male) individuals with PKU whose response to treatment with BH4 was unknown. The original baseline sample included 32 participants with PKU [4], but in this report 1 participant was excluded due to failure to complete screening for response to BH4. Participants were recruited through metabolic clinics at Washington University (WU; n = 13), University of Missouri (MU; n = 8), University of Florida (n = 4), St. Louis University (n = 3), New York Medical College (n = 2), and University of Nebraska (n = 1). All were diagnosed and treated early through dietary restriction of Phe intake.
Findings from these individuals with PKU were compared with those of demographically-matched healthy controls without PKU (n = 12; 4 female; 8 male) recruited from the St. Louis community. No participant had a history of major medical, psychiatric, or learning disorder unrelated to PKU. Age ranged from 6 to 35 years (M = 17.6, SD = 8.8) for the PKU group and 7 to 33 years (M = 17.8, SD = 8.0) for the control group. Education ranged from 0 to 18 years (M = 9.1, SD = 4.7) for the PKU group and 1 to 16 years (M = 10.3, SD = 4.8) for the control group. In terms of race/ethnicity, 3% of the PKU group and 8% of the control group were members of a minority group. There were no significant differences in gender, age, education, or race/ethnicity between the PKU and control groups (p > .05 in all instances).
2.1.2. Participants at screening
Immediately following baseline assessment, all 31 individuals with PKU began treatment with BH4 at a dose of 20mg/kg/day. Over the next four weeks, screening for response to BH4 occurred through weekly assessment of blood Phe; for several participants the screening period was somewhat longer due to factors such as illness during the screening period. Individuals with PKU were considered to be BH4 responders if their average decrease in Phe during screening was at least 20%.
2.1.3. Participants at follow-up
Responders who continued treatment with BH4 were asked to return approximately six months later for follow-up evaluations using DTI and executive abilities measurements identical to those administered at baseline. A total of 12 BH4 responders (3 female; 9 male) participated in follow-up evaluations. Findings were compared with those of a subset of healthy controls who continued in the study (n = 9; 2 female; 7 male). Age ranged from 8 to 35 years (M = 18.2, SD = 9.6) for the BH4 responder group and 7 to 33 years (M = 17.8, SD = 9.3) for the control group. Education ranged from 3 to 18 years (M = 9.2, SD = 4.5) for the BH4 responder group and 1 to 16 years (M = 9.7, SD = 5.2) for the control group. In terms of race/ethnicity, 8% of the BH4 responder group and 11% of the control group were members of a minority group. There were no significant differences in gender, age, education, or race/ethnicity between the BH4 responder and control groups (p > .05 in all instances).
2.2. Procedures
Approval to conduct this study was obtained from institutional review boards for the protection of human subjects at WU and MU, and all DTI and executive abilities data were collected at these institutions. Informed consent/assent was obtained for all participants prior to administration of any study procedures. Participants typically completed DTI and executive abilities evaluations in a single session lasting approximately four hours.
2.2.1. Diffusion tensor imaging
Neuroimaging procedures are described in detail elsewhere [4]. Briefly, data were acquired using a Siemens Tim Trio 3.0T imaging system (Erlangen, Germany). DTI was acquired using an echo planar imaging (EPI) sequence along 25 non-collinear diffusion gradients. Diffusion weighted images were registered to weighted structural images and then to an in-house atlas at WU. Parametric maps were then generated for MD; fractional anisotropy was not examined because our previous findings indicated no significant difference between PKU and control groups [4].
MD was examined using two analytic approaches: ROI analysis and voxel-wise TBSS analysis. We focused on the following 10 ROIs to sample across a variety of brain regions: prefrontal cortex, centrum semiovale, posterior parietal-occipital cortex, optic radiation, putamen, corpus callosum (genu, body, splenium), thalamus, and hippocampus. Voxel-wise analysis was conducted to permit examination of the white matter across brain regions without rigid adherence to neuroanatomical boundaries.
2.2.2. Executive abilities
Executive abilities were assessed using two tasks measuring working memory and two tasks measuring strategic processing that are detailed elsewhere [4]. To evaluate working memory, 2-back and recognition span tasks were administered. Variables of interest from these tasks included the average number of errors made across two conditions (letter and location) of the 2-back task and the average number of items correctly recalled across two conditions (shape and location) of the recognition span task. To evaluate strategic processing, word-list learning and verbal fluency tasks were administered. Variables of interest from these tasks included number of words correctly recalled and semantically clustered on the fifth learning trial of the word-list learning task and number of words correctly generated on the food/drink verbal fluency task. General intellectual ability (IQ) was assessed using the Wechsler Abbreviated Scale of Intelligence (Psychological Corporation).
3. Results
The statistical approaches used for analyzing data were t-tests, paired samples t-tests, and Pearson correlations. Given the paucity of data describing white matter integrity and executive abilities following treatment with BH4, the statistical significance level was set at p < .05 and control for multiple comparisons was not conducted so that important patterns of results were not obscured. To limit the number of analyses, as noted earlier, conditions of some executive tasks were combined and only selected variables from other executive tasks were analyzed. Age was not controlled in analyses because there was no significant difference in age between the PKU and control groups, and in previous analyses of baseline DTI and executive abilities data we did not find significant interactions between age and group [4].
Results regarding changes in Phe from baseline through screening are described for the entire baseline sample of 31 participants with PKU. Results regarding changes in DTI, executive abilities, and Phe from baseline through follow-up are described for the subsample of 12 individuals with PKU who were BH4 responders and continued in the study to follow-up. Comparisons between these 12 BH4 responders and the subsample of 9 controls completing follow-up are also described.
3.1. Blood Phe levels
3.1.1. Blood Phe at baseline and screening
Phe at baseline and average Phe during the screening period for the entire sample of 31 participants with PKU are reported in the upper section of Table 1. Response to BH4 was determined by comparing baseline Phe with average Phe during the screening period. Results of a paired samples t-test indicated that there was a significant reduction in Phe of 27% from baseline to screening [t(30) = 3.51, p < .001].
Table 1.
Phe (μmol/L) at baseline and average Phe during screening for response to BH4.
| Baseline Sample (n = 31) | ||
|---|---|---|
|
| ||
| Baseline | Screening | |
| Phe mean (SD)* | 697 (403) | 512 (422) |
| Phe range | 54 - 1405 | 26 - 1542 |
|
| ||
| BH4 Responder Sample (n = 19) | ||
|
| ||
| Baseline | Screening | |
|
|
|
|
| Phe mean (SD)* | 728 (344) | 378 (284) |
| Phe range | 115 - 1398 | 26 - 881 |
Note:
significant difference between baseline and screening, p <.001
In 19 of 31 (61%) cases, participants were considered responders to BH4 on the basis of a reduction in Phe of at least 20%. Among these BH4 responders, results of a paired samples t-test indicated that there was a significant reduction in Phe of 48% from baseline to screening [t(18) = 6.96, p < .001]. In the lower section of Table 1, Phe at baseline and average Phe during the screening period for these BH4 responders are reported.
3.1.2. Blood Phe at follow-up in BH4 responders
For the BH4 responder subsample of 12 individuals with PKU who continued to follow-up, Phe at baseline, average Phe during the screening period, and average Phe during the BH4 treatment period are reported in Table 2. As expected, results of a paired samples t-test revealed a significant reduction in Phe of 51% [t(11) = 5.43, p < .001] from baseline to screening. To determine response to BH4 during the treatment period, comparison was made between baseline Phe and average Phe from the time BH4 treatment was initiated through follow-up (including the screening period). Results of a paired samples t-test indicated that a significant reduction in Phe of 37% was maintained during the treatment period [t(11) = 3.56, p < .005].
Table 2.
Phe (μmol/L) at baseline, average Phe during screening, and average Phe during BH4 treatment period.
| Follow-up BH4 Responder Sample (n = 12) | |||
|---|---|---|---|
|
| |||
| Baseline | Screening | Follow-up | |
| Phe mean (SD)*, ** | 653 (322) | 317 (237) | 409 (256) |
| Phe range | 200 - 1108 | 51 - 881 | 136 - 897 |
Note:
significant difference between baseline and screening, p < .001;
significant difference between baseline and follow-up, p < .005.
3.2. Diffusion tensor imaging in BH4 responders
DTI follow-up was completed for the 12 BH4 responders. Changes from baseline to follow-up were examined using paired samples t-tests. MD and statistical findings for each of the 10 ROIs at baseline and follow-up are reported in Table 3 (as well as baseline MD for the controls, which will be discussed later). Significant improvements (i.e., increases) from baseline to follow-up for the BH4 responders were found in 3 of the 10 ROIs examined, including the centrum semiovale, posterior parietal-occipital cortex, and body of the corpus callosum. It is also notable that, in absolute terms, MD increased in 8 of the 10 ROIs and remained the same in the other 2 ROIs; in no instance did MD decrease (i.e., worsen).
Table 3.
Mean (SD) diffusion tensor imaging (DTI) mean diffusivity (MD) region of interest (ROI) values in BH4 responders and controls, along with statistical findings.
| ROI | PKU (n = 12) | Control (n = 12) | Statistical Findings | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Baseline | Follow-up | Baseline | PKU Baseline vs PKU Follow-up | PKU Baseline vs Control Baseline | P KU Follow-up vs Control Baseline | |
| Prefrontal | .70 (.03) | .71 (.03) | .75 (.05) | ns | t(19)=2.49, p<.05 | t(19)=2.34, p<.05 |
| Centrum Semiovale | .66 (.07) | .68 (.06) | .74 (.03) | t(11)=3.03, p<.05 | t(19)=2.86, p<.01 | t(19)=2.43, p<.05 |
| Parietal Occipital | .72 (.08) | .75 (.10) | .84 (.06) | t(11)=2.65, p<05 | t(19)=3.36, p<005 | t(19)=2.26, p<.05 |
| Optic Radiation | .77 (.08) | .77 (.08) | .83 (.03) | ns | t(19)=2.31, p<05 | t(19)=2.15, p<05 |
| Putamen | .68 (.03) | .69 (.04) | .72 (.03) | ns | t(19)=2.99, p<01 | ns |
| Genu of CC | .71 (.07) | .71 (.09) | .82 (.08) | ns | t(19)=3.40, p<005 | t(19)=3.01, p<01 |
| Body of CC | .78 (.08) | .85 (.10) | .86 (.07) | t(11)=2.35, p<05 | t(19)=2.17, p<05 | ns |
| Splenium of CC | .70 (.05) | .71 (.06) | .68 (.04) | ns | ns | ns |
| Thalamus | .71 (.04) | .73 (.05) | .73 (.04) | ns | ns | ns |
| Hippocampus | .83 (.04) | .84 (.05) | .84 (.02) | ns | ns | ns |
Notes: CC = Corpus Callosum; ns = not significant.
Voxel-wise analyses of MD were also conducted for the BH4 responders at baseline and follow-up using paired samples t-tests in Randomise (FSL, FMRIB, Oxford, UK). As shown in Figure 1, MD improved (i.e., increased) from baseline to follow-up across multiple brain regions.
Figure 1.
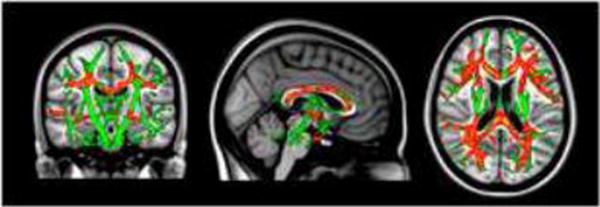
Diffusion tensor imaging (DTI) mean diffusivity (MD) voxel-wise findings demonstrating significant increase (i.e., improvement) in MD from baseline to follow-up in BH4 responders.
Notes: Green = TBSS skeleton; Red = Baseline to follow-up increase in MD, p < .01; Yellow = Baseline to follow-up increase in MD, p < .05.
In addition to comparisons within the PKU BH4 responder group, MD was examined in relation to findings from the control group at baseline; neuroimaging was not repeated for the control group after six months because it was not expected that changes would occur in a control group within our age range over a six month period [11]. Not surprisingly, as shown in Table 3, t-tests revealed significant differences between the BH4 responder and control groups at baseline for the seven ROIs previously identified as different in our earlier report [4]. These ROIs included the prefrontal cortex, centrum semiovale, posterior parietal-occipital cortex, optic radiation, putamen, genu of the corpus callosum, and body of the corpus callosum.
Of greater interest, in comparing MD for the BH4 responder group at follow-up with MD for the control group at baseline, between-group differences in the putamen and body of the corpus callosum that were identified at baseline were no longer significant (see Table 3). The considerable improvement in MD for the body of the corpus callosum is particularly intriguing. MD in this region increased from 0.78 to 0.85 for the BH4 responder group, which is almost identical to the value of 0.86 for the control group.
3.3. Executive abilities in BH4 responders
Changes in executive abilities and IQ from baseline to follow-up for the 12 BH4 responders were examined using paired samples t-tests (scores are reported in Table 4). There were no significant changes from baseline to follow-up for any variable examined.
Table 4.
Mean (SD) scores from tasks assessing executive abilities and IQ in BH4 responders and controls.
| Executive Variable | PKU (n = 12) | Control (n = 9) | ||
|---|---|---|---|---|
|
|
|
|||
| Baseline | Follow-up | Baseline | Follow-up | |
| 2-Back Errors* | 8.8 (6.2) | 7.4 (4.3) | 3.4 (2.6) | 3.1 (3.0) |
| Recognition Span | 5.4 (0.6) | 5.2 (0.8) | 5.8 (1.0) | 6.1 (0.8) |
| Word List Learning | 13.2 (3.1) | 14.3 (2.6) | 16.0 (1.7) | 14.8 (2.8) |
| Word List Clustering | 10.3 (5.0) | 11.9 (4.1) | 13.9 (3.9) | 13.2 (5.0) |
| Verbal Fluency | 19.2 (4.0) | 17.1 (4.5) | 21.7 (7.6) | 22.0 (8.3) |
| IQ | 102 (15) | 105 (15) | 110 (10) | 114 (10) |
Note:
significantly poorer performance for BH4 responders than controls at baseline and follow-up, p < .05.
In addition to comparisons within the BH4 responder group, executive abilities and IQ were examined in relation to findings from the nine controls who received executive abilities evaluation at both baseline and follow-up (scores are reported in Table 4). To do so, repeated measures analysis of variance was conducted, with time of evaluation (baseline, follow-up) as the within subjects factor and group (BH4 responder, control) as the between subjects factor. The interaction between these variables was also examined.
Results revealed a significant main effect of group on the working memory 2-back task, with a greater number of errors for the BH4 responder than control group [F(1, 18) = 7.08, p < .05]. Post-hoc analyses verified that the BH4 responder group made a greater number of errors on the 2-back task than the control group at both baseline [t(18) = 2.43, p < .05] and follow-up [t(18) = 2.55, p < 05] evaluation. There were no other significant main effects or interactions for the executive tasks. For IQ, there was a significant main effect of time of evaluation, with higher IQ at follow-up than at baseline [F(1, 19) = 5.76, p < .05]; there was no significant effect of group or interaction between time of evaluation and group for IQ.
3.4. Relationships between blood Phe, MD, and executive abilities in BH4 responders
At baseline, Phe was significantly correlated with MD for the centrum semiovale (r = -.72, p < .01), posterior parietal-occipital cortex (r = -.69, p < .05), optic radiation (r = -.63, p < .05), genu of the corpus callosum (r = -.66, p < .05), and hippocampus (r = -.71, p < .01). At follow-up, average Phe during the BH4 treatment period was significantly correlated with MD for the centrum semiovale, (r = -.83, p < .001), posterior parietal-occipital cortex (r = -.81, p < .005), and optic radiation (r = -.84, p < .001). In all instances, MD increased as Phe decreased; in other words, MD values more closely approximated normality when Phe levels were lower. There were no significant correlations between Phe and executive abilities or IQ at either baseline or follow-up (p > .05 in all instances).
4. Discussion
Very little research has been conducted to determine whether white matter abnormalities in the brain and deficits in cognition are reversible following improvements in Phe control in individuals with PKU. BH4 is a pharmaceutical agent that lowers blood Phe levels in individuals with PKU who are responders. In the present study, we evaluated blood Phe, microstructural white matter integrity (i.e., MD), and executive abilities (i.e., working memory and strategic processing) in individuals with PKU before (i.e., baseline) and after (follow-up) six months of treatment with BH4.
Turning first to our findings regarding response to BH4, we found that average Phe during a screening period of four weeks decreased by 27% in comparison with Phe at baseline in our sample of individuals with PKU. Reductions in Phe of at least 20% occurred in 61% of cases, with an average reduction in Phe of 48% from baseline to screening in these BH4 responders. Among the BH4 responders who continued in our study, average Phe during a treatment period of six months decreased by 37% in comparison with Phe at baseline. These results indicate that treatment with BH4 facilitated the maintenance of lower Phe in responders during the course of our study.
With regard to microstructural white matter integrity, baseline DTI findings verified those of our previous report [4] and those of other investigators [12, 13, 14, 15, 16, 17, 18]. That is, widespread reductions in MD were identified for our PKU group prior to treatment with BH4 in comparison with our control group. Reduced MD for the PKU group was also significantly associated with higher Phe. Although speculative, prior research has suggested that higher Phe may be related to reductions in MD due to the accumulation of intracellular debris within the axons of individuals with PKU, which restricts the diffusion of water molecules [14].
Of particular relevance to the current study, following six months of treatment with BH4, MD in BH4 responders increased (i.e., improved) significantly across a number of brain regions, including the centrum semiovale, posterior parietal-occipital cortex, and body of the corpus callosum. In fact, MD in the body of the corpus callosum appeared to normalize for the BH4 responders, reaching a value comparable to that of controls. These findings suggest that diffusion of water molecules along axons improves when Phe is reduced following treatment with BH4. These findings are also consistent with a previous report showing that white matter abnormalities identified using structural MRI in individuals with PKU are at least partially reversible following improvements in Phe control [19].
In terms of executive abilities, in comparison with controls, our group of BH4 responders with PKU demonstrated poorer working memory performance both prior to and following treatment with BH4. Six months of treatment with BH4 was not associated with improvements in executive abilities. The reasons for the discrepancy between DTI and executive follow-up findings remain unclear, but it is possible that six months of improved Phe control is too brief for significant changes in executive abilities to follow improvements in white matter integrity. Another possibility is that the executive deficits associated with PKU are not solely the result of white matter compromise but instead are more strongly related to a different effect of elevated Phe on the brain. If this is the case, it would not be expected that improvements in executive abilities would accompany improvements in white matter integrity.
This last finding points to the fact that there were a number of limitations to our study that need to be addressed through future research. For example, further research is required to determine whether BH4 treatment-related improvements in Phe control and white matter integrity are followed by improvements in executive abilities when tested over a longer period of treatment. Similarly, it is possible that further improvements in white matter integrity may be identified when improved Phe control is extended over a longer period. In addition, research is needed to determine whether compromises in the white matter versus other aspects of brain structure and function (e.g., neurotransmitter disregulation) are more strongly related to the executive deficits associated with PKU.
It should also be noted that our final sample of 12 BH4 responders with PKU was small, which may have limited our ability to detect additional treatment-related changes in white matter integrity beyond those identified, as well as improvements in executive abilities. Further, it was not possible to conduct follow-up DTI and executive abilities evaluations in the BH4 nonresponders with PKU or to conduct follow-up DTI evaluation in controls due to funding limitations. In the future, it will be important to reassess nonresponders and controls at intervals comparable to those of responders to fully understand the extent of BH4 treatment-related changes in responders. Finally, future research is needed to determine whether improvements in Phe control in the absence of BH4 treatment result in changes in microstructural white matter integrity that are similar to those identified in the current study.
In spite of these limitations, our findings clearly indicate that treatment with BH4 was beneficial from two key perspectives. First, Phe was significantly reduced in BH4 responders following initiation of treatment with BH4 and over a six month treatment period. Second, BH4-related reductions in Phe were accompanied by improvements in white matter integrity across a number of brain regions. Overall, BH4 appears to be an important component of PKU treatment that may serve to optimize neural outcomes in responders.
Highlights.
Phe decreased by 37% during 6 months of BH4 treatment in responders with PKU
Microstructural white matter integrity improved after 6 months of BH4 treatment
Executive abilities did not change after 6 months of BH4 treatment
First unified study of BH4 treatment, Phe control, white matter, and executive
Acknowledgments
This research was supported by an Investigator Sponsored Trial grant from BioMarin Pharmaceutical Inc. This research was also supported by a grant from the National Institute on Drug Abuse (T32DA007261) and the Human Clinical Core of the Washington University Intellectual and Developmental Disabilities Research Center which is funded by the National Institute of Child Health and Human Development (P30HD062171) and the James S. McDonnell Foundation. Drs. White and Christ serve as consultants to BioMarin Pharmaceutical Inc., and Dr. White serves as a consultant to Merck Serono S.A., but the content of this article has not been influenced by these relationships. The authors thank those who participated in our study for their contributions to our research. We also thank Suzin Blankenship and Laurie Sprietsma for their contributions to study management, as well as the physicians, faculty, and staff of Washington University, University of Missouri, University of Florida, St. Louis University, New York Medical College, and University of Nebraska who generously contributed through recruitment and phenylalanine monitoring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Paine RS, Hsia DY. The dietary phenylalanine requirements and tolerances of phenylketonuric patients. AMA J Dis Child. 1957;94:224–230. doi: 10.1001/archpedi.1957.04030040006002. [DOI] [PubMed] [Google Scholar]
- 3.Moyle JJ, Fox AM, Bynevelt M, Arthur M, Burnett JR. A neuropsychological profile of off-diet adults with phenylketonuria. J Clin Exp Neuropsychol. 2007;29:436–441. doi: 10.1080/13803390600745829. [DOI] [PubMed] [Google Scholar]
- 4.Antenor-Dorsey JV, Hershey T, Ruttlin J, Shimony JS, McKinstry RC, Grange DK, Christ SE, White DA. White matter integrity and executive abilities in individuals with phenylketonuria. Mol Genet Metab. 2013 doi: 10.1016/j.ymgme.2013.03.020. http://dx.doi.org/10.1016/j.ymgme.2013.03.020. [DOI] [PMC free article] [PubMed]
- 5.Anderson PJ, Leuzzi V. White matter pathology in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S3–S9. doi: 10.1016/j.ymgme.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Brumm VL, Grant ML. The role of intelligence in phenylketonuria: a review of research and management. Mol Genet Metab. 2010;99(Suppl 1):S18–S21. doi: 10.1016/j.ymgme.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Christ SE, Huijbregts SC, De Sonneville LM, White DA. Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol Genet Metab. 2010;99(Suppl 1):S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: intelligence and executive function. Dev Neuropsychol. 2008;33:474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- 9.van Spronsen FJ, Enns GM. Future treatment strategies in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S90–S95. doi: 10.1016/j.ymgme.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Gassio R, Vilaseca MA, Lambrushini N, Boix C, Fusté ME, Campistol J. Cognitive functions in patients with phenylketonuria in long-term treatment with tetrahydrobiopterin. Mol Genet Metab. 2010;99(Supp 1):S75–S78. doi: 10.1016/j.ymgme.2009.10.187. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BCP, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 12.Dezortova M, Hajek M, Tintera J, Hejcmanova L, Sykova E. MR in phenylketonuria-related brain lesions. Acta Radiol. 2001;42:459–466. doi: 10.1080/028418501127347179. [DOI] [PubMed] [Google Scholar]
- 13.Kono K, Okano Y, Nakayama K, Hase Y, Minamikawa S, Ozawa N, Yokote H, Inoue Y. Diffusion-weighted MR imaging in patients with phenylketonuria: relationship between serum phenylalanine levels and ADC values in cerebral white matter. Radiology. 2005;236:630–636. doi: 10.1148/radiol.2362040611. [DOI] [PubMed] [Google Scholar]
- 14.Leuzzi V, Tosetti M, Montanaro D, Carducci C, Artiola C, Carducci C, Antonozzi I, Burroni M, Carnevale F, Chiarotti F, Popolizio T, Giannatempo GM, D'Alesio V, Scarabino T. The pathogenesis of the white matter abnormalities in phenylketonuria. A multimodal 3.0 tesla MRI and magnetic resonance spectroscopy (1H MRS) study. J Inherit Metab Dis. 2007;30:209–216. doi: 10.1007/s10545-006-0399-4. [DOI] [PubMed] [Google Scholar]
- 15.Scarabino T, Popolizio T, Tosetti M, Montanaro D, Giannatempo GM, Terlizzi R, Pollice S, Maiorana A, Maggialetti N, Carriero A, Leuzzi V, Salvolini U. Phenylketonuria: white-matter changes assessed by 3.0-T magnetic resonance (MR) imaging, MR spectroscopy and MR diffusion. Radiol Med. 2009;114:461–474. doi: 10.1007/s11547-009-0365-y. [DOI] [PubMed] [Google Scholar]
- 16.Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn Reson Med. 2007;58:1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- 17.White DA, Connor LT, Nardos B, Shimony JS, Archer R, Snyder AZ, Moinuddin A, Grange DK, Steiner RD, McKinstry RC. Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: A DTI study of the corpus callosum. Mol Genet Metab. 2010;99(Suppl 1):S41–S46. doi: 10.1016/j.ymgme.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng H, Peck D, White DA, Christ SE. Tract-based evaluation of white matter damage in individuals with early-treated phenylketonuria. J Inherit Metab Dis. doi: 10.1007/s10545-013-9650-y. in press. [DOI] [PubMed] [Google Scholar]
- 19.Cleary MA, Walter JH, Wraith JE, White F, Tyler K, Jenkins JP. Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J Pediatr. 1995;127:251–255. doi: 10.1016/s0022-3476(95)70303-9. [DOI] [PubMed] [Google Scholar]


