Abstract
This report compares cellular localization of fesselin in chicken smooth, skeletal and cardiac muscle tissues using affinity purified polyclonal fesselin antibodies. Western blot analyses revealed large amounts of fesselin in gizzard smooth muscle with lower amounts in skeletal and cardiac muscle. In gizzard, fesselin was detected by immunofluorescence as discrete cytoplasmic structures. Fesselin did not co-localize with talin, vinculin or caveolin indicating that fesselin is not associated with dense bands or caveolar regions of the cell membrane. Immunoelectron microscopy established localization of fesselin within dense bodies. Since dense bodies function as anchorage points for actin and desmin in smooth muscle cells, fesselin may be involved in establishing cytoskeletal structure in this tissue. In skeletal muscle, fesselin was associated with desmin in regularly space bands distributed along the length of muscle fibers suggesting localization to the Z-line. Infrequently, this banding pattern was observed in heart tissues as well. Localization at the Z-line of skeletal and cardiac muscle suggests a role in contraction of these tissues.
Keywords: Fesselin, smooth muscle, dense bodies, immunofluorescence, electron microscopy
Introduction
Fesselin is a proline-rich actin-binding protein that was first isolated from turkey gizzard muscle (Leinweber et al. 1999). Fesselin is an avian homologue of mammalian synaptopodin 2 (Schroeter et al. 2008) and shares several biophysical features with synaptopodin and synaptopodin 2 (myopodin) such as an isoelectric point of 9.3 and similar molecular weights (Leinweber et al. 1999). In vitro studies indicate that fesselin stimulates actin polymerization by increasing actin nucleation (Beall and Chalovich 2001). Fesselin also stimulates F-actin bundling (Leinweber et al. 1999). Fesselin binds to Ca2+-calmodulin (Schroeter and Chalovich 2004; Kolakowski et al. 2004) and this interaction regulates actin polymerization (Schroeter and Chalovich 2004). While Ca2+-calmodulin regulates the interaction of fesselin with G-actin it has little effect on its interaction with F-actin (Schroeter and Chalovich 2004). Fesselin also inhibits the actin activation of S1 ATPase activity (Schroeter and Chalovich 2005).
Furthermore fesselin binds to several actin binding proteins including myosin (Schroeter and Chalovich 2005) and α-actinin (Pham and Chalovich 2006). Western blots using crude polyclonal antibody revealed fesselin expression in mammalian smooth, skeletal and cardiac muscle tissues. The presence of fesselin in muscle tissue and its interactions with muscle proteins suggested a role for this protein in regulation of actin filament formation and regulation of contraction. However, it is unknown if subcellular localization of fesselin supports this hypothesis.
In this report immunofluoresence and electron microscopic immunogold methods were utilized to establish the localization of fesselin in avian muscle.. Our findings indicate that fesselin is localized to dense bodies in chicken smooth muscle cells. In addition fesselin was localized to Z-lines of avian skeletal and cardiac muscles. These results are consistent with the proposed function of fesselin.
Materials and Methods
Tissue Samples
Fresh tissue samples (gizzard, thigh, breast, heart and liver) were recovered from mature chickens at the time of death. Procedures used to obtain these tissues were accomplished in compliance with the guidelines of the East Carolina University Committee on Animal Care.
Immunocytochemistry
For immunofluorescence microscopy samples were embedded in OCT (Sakura Finetek USA, Inc, Torrance CA), quick-frozen in dry ice cooled isopentane and stored at −70°C. Sections (3 μm) were cut on a cryostat and thaw mounted on silane (Sigma, St. Louis, MO) coated slides. Sections were fixed in a solution of 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4, and rinsed in TBS (10 mM Tris, pH 8.0, 150 mM NaCl) containing 0.1 %Tween (TTBS). Nonspecific binding sites were blocked by incubation in TTBS containing 10% normal donkey serum and 0.1% bovine serum albumin. Sections were incubated with primary antibody for 2–3 hours at room temperature, rinsed twice with TTBS, and specifically bound antibodies were detected by incubation with rhodamine red X-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA; dilution 1:200). The fesselin polyclonal antibody (rabbit anti-fesselin; Leinweber et al., 1999) was affinity purified by coupling highly purified fesselin to CNBr-activated Sepharose™ 4 Fast Flow (Amersham Bioscience). The anti-fesselin IgG was eluted with 0.1 M glycine pH 2.7 and directly neutralized with Tris-HCl pH 8.0. The purified anti-fesselin antibodies were tested for specificity by Western blots of whole tissue extracts (Figure 1, lane A). MALDI-TOF mass spectroscopy fingerprint analysis was used to establish the identity of the detected proteins. Specificity of detected immunocytochemical staining was evaluated by treating adjacent sections with nonimmune rabbit serum and preabsorbing the primary antibody with 10 μM purified fesselin. Tissue was also stained with mouse monoclonal antibodies to desmin (Sigma, St. Louis, MO; 1:200 dilution), talin (Sigma, St. Louis, MO; 1:200), vinculin (Chemicon, Temecula, CA; 1:2000), calveolin-1 (BD Biosciences, San Jose, CA; 1:100) and myomesin (Grove et al. 1984; 1:10). The secondary antibody (1:100 dilution) was FITC-labeled donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Non-specific staining was evaluated by omitting the primary antibody. Stained tissue sections were viewed and photographed using a Zeiss Photomic III or Nikon E600 research microscope or a Zeiss LSM 510 confocal microscope. Histological measurements were obtained using iTEM Universal TEM Imaging Software and at least 15 measurements were performed for each variable on each of three separate digital images.
Figure 1.
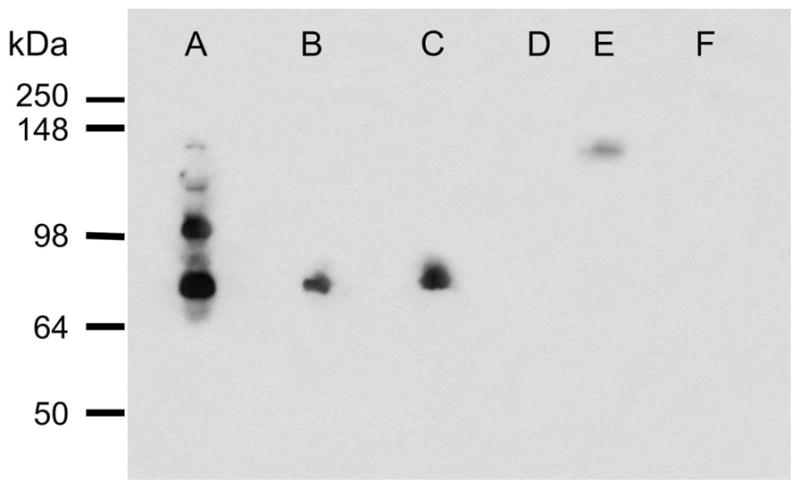
Western blot analysis of fesselin immunoreactivity. Gizzard (A), thigh (B), breast (C) and heart (D, E) are shown. Lane (E) was loaded with 10-times the amount of protein used in other lanes. Pancreas extract (F) was used as a negative control tissue.
For immunoelectron microscopy, gizzard tissues were cut (1–3 mm cubes) and fixed for 2–4 hr by immersion in 2% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.3). Tissues were dehydrated in a graded series of ethanol solutions and embedded in L.R. White embedding media (London Resin Company Limited, England). Sections (80 nm) were collected on nickel grids (200 mesh) and incubated in PBS. Nonspecific binding was blocked by incubation in 1% ovalbumin (OA)-PBS (30 min) followed by a brief rinse in PBS. Sections were incubated in fesselin antibody (1:15 in 1% OA-PBS) for 1 hr, washed (PBS), incubated in the protein-A gold (10 nm diameter; Electron Microscope Science, Hatfield, PA) solution (1:10 in 1% OA-PBS) for 30 minutes and washed. Control grids were processed similarly without fesselin antibody. Sections were post-stained with 2% uranyl acetate and viewed with a JEOL 1200 EX electron microscope operated at 60 kV accelerating voltage. Images were recorded using a SIS MegaView III CCD camera.
Western Blotting
Snap frozen tissues were ground under liquid nitrogen to a fine powder and suspended in a 2X SDS-gel loading buffer (8 M urea, 0.1 M Tris pH 6.8, 2% SDS, 0.035 M dithiothreitol, and protease inhibitor cocktail (SIGMA P2714) equivalent to 0.1 mg USP pancreatin/ml). The suspended tissues were incubated for 1 hr on ice, and clarified by centrifugation (20,800 g for 10 min). Protein concentrations were determined using a Bradford assay with a BSA standard. Proteins (50–500 μg) were separated by SDS-PAGE (10%), transferred to nitrocellulose membranes, and probed with affinity purified anti-fesselin antibodies (1:20,000). As secondary antibody a horseradish peroxidase conjugated donkey anti-rabbit antibody (Amersham Biocience) was used. Fesselin immunoreactive proteins were identified using enhanced chemiluminescence (Pierce ECL Western Blotting Substrate).
Results
Gizzard Smooth Muscle
Affinity purified anti-fesselin antibody reacted in the Western blot with 4 bands extracted from gizzard tissue (Figure 1). The apparent molecular masses of the dominant immuno-reactive bands were about 80 and 100 kDa. MALDI-TOF MS fingerprint analysis confirmed the presence of fesselin in each band detected by the anti-fesselin antibody. The presence of multiple species of fesselin in gizzard smooth muscle is due to different splicing of the fesselin gene (Schroeter et al., 2008).
Light microscopic immunocytochemistry showed strong immunofluorecence in the form of punctuate inclusions in smooth muscle cells of the gizzard (Figure 2A). The fesselin stained structures measured 1.28±0.01 by 0.31±0.03 μm and had a lateral spacing of 0.54±0.03 μm (data not shown). Their size and distribution was consistent with those previously reported (reviewed in Gabella, 1997) for cytoplasmic dense bodies. When the primary antibody was omitted or preabsorbed there was no specific staining of gizzard tissue. Staining was also not detected in liver which was used as negative tissue control (data not shown).
Figure 2.
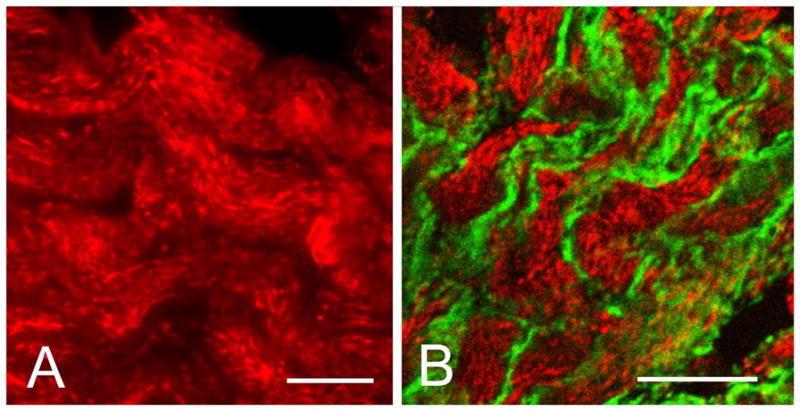
Immunocytochemical localization of fesselin in chicken gizzard. (A) Specific staining was observed primarily as punctuate rod-like intracellular inclusions similar in appearance and distribution to that of cytoplasmic dense bodies. (B) Fesselin (red) did not co-localize with the plasma membrane (dense plaques) associated protein talin (green). Bar = 10 μm.
Additional evidence that the fesselin-staining structures were dense bodies was obtained by examining the relative localization of fesselin to proteins of known cellular location. Neither talin (Fig. 2B) nor vinculin (data not shown) co-localized with fesselin. Both proteins are markers of dense plaques). Furthermore, caveolin, the main component of caveolae, (data not shown) did not co-localize with fesselin. These results suggest that fesselin is localized in cytoplasmic dense structures that are distinct from dense plaques and caveolae.
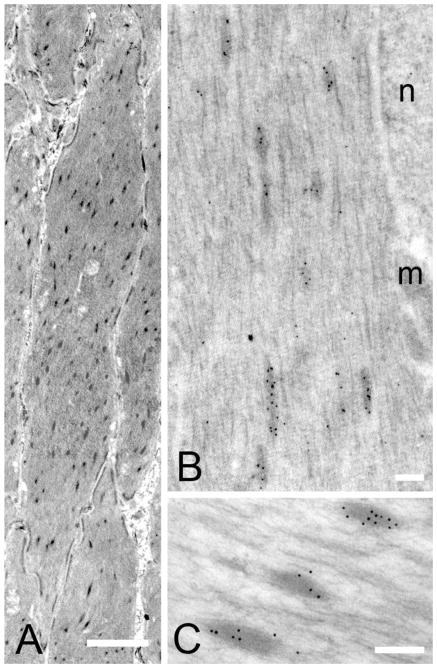
Immunoelectron microscopy was used to confirm that the fesselin-staining structures were dense bodies. Figure 3A shows that large numbers of dense bodies were present in avian gizzard sections. Staining thin sections from LR White embedded tissue using the specific fesselin antibody and protein-A gold showed the presence of numerous gold particles within the boundaries of the dense bodies with few gold particles in the cytoplasm or in other structures (Fig. 3B, C). We took particular note of nuclei and membrane-associated dense plaques; gold particles were not observed in these structures.
Figure 3.
Transmission electron micrographs of gizzard tissues. (A) Dense bodies were observed as dark elongated structures throughout the cytoplasm. Bar= 20 μm. (B–C) Fesselin was detected in association with dense bodies and there was no evidence that fesselin is confined to a particular region of these structures. nucleus (n) mitochondria (m). Bar =2μm.
Skeletal and Cardiac Muscle
Fesselin was detected as a pattern of regularly spaced bands along the longitudinal axis of skeletal muscle fibers from both white and dark skeletal muscle (Figure 4). Dual staining of fesselin and desmin revealed a close association of these two proteins and indicated that fesselin is localized to the Z-line in skeletal muscle (Figure 4A). Fesselin was not co-localized with the M-line associated protein, myomesin (Figure 4B).
Figure 4.
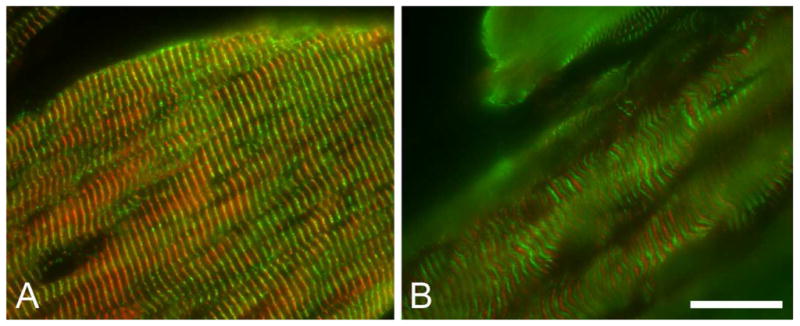
Immunocytochemical localization of fesselin in skeletal muscle. Localization of (A and B) fesselin (red), desmin (A: green) and myomesin (B: green) in thigh muscle are shown. Exclusion of fesselin from myomesin containing M-lines places fesselin at Z-lines.
Western blot analysis showed that the fesselin content of cardiac muscle is much lower than in skeletal or smooth muscle tissue (Figure 1, lane D–E). Furthermore, the band corresponding to fesselin migrated in the SDS gel with a higher apparent molecular mass than that from skeletal muscle tissue. Nevertheless, fesselin was observed by immunocytochemistry to have a banding pattern similar to that for skeletal muscle, (Figure 1G–H). Cardiac tissue probed with anti-fesselin and anti-myomesin antibodies did not show an overlap but resulted in an alternating banding pattern. However co-localization was observed for desmin and fesselin indicating that fesselin is also localized to the Z-line in cardiac tissue (data not shown).
Discussion
The data presented here are consistent with localization of fesselin to dense bodies in gizzard smooth muscle cells. Gabella (1997) described visceral smooth muscle dense bodies as rigid structures associated with thin and intermediate filaments that are variable in size with dimensions up to 1.2 μm long and 0.3 μm wide. The size of the intracellular structures identified in the current study by immunofluorescence is consistent with those for dense bodies. Immunoelectron microscope evaluation of fesselin confirmed association of this protein with numerous dense bodies found in this tissue. The close association of dense bodies to thin (Bond and Somlyo 1982) and intermediate filaments (Cooke 1976; Small and Sobieszek 1977, 1980) has lead to the hypothesis that these structures are integral parts of the smooth muscle cell cytoskeleton and contractile apparatus. Bond and Somylo (1982), and Sachiko et al. (1983) demonstrated that dense bodies serve as anchorage sites for thin filaments similar to the association of thin filaments at the Z-lines of skeletal muscle. Those studies supported the hypothesis that dense bodies in association with thin and thick filaments constitute the contractile unit of vertebrate smooth muscle and that these contractile units are interconnected by intermediate (10nm) filaments. This concept was supported by the observation that dense bodies in skinned toad stomach cells are arranged in semi-rigid groups which move toward each other during muscle contraction (Kargacin et al. 1989).
We also observed fesselin in the Z-lines of skeletal and cardiac muscle. This is reasonable as dense bodies are functionally analogous to Z-lines (Ashton et al. 1975; Bois 1973; Schollmeyer et al. 1976). Therefore, fesselin may have a role in organizing actin filaments in all muscle types. While fesselin is in both dense bodies and Z-lines the distribution of fesselin forms is different in these structures. Electrophoresis and Western blotting of smooth muscle tissue extracts shows several fesselin forms that vary in molecular mass. The major forms detected in gizzard have molecular masses of 79 and 103 kDa. We have so far only detected the 79 kDa species in skeletal muscle tissue extracts. Heart tissue contains a form of fesselin that is larger than 103 kDa and that has not yet been characterized.
MALDI-TOF mass spectral analysis of several of the fesselin forms indicate that they result from alternative splicing (unpublished data). Furthermore, as will be discussed later, fesselin is avian synaptopodin 2 or myopodin. De Ganck et al. (2008) observed 3 splice forms of synaptopodin 2 mRNA consistent with our observation. At present, the specific properties of each form important for the formation of dense bodies and Z-lines are unknown.
A recent publication has shown that fesselin is a member of the synaptopodin protein family (Schroeter et al. 2008) and is homologous to mammalian synaptopodin 2, better known as myopodin. Myopodin is localized to the Z-line of skeletal and cardiac muscle (Weins et al. 2001) where it is believed to be involved in anchoring actin filaments. That location is consistent with our observation of fesselin being localized to Z-lines in skeletal and cardiac tissue. While localization of members of the synaptopodin 2 subfamily in skeletal and cardiac tissue is well established there are no reports of localization in smooth muscle tissue. Localization of fesselin in dense bodies of smooth muscle cells suggests that fesselin has the same function in smooth muscle dense bodies and in z-lines of muscles. The localization of fesselin suggests an important role in the organization of actin filaments in muscle.
Localization of fesselin to centers of actin organization is consistent with our earlier observation that fesselin stimulates actin polymerization (Beall and Chalovich 2001). Fesselin increases the rate of actin nucleation and this function is regulated by Ca2+-calmodulin (Beall and Chalovich 2001; Schroeter and Chalovich 2004). Fesselin also stimulates bundling of filamentous actin, although Ca2+-calmodulin does not regulate this action (Schroeter and Chalovich 2004). This bundling may also be important in the organization of actin filaments.
Smooth muscle dense plaques link the cytoskeleton with the plasma membrane and are considered vital components of the cellular cytoskeleton (Small and Gimona 1998). Association of multiple cytoskeleton components in dense plaques and their proposed linkage to the extracellular matrix (Small and North 1993) and ultimately to adjacent cells (Gabella 1997) suggest a central role for these structures in transmission of contractile forces among smooth muscle cells. Dense plaques contain some of the same components found in dense bodies including α-actinin, desmin, calponin, and filamin. In addition, dense plaques contain proteins that are not found in dense bodies including vinculin and talin. We did not observe co-localization of fesselin with vinculin- (not shown) or talin-containing structures (Fig. 2B). We also failed to detect co-localization of fesselin with the caveolae specific protein caveolin-1 (not shown). Fesselin is one of very few proteins that have been reported to be localized to dense bodies but not to dense plaques. Therefore, fesselin is a specific marker for dense bodies. An interesting remaining question is which property of fesselin makes it uniquely suited to the function of dense bodies.
In summary, the data presented here establish the localization of fesselin to dense bodies in avian smooth muscle tissue as well as to the Z-line of skeletal and cardiac muscle. The close relationship of this protein to thin and intermediate filaments associated with these structures suggests an important role in cytoskeletal and contractile element assembly and function.
Acknowledgments
This work was supported by grant AR035216 from the National Institutes of Health to J.M.C. Parts of this work were presented at the 51st Annual Biophysical Society Meeting, Baltimore, MD; March 2007.
Abbreviations
- MALDI-TOF MS
matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy
- FITC
fluorescein isothiocyanate
Reference List
- Ashton FT, Somlyo AV, Somlyo AP. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975;98:17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- Beall B, Chalovich JM. Fesselin, a Synaptopodin-like protein, stimulates actin nucleation and polymerization. Biochem. 2001;40:14252–14259. doi: 10.1021/bi011806u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois RM. The organization of the contractile apparatus of vertebrate smooth muscle. Anat Rec. 1973;177:61–77. doi: 10.1002/ar.1091770107. [DOI] [PubMed] [Google Scholar]
- Bond M, Somlyo AV. Dense bodies and actin polarity in vertebrate smooth muscles. J Cell Biol. 1982;95:403–413. doi: 10.1083/jcb.95.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976;68:539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. Modulation of solute permeability in microvascular endothelium. Fed Proc. 1986;45:77–83. [PubMed] [Google Scholar]
- De Ganck A, De Corte V, Staes A, Gevaert K, Vandekerckhove J, Gettemans J. Multiple isoforms of the tumor suppressor myopodin are simultaneously transcribed in cancer cells. Biochem Biophys Res Comm. 2008;370:269–273. doi: 10.1016/j.bbrc.2008.03.086. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993;120:1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Morphology of smooth muscle. In: Kao CY, Carsten ME, editors. Cellular Aspect of Smooth Muscle Function. Cambridge University; Cambridge Press: 1997. pp. 1–47. [Google Scholar]
- Grove BK, Kurer V, Lehner C, Doetschman TC, Perriard J-C, Eppenberger HM. A new 185,000-dalton skeletal muscle protein detected by monoclonal antibodies. J Cell Biol. 1984;98:518–524. doi: 10.1083/jcb.98.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin GJ, Cooke PH, Abramson SB, Fay FS. Periodic organization of the contractile apparatus in smooth muscle revealed by the motion of dense bodies in single cells. J Cell Biol. 1989;108:1465–1475. doi: 10.1083/jcb.108.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowski J, Wrzosek A, Dabrowska R. Fesselin is a target protein for calmodulin in a calcium-dependent manner. Biochem Biophys Res Commun. 2004;323:1251–1256. doi: 10.1016/j.bbrc.2004.08.224. [DOI] [PubMed] [Google Scholar]
- Leinweber BD, Fredricksen RS, Hoffman DR, Chalovich JM. Fesselin: a novel synaptopodin-like actin binding protein from muscle tissue. J Muscle Res Cell Motil. 1999;20:539–545. doi: 10.1023/a:1005597306671. [DOI] [PubMed] [Google Scholar]
- North AJ, Gimona M, Lando Z, Small JV. Actin isoform compartments in chicken gizzard smooth muscle cells. J Cell Sci. 1994;107:445–455. doi: 10.1242/jcs.107.3.445. [DOI] [PubMed] [Google Scholar]
- Popescu LM, Diculescu I. Calcium in smooth muscle sarcoplasmic reticulum. J Cell Biol. 1975;67:911–918. doi: 10.1083/jcb.67.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Jr, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Pham M, Chalovich JM. Smooth muscle α-actinin binds tightly to fesselin and attenuates its activity toward actin polymerization. J Muscle Res Cell Motil. 2006;27:45–51. doi: 10.1007/s10974-005-9053-2. [DOI] [PubMed] [Google Scholar]
- Sachiko T, Tsukita S, Ishikawa H. Association of actin and 10 nm filaments with the dense body in smooth muscle cells of the chicken gizzard. Cell Tissue Res. 1983;229:233–242. doi: 10.1007/BF00214972. [DOI] [PubMed] [Google Scholar]
- Schollmeyer JE, Furch LT, Goll DE, Robson RM, Stromer MH. Localization of contractile proteins in smooth muscle cells and in normal and transformed fibroblasts. In: Goldman AR, Pollard T, Stromer MH, editors. Cell Motility. Cold Spring Harbor Cold Spring Harbor Laboratory; 1976. pp. 361–385. [Google Scholar]
- Schroeter M, Chalovich JM. Ca2+-calmodulin regulates fesselin induced actin polymerization. Biochem. 2004;43:13875–13882. doi: 10.1021/bi0487490. [DOI] [PubMed] [Google Scholar]
- Schroeter MM, Chalovich JM. Fesselin binds to actin and myosin and inhibits actin-activated ATPase activity. J Muscle Res Cell Motil. 2005;26:183–189. doi: 10.1007/s10974-005-9009-6. [DOI] [PubMed] [Google Scholar]
- Schroeter MM, Beall B, Heid H, Chalovich JM. The actin binding protein, Fesselin, is a member of the synaptopodin family. Biochem Biophys Res Commun. 2008;371:582–586. doi: 10.1016/j.bbrc.2008.04.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV. Structure-function relationship in smooth muscle: the missing links. BioEssays. 1995;17:785–792. doi: 10.1002/bies.950170908. [DOI] [PubMed] [Google Scholar]
- Small JV, Gimona M. The cytoskeleton of the vertebrate smooth muscle cell. Acta Physiol Scand. 1998;164:341–348. doi: 10.1046/j.1365-201X.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- Small JV, North AJ. Architecture of the smooth muscle cell. In: Schwartz SM, Machem R-P, editors. Vascular Smooth Muscle Cell. Academic Press; San Diego: 1995. pp. 169–185. [Google Scholar]
- Small JV, Sobieszek A. Studies on the function and composition of the 10 nm (100Å) filaments of vertebrate smooth muscles. J Cell Sci. 1977;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Small JV, Sobieszek A. The contractile apparatus of smooth muscle. Int Rev Cytol. 1980;64:241–306. doi: 10.1016/s0074-7696(08)60239-9. [DOI] [PubMed] [Google Scholar]
- Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J Cell Biol. 2001;155:393–404. doi: 10.1083/jcb.200012039. [DOI] [PMC free article] [PubMed] [Google Scholar]