Abstract
Moderate alcohol consumption has been linked to an approximate 30-50% increased risk in breast cancer. Case-control and cohort studies have consistently observed this modest increase. We highlight recent evidence from molecular epidemiologic studies and studies of intermediate markers like mammographic density that provide additional evidence that this association is real and not solely explained by factors/correlates of the exposure and outcome present in non-randomized studies. We also review evidence from studies of higher risk women including BRCA1 and BRCA2 mutation carriers. Given the incidence of heart disease is higher than breast cancer and modest alcohol consumption is associated with reduced risk of heart disease, we examine the latest evidence to evaluate if alcohol reduction should be targeted to women at high risk for breast cancer. We also review the most recent evidence on the effect of alcohol use on tumor recurrence and survival for those diagnosed with breast cancer.
Keywords: Alcohol, Alcohol dehydrogenase (ADH), BRCA1, BRCA2, Breast cancer, Mammographic density, Risk, Prevention, Screening, Review
Introduction
Extensive epidemiologic data have linked alcohol consumption to risk of breast cancer (reviewed in [1-5]). The overall estimated association is an approximate 30-50% increase in breast cancer risk from 15-30 grams/day of alcohol consumption (about 1-2 drinks/day) [6-8•]. Given the level of alcohol consumption in the U.S. population, modifying this behavior could have a large impact on breast cancer incidence [9].
Despite variability in defining light, moderate and heavy alcohol intake, studies have found a consistent modest association between higher alcohol intake and increased breast cancer risk (reviewed in [1-3]. A meta-analysis of 53 studies reported that compared with women who abstained from alcohol consumption, the relative risk of breast cancer increased by 32% (95% CI 1.19-1.45) for those with intake of 35-44 g/day (approximately 3-4 drinks per day), and by 46% (95% CI 1.33-1.61) for ≥45 g/day of alcohol use (approximately more than 4 drinks per day) [10]. Overall, the relative risk of breast cancer is increased by 7% for each additional 10 grams of alcohol consumed per day [10]. More recently, a summary of data for lighter drinking supported that even in women who drink ≤12.5 g/day (≤1 drink/day) there was a 5% increase in risk of breast cancer compared to non-drinkers (95% CI 1.02-1.08) [1].
Cell culture, animal, and human studies reveal multiple plausible pathways by which alcohol may play a role in breast cancer pathogenesis [5, 3]. The most commonly explored pathways stem from both the carcinogenic role of ethanol metabolites as well as the role of alcohol in altering estrogen levels [1-4]. Acetaldehyde (AA) is the primary ethanol metabolite suggested to have a possible role in breast cancer pathogenesis. Eighty-percent of ethanol is metabolized by alcohol dehydrogenase (ADH) to AA [11-14]. This mainly happens in the liver where approximately 97% of ADH is localized; however, ADH is also expressed and regulated in breast and other tissues [12-14, 11]. AA can bind proteins and DNA, interfering with the anti-oxidative defense system and DNA synthesis and repair. Moreover, by decreasing the availability of methyl donors, it can indirectly modify epigenetic histones and DNA methylation creating genomic instability [2, 3, 15]. Higher intake of alcohol has also been shown to increase circulating estrogen levels (reviewed in [1, 4, 2]). Estrogen is thought to induce hormone-receptor mediated cell proliferation and cause genetic alterations including aneuploidy [2, 4]. Ethanol has also been hypothesized to play a direct role in breast cancer tumorigenesis by down-regulating the tumor suppressor gene BRCA1, resulting in increased transcriptional activity of ERα, a key estrogen receptor, leading to increased cell proliferation and greater opportunity for genetic damage [16].
Despite the strong biologic rationale, human data on alcohol and breast cancer risk are from non-randomized studies, and since the magnitude of the overall association is relatively modest at about 30-50% increase in breast cancer risk from moderate alcohol consumption [6-8], it is critical to rule out the role of bias in explaining these results. Importantly, as alcohol has been associated with breast cancer risk in both case-control and cohort studies (reviewed in [3, 1]), it is less likely that selection bias or information bias are plausible explanations for this association as these two biases operate differently in these study designs. However, the observed results can still be explained by confounding of unmeasured or poorly measured antecedents of alcohol consumption and breast cancer. For example, if moderate alcohol drinkers were more likely to have higher caloric intake or less likely to exercise, then the increased breast cancer risk may partially or even fully be explained by these other factors. Although confounding cannot be ruled out through observational epidemiologic studies, we can leverage information from molecular epidemiologic studies and studies of intermediate markers to help exclude confounding as a likely explanation of the observed findings. This is because it is less likely that potential confounders of the alcohol and breast cancer association also map to different intermediate and genetic markers associated with this pathway. Since we know that alcohol may increase breast cancer risk through acetaldehyde as well as through altering endogenous hormone levels, we review the latest epidemiologic evidence for 1) gene-environmental interactions with alcohol metabolizing genes and breast cancer and 2) alcohol and mammographic breast density, the strongest intermediate marker for breast cancer.
Alcohol metabolizing genes and breast cancer
Ethanol is metabolized by ADH to AA and subsequently removed by aldehyde dehydrogenase (ALDH) to acetate [17]. The genes that encode for ADH or ALDH are polymorphic, and depending on the activity of the enzymes they encode, determine the rate of ethanol metabolism and the concentration of intermediate metabolites including the carcinogenic AA [17]. If the observed association between alcohol consumption and breast cancer is attributable to an unmeasured or a poorly measured confounder, we would not expect the strength of the alcohol and breast cancer association to change depending on the genotype of the enzymes that regulate ethanol metabolism unless the confounder also mapped to the genotype. Any modification of the association between alcohol intake and breast cancer by the activity of enzymes therefore offers additional support that the association is causal and not explained by confounding.
Since AA is thought to play a central role in alcohol-associated carcinogenesis and its levels are primarily determined by the activity of genes encoding ADH, several studies have evaluated the effect modification of the association between alcohol and breast cancer risk by the genes encoding ADH isozymes [18-28]. There are several classes of human ADH genes and it’s the class I ADH isozymes that are primarily involved in ethanol oxidation. The genes that encode these enzyme types are ADH1A, ADH1B, and ADH1C (former nomenclature ADH1, ADH2 and ADH3, respectively). ADH1B polymorphisms are primarily found among Asians and the genetic variant ADH1B*2 has almost 40 times more enzymatic activity compared to the wild-type ADH1B*1 [29, 17]. Therefore, having ADH1B*2 results in prolonged exposure to AA and is thought to increase breast cancer risk [17]. Several studies have reported that the type of ADH1B allele present modifies the association between alcohol and breast cancer [24, 26, 27]. However, AA is also responsible for the side effects associated with alcohol consumption. Therefore, homozygous recessive carriers of ADH1B*2 have such severe side effects from alcohol consumption that they are often alcohol abstainers, making this genotype less commonly studied due to the small numbers who consume alcohol [17].
In addition to ADH1B, ADH1C also effects enzymatic activity but to a lesser extent: ADH1C*1 allele has almost 2.5 times more enzymatic activity compared to ADH1C*2. Because polymorphisms in ADH1C gene are more common in Caucasians than polymorphisms in ADH1B, they have been more frequently studied in U.S. populations [17, 29]. Individuals with the ADH1C*1/1, ADH1C*1/2, or ADH1C*2/2 genotypes are considered fast, intermediate, or slow metabolizers of alcohol, respectively [17]. In a population based study among 1,047 breast cancer cases and 1,101 controls, we observed that ADH1C*1/1 carriers who consumed moderate levels of alcohol (15-30 grams/day) were at almost twice the risk of having breast cancer compared to nondrinkers (OR 1.97, 95% CI 1.10-3.54) [21]. Another case-control study (N=315 cases and N=356 controls) also reported increased breast cancer risk in ADH1C*1/1 carriers, especially among premenopausal women with alcohol intake above the median (OR 3.6, 95% CI 1.5-8.8) [19]. With some exceptions [18, 22-24], these findings [21, 19] and results from other studies showing interaction of the ADH1C genotype with alcohol-breast cancer association [20, 25] give additional support that this association is likely causal as it is less likely that unknown or poorly measured confounders also differ by the ADH1C genotype.
Alcohol and breast density marker
In addition to the carcinogenic role of its metabolites, alcohol has been shown to alter estrogen levels, which may lead to changes in breast density, affecting breast cancer risk [30, 31]. An intermediate marker of breast cancer risk that has also been linked with many hormonal breast cancer risk factors is mammographic density, a measure of epithelial and connective tissue in the breast. Higher density confers a 4-6 fold increase in breast cancer risk (reviewed in [30, 31]). Moreover, alcohol use has been shown to modify the mammographic density-breast cancer association in a dose-response way [32-34]. Associations between alcohol use and intermediate markers such as breast density provide further evidence that alcohol is truly associated with breast cancer and that this association cannot be explained by bias alone.
Epidemiologic studies have used different measures to assess mammographic density with earlier studies using qualitative measures such as the Wolfe parenchymal patterns [35, 36], and more recent studies using more quantitative methods that either use categories such as the Breast Imaging Reporting and Data System (BI-RADS) [37] or use a continuous measure ranging from 0-100% based on computer threshold programs [38, 39, 31].
Wolfe patterns classify breast density into four categories: predominately fat (N1), ductal prominence in <25% of breast (P1), ductal prominence in >25% of breast (P2), and extensive dysplasia (DY) [38, 39, 31]. There have been at least four studies that have assessed breast density using Wolfe patterns [40-43], with one large European study (N=1,668) reporting a 31% increase in higher density with higher consumption of alcohol (Wolfe pattern P2/DY compared to N1/P1) [43]. Other studies that have used Wolfe patterns have generally failed to find significant associations [40-42]. This may be because Wolfe patterns generally reflect much larger changes in density than the more quantitative measures.
Other recent studies that use more quantitative measures of density are reviewed in Table 1. With a few exceptions [44-46], studies examining alcohol consumption and breast density have generally found positive associations with at least six studies reporting a statistically significant association [47-52], and another three reporting a positive trend that was not statistically significant [53-55]. A large Spanish study (N=3,568) found a positive association between alcohol consumption (>10 grams/day) and higher percent density compared to non-drinkers among postmenopausal women (≥10% density (ordinal scale) vs. <10%: OR 1.26, 95% CI 1.03-1.53) [53]. The Minnesota Breast Cancer Family study also reported in a series of studies, positive associations between alcohol consumption and higher density [50, 51, 55]. In one of these studies (N=1,900), compared to never users, daily drinkers had higher mean percent density among both pre- (43% vs. 32%, Ptrend 0.01) and postmenopausal women (34% vs. 29%, Ptrend 0.02) [50]. The longitudinal Fernald Community Cohort (N=1,125) study measured density using BIRADS [47]. BI-RADS uses the following four groups to categorize breast density from the least dense to the most dense: almost entirely fat (I), scattered fibroglandular densities (II), heterogeneously dense (III), and extremely dense (IV) [37]. In this study, the authors defined low density (BIRADS I) and high density (BI-RADS IV) as sustained low or high densities throughout the cohort’s follow-up. They found that among both pre- and postmenopausal women, ever users of alcohol were at almost twice the risk of having high breast density (BIRADS density IV vs. I: OR 2.0; 95% CI 1.4-2.8) compared to never users [47•].
Table 1.
Studies reporting the association between alcohol intake and mammographic density
| Study | Study Population |
Years Recruited |
Age (years) | Total, n |
Prevalence of Exposure |
Results: Main Effectsa | Adjustments |
|---|---|---|---|---|---|---|---|
|
BI-RADS DENSITY
| |||||||
| Jeon et al. 2011 [48] |
Mammography Screening (Korea) |
2008 | 40-80 mean 50.6 |
516 | 30% current/past users |
Outcome: BI-RADS (III/IV versus I/II) Versus never users: current/past users OR 1.36, 95% CI 0.87- 2.14 |
age, BMI, menopausal status, age at menarche, parity, family history, OC use, education |
|
| |||||||
| Yaghjyan et al. 2012 [47] |
Fernald Community Cohortb (USA) |
1990-2008 | mean 51.3 | 1125 | 21% ever users |
Outcome: BI-RADS (IV versus I) Versus never users: users OR 2.0, 95% CI 1.4-2.8 |
age at mammogram, BMI at mammogram, menopausal status, age at menarche, age at first birth, parity, family history, smoking, |
|
CONTINUOUS MEASURE OF DENSITY
| |||||||
| Boyd et al. 1995 [49] |
Hospital based sample of pre- menopausal women (Canada) |
not available | 29-51 mean 43.0 |
273 | not available | Outcome: Percent Density Positive correlation between mammographic density and alcohol consumption in grams (ß (SE) 2.34 (0.81), P=0.004). |
BMI, skinfolds, parity, Apo B, malondialdehyde |
| Cabanes et al. 2011 [53] |
Spanish breast cancer screening program network (Spain) |
2007-2008 | 45-68 | 3568 | 63% ever users 58% current users 17% consume >10 grams/day |
Outcome: Percent Density (ordinal scale: <10%, 10-25%, 25-50%, 50-75%, >75%) Versus non-current users: users OR 1.13, 95% CI 0.99-1.28 <10 g/day OR 1.11, 95% CI 0.97-1.27 ≥10 g/day OR 1.18, 95% CI 0.99-1.41 Versus never users: former drinker OR 0.98, 95% CI 0.73-1.32 current user OR 1.13, 95% CI 0.99-1.28 Versus alcohol initiation after menarche: before/at menarche OR 0.66, 95% CI 0.42-1.03 |
age at mammogram, BMI, menopausal status, parity, HRT use, smoking, screening program |
|
| |||||||
| Vachon et al. 2000 [51] |
All studies include women from the Minnesota breast cancer family registry (US)c |
1944-2001 | 40-90 mean 61.4 |
1508 | mean 4 g/day | Outcome: Percent Density (visual estimation)d Among Premenopausal users (mean density): Non-current users 39%, 95% CI 34-43 ≤3.9 g/day 45%, 95% CI 40-50 >3.9 g/day 42%, 95% CI 38-46 Ptrend=0.08 Among Postmenopausal users (mean density): Non-current users 31%, 95% CI 26-30 ≤3.9 g/day 32%, 95% CI 27-31 >3.9 g/day 33%, 95% CI 28-31 Ptrend=0.09 Among red wine users, there was no effect among premenopausal women (Ptrend=0.42), but an inverse association among postmenopausal women (Ptrend=0.02) Among white wine users, there was no effect among premenopausal women (Ptrend=0.62), but a positive association among postmenopausal women (Ptrend<0.01) |
age, age2, BMI, WHR, age at menarche, age at first birth and parity combined, family history, OC use (premenopausal only), HRT use (postme- nopausal only), smoking, physical activity, caloric intake; beer, red wine, white wine, and liquor mutually adjusted |
| 2005 [55] | mean 60.4 at mammogram |
1893 | 21% users before age 18 86% adult users |
Outcome: Percent Density (computer assisted)d Use before age 18 (adolescent alcohol use): Never users 21.2, 95% CI 20.8-21.7 Ever users 21.0, 95% CI 20.1-21.9 ≤6 times/year 20.2, 95% CI 18.9-21.4 >6 times/year 22.0, 95% CI 20.7-23.3 Among daily/weekly adult users (mean density): adolescent non-users or ≤6 times/year 23.2%, 95% CI 22.2-24.2 adolescent >6 times/year 25%, 95% CI 23-27 Ptrend=0.24 |
age, BMI, age at menarche, age at first birth and parity, smoking, OC use, education, HRT use, menopausal status, adolescent and adult smoking; correlated frailty score based on kinship; frequency of adult alcohol use (where appropriate) |
||
| Gapstur et al. 2003 [45] |
Chicago Breast Health Project, Hispanic women (US) |
2000-2002 | 40-76 | 296 | 17% users | Outcome: Percent Density (computer assisted) Univariate associations (mean density): Non-users 17.4% <1 drink/week 17.8% ≥1 drinks/week 22.2% P=0.27 |
age, BMI menopausal status, age at menopause, age at menarche, age at first birth, parity, family history, HRT use, smoking, education |
|
| |||||||
| Maskarinec et al. 2006 [54] |
The Multiethnic Cohort (MEC), Caucasian, Japanese, and Hawaiian women (US) |
1993-1996 | mean 57.3 | 1250 | mean 2.3 drinks/week |
Outcome: Percent Density (computer assisted) mean density: Non-users 33.1% <1 drink/day 34.6% ≥1 drink/day 33.8% Ptrend=0.96 |
age, BMI, menopausal status, age at menarche, age at birth, parity, family history, HRT use, case or experimental group status, ethnicity |
| Breast, Estrogens, and Nutrition (BEAN) Study (US) |
2000-2001 | mean 43.0 | 217 | mean 2.0 drinks/week |
Outcome: Percent Density (computer assisted) mean density: Non-users 44.5% <1 drink/day 45.3% ≥1 drink/day 46.1% Ptrend=0.22 |
MEC matched on ethnicity and 5-year age intervals |
|
| Flom et al. 2009 [52] |
New York Women’s Birth Cohort, non- Hispanic white, non-Hispanic Black, and Hispanic womene (US) |
1959-1963 | mean 42.4 at mammogram |
151 | 53% current users 71% ever users |
Outcome: Percent Density and Absolute Dense Area (computer assisted)f Versus non-current users: 1-6 drinks/week ß −0.09, 95% CI −4.79-4.60 ≥7 drinks/week ß 12.32, 95% CI 4.28-20.36 Versus never users: ≤4 g/day ß 3.88, 95% CI −1.82-9.58 >4 g/day ß −0.40, 95% CI −6.07-5.27 Versus non-current red wine users: Inverse association during the following age periods (years): ≤20, 20-29, 30-39, 40-49 |
age, BMI; beer, red wine, white wine, and liquor mutually adjusted |
|
| |||||||
| Qureshi et al. 2012 [46] |
Norwegian Breast Cancer Screening Program among postmenopausal women (Norway) |
2004 | 50-69 mean 58.4 |
2251 | 87% current users |
Outcome: Percent Density and Absolute Dense Area (computer assisted)f mean percent density: Non-users 18.3%, 95% CI 17.3-19.6 1-<7 g/week 18.3%, 95% CI 17.5-19.3 7-14 g/week 18.3%, 95% CI 17.6-19.0 15-30 g/week 18.2%, 95% CI 17.6-18.8 31-60 g/week 18.2%, 95% CI 17.5-18.8 61-90 g/week 18.2%, 95% CI 17.2-18.9 >90 g/week 18.2%, 95% CI 16.9-19.0 Ptrend=0.91 No effect on density by alcohol type (beer, red wine, white wine, or liquor) |
age at mammogram, BMI, age at menarche, age at first birth, parity, HRT use, education; beer, red wine, white wine, and liquor mutually adjusted |
Abbreviations: BMI, body mass index; BI-RADS, Breast Imaging-Reporting and Data System; CI, confidence interval; g, grams; HRT, hormone replacement therapy; OC, oral contraceptive; OR, odds ratio; US, United States
Measures of mammographic density: BI-RADS, four categories from the least dense to most dense: almost entirely fat (category I), scattered fibroglandular densities (category II), heterogeneously dense (category III), and extremely dense (category VI); Semi-quantitative Boyd’s scale, six categories from the least dense to most dense: 0% (A), <10% (B), 10-25% (C), 25-50% (D), 50-75% (E), and >75% (F) [38]; Percent density, defined as the percentage of dense tissue; absolute density, defined as the total dense area in cm2.
The Fernald Medical Monitoring Program (FMMP) was a community based surveillance program for residents living in close proximity to the former uranium processing plant in Fernald, Ohio. Women who were exposed to uranium/radiation were not eligible for the study.
The Minnesota Breast Cancer Family registry recruited women from 1944-1952 and from 1990-1996 information for enrolled families were updated. From 2001-2006, a questionnaire was sent to update cancer and new exposure data. Another report was published from this cohort in the same year and found similar results and is referred to in the text [50].
Visual density estimation was done by trained radiologists. Training included a quantitative estimation of phantom mammograms of known densities. Other percent density measurements employed a computer assisted quantitative program.
The New York Women’s Birth Cohort is an adult follow-up study of women enrolled in the National Collaborative Perinatal Project (NCPP). The adult follow-up was from 2001-2006.
Only displaying results for percent density. There are no differences in inferences with absolute density.
Although studies assessing density are generally among postmenopausal women when mammography screening is more common; studies with predominantly premenopausal populations have also found positive associations between alcohol use and higher density [48, 47, 49, 50, 52, 43]. In particular, we conducted a study in a racially diverse (26% non-Hispanic white, 34% non-Hispanic Black, 40% Hispanic) urban birth cohort where 91% women were premenopausal and found higher percent density among those consuming ≥7 drinks/week compared to non-drinkers (12.3%, 95% CI 4.3-20.4) [52]. Similar results were seen in the Multiethnic Cohort, which had a higher proportion of Japanese and Hawaiian women (>1 drink/day vs. <1 drink/month: mean density 46.1% and 44.5%, Ptrend 0.22) [54]. In addition to examining whether the alcohol-breast density association varies by menopausal status, three studies have looked at interaction by hormone replacement therapy (HRT) status [47, 54, 46] with two studies, the Fernald Community Cohort and the Multiethnic Cohort, suggesting a stronger association among HRT users [47, 54]; similar to the alcohol and breast cancer literature.
Fewer studies have fully addressed the question of timing and type of alcohol. In contrast to the studies examining adult alcohol use, the three studies that looked at adolescent alcohol use and adult breast density failed to report significant associations [52, 55, 53] (Table 1). This may be because unlike assessing current or lifetime alcohol use, examining past alcohol use is subject to recall bias, which would lead to underestimation of the strength of the true association. In addition to the timing of alcohol, four studies have assessed the strength of the alcohol-density association by the type of alcohol consumed [46, 51, 43, 52] (Table 1). One study suggested that density is positively associated with white wine [51] and two studies found an inverse association with red wine [51, 52]. However, in a large Norwegian cohort of postmenopausal women (N=2,251), there was no effect of alcohol on percent density overall (>90 g/week vs. 0 g/week: 18.2% and 18.3%, Ptrend 0.91) or by alcohol types (beer, red wine, white wine, or liquor) [46]. This may be because the Norwegian cohort consumed an average of 6 g/day of alcohol (approximately half a serving/day) [46]; which is lower than the studies that observed an effect [52, 43].
Although a number of studies reported a positive association between alcohol consumption and density, not all were statistically significant. Reasons for this could be small sample size [56, 41, 40, 45] or low levels of alcohol use [45, 46, 44]. Another important reason could be the use of categorical rather than continuous measures to assess density. Other established factors associated with breast cancer risk such as HRT or tamoxifen use show only modest changes in breast density [30, 57]; therefore, finding significant associations between alcohol use and density may require more sensitive measures of breast density.
In summary, studies examining alcohol consumption and breast density provide additional evidence that the association between alcohol intake and breast cancer risk is causal. Unlike assessing breast cancer as the outcome, studies examining breast density are less likely to have differential information bias as women reporting alcohol use would most likely be unaware of their breast density status. Studies of intermediate markers like density provide further evidence that alcohol can affect breast cancer risk through hormonal pathways [31, 58]. Future work to confirm that reducing alcohol consumption reduces breast density and risk accordingly over time, as is the case for HRT cessation [59], would be the most robust way to confirm this hypothesis.
Considerations for clinical recommendations on alcohol consumption for breast cancer prevention
Existing epidemiologic evidence, complemented further by studies of genetic and intermediate markers, strongly suggests that alcohol use may increase breast cancer risk. High alcohol consumption has also been associated with a number of other adverse health conditions including liver diseases such as alcoholic hepatitis and cirrhosis, cardiovascular diseases such as high blood pressure and cardiomyopathy, and other cancers such as that of mouth, pharynx, larynx, esophagus, and colon [60]. Alcohol abuse also leads to violent crimes, automobile accidents, psychiatric problems, and for a pregnant woman, increases the risk of birth defects [60]. However, modest alcohol consumption has been consistently associated with a decreased risk of coronary heart disease (CHD). In a recent meta-analysis, among women, current drinkers had a 29% reduced risk of CHD incidence and a 21% reduced risk of CHD mortality compared to non-current drinkers [61].
Based on the overall evidence regarding health effects of alcohol use, the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the American Heart Association (AHA), and the Health and Human Services (HHS) recommend that women should not consume >1 drink/day and >7 drinks/week [62-64]. Despite these guidelines, data from the National Health and Nutrition Examination Survey (NHANES) demonstrate that approximately 8% of women are heavy drinkers (>7 drinks/week) and this number has remained fairly stable from 1999 to 2010 [65]. According to the 2009-2010 NHANES, 51% of US women are current drinkers consuming more than 12 drinks in the past one year (Figure). Among current drinkers, 41% are moderate drinkers (≤7 drinks/week) and 10% are heavy drinkers (>7 drinks/week). Prevalence of heavy drinking is higher among non-Hispanic white women (12%) as compared to non-Hispanic Black (7%) or Hispanic women (4%). Among non-Hispanic Black and Hispanic women, younger women (20-39 years) tend to drink more as compared to older women (≥60 years).
Figure. Prevalence (percentage) of alcohol consumption among US women, by Race and Ethnicity (2009-2010).
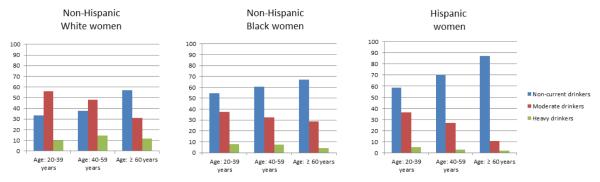
Prevalence (percentage) of alcohol consumption among US women 20 years and older stratified by race-ethnicity and age group (data from the National Health and Nutrition Examination Survey, 2009-10). Definitions: Non-current drinkers, ≤ 12 drinks in the past 12 months; Moderate drinkers, > 12 drinks but ≤ 7 drinks/week in the past 12 months; heavy drinkers, > 7 drinks/week in the past 12 months.
One of the goals of the Healthy People 2020 initiative is to reduce the average annual alcohol consumption by 10% over a period of 10 years [66]. Meeting this goal will require public health practitioners and clinicians to develop effective strategies to modify alcohol behavior among those who drink more than one drink a day. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) reported that over a period of three years, among women who were current drinkers at baseline, 11% reported abstaining from alcohol during the 3 year study period [67]. Among women who were current drinkers who did not exceed drinking limits at baseline, 20% reported exceeding drinking limits in the past year [67]. These findings reflect that though the trends of alcohol use may appear stable, women change their drinking habits over time.
What about women at higher-breast cancer risk
Assuming that effective strategies can be implemented to reduce heavy drinking to moderate levels for the majority of women who are at average risk of breast cancer, the potential cardiovascular benefit from modest intake may outweigh the increased risk of breast cancer and other alcohol-related adverse health outcomes, given the high incidence of cardiovascular disease. The prevention challenge in women at high risk of breast cancer is that the cardioprotective benefits of alcohol among these women may be of less consequence. The empirical evidence however, has not shown that women at high risk of breast cancer who consume alcohol are at particularly higher risk due to their alcohol intake. For example, in a study among 89,538 US women that defined breast cancer risk by age and a set of other risk factors, compared to non-drinkers, alcohol consumption was associated with an increased breast cancer risk in women who were at low (RR 2.5, 95% CI 1.5-4.2) as well as high risk (RR 1.5, 95% CI 1.1-1.9) [68]. Additional studies have examined risk by family history (broadly defined as ever/never for an affected mother or sister). These studies also found that alcohol use was not associated with an increased risk in women with a family history of breast cancer [8, 69]. Table 2 summarizes more recent studies of women at higher risk of breast cancer from high risk family based registries and from women who are BRCA1 or BRCA2 mutation carriers. In the Minnesota Family study (132 high risk families), compared to never drinkers, first-degree relatives who consumed alcohol daily had a greater risk of developing breast cancer (RR 2.71, 95% CI 1.33-5.53), with no association among second-degree relatives (RR 1.15, 95% CI 0.62-2.13) [70]. In contrast, using data from 811 sibling-sets from families at high risk of breast cancer from the Breast Cancer Family Registry, we did not observe an association for either moderate (<7 drinks/week) or high (≥7 drinks/week) alcohol intake (moderate OR 0.91, 95% CI 0.73-1.14; high OR 0.85, 95% CI 0.63-1.14 compared to non-drinkers) [22].
Table 2.
Association between Alcohol Intake and Breast Cancer Incidence among Women at High Risk for Breast Cancer
| Study | Study Popula- tion |
Years Recruited |
Age (years) | Sample Size |
Prevalence of Exposure |
Results | Adjustments |
|---|---|---|---|---|---|---|---|
| Vachon et al. 2001 [70] |
Multigenerational breast cancer families (USA)a |
1944-1952 | Not provided | 132 high risk families |
76% ever users |
Versus never users, first degree relatives: <weekly users RR 1.08, 95% CI 0.72-1.63 weekly users RR 1.02, 95% CI 0.48-2.18 daily users RR 2.71, 95% CI 1.33-5.53 Versus never users, second degree relatives: <weekly users RR 1.02, 95% CI 0.75-1.40 weekly users RR 0.86, 95% CI 0.55-1.34 daily users RR 1.15, 95% CI 0.62-2.13 Versus never users, marry-ins: <weekly users RR 1.48, 95% CI 1.01-2.15 weekly users RR 1.07, 95% CI 0.62-1.84 daily users RR 0.85, 95% CI 0.40-1.81 |
age, smoking, birth cohort, familial clustering, type of information (self- respondents or surrogate) |
| Terry et al. 2007 [22] |
Sibling-based study of high breast cancer risk (USA, Canada)b |
1996-2005 | Control: mean 47.8 (among unaffected sisters) |
811 sibling- sets |
49% current user among unaffected sisters |
Versus non-current users: <7 drinks/week OR 0.91, 95% CI 0.73-1.14 ≥7 drinks/week OR 0.85, 95% CI 0.63-1.14 Versus ADH1B slow genotype (ADH1B*1/1): ADH1B*1/2 OR 1.15, 95% CI 0.80-1.66 ADH1B*2/2 OR 0.91, 95% CI 0.40-2.11 Versus ADH1C fast genotype (ADH1C*1/1): ADH1C*1/2 OR 1.04, 95% CI 0.81-1.33 ADH1C*2/2 OR 1.04, 95% CI 0.71-1.52 No interaction between ADH1B or ADH1C genotypes and alcohol |
Sibling based study adjusted for age |
| McGuire et al. 2006 [72] |
BRCA1 or BRCA2 mutation carriers (USA, Canada, and Australia)c |
1996-2005 | <50 | 804 | 47% ever users |
Versus never users: users BRCA1 OR 1.06, 95% CI 0.73-1.52 users BRCA2 OR 0.66, 95% CI 0.45-0.97 Versus never users: 1-4 g/day BRCA1 OR 0.63, 95% CI 0.34-1.18 >4 g/day BRCA1 OR 1.14, 95% CI 0.77-1.69 1-4 g/day BRCA2 OR 0.41, 95% CI 0.22-0.77 >4 g/day BRCA2 OR 0.79, 95% CI 0.52-1.18 No increased risks by alcohol type (beer, wine, or spirits) |
age, parity, family history, smoking; Stratified on age and study sites |
| Nkondjock et al. 2006 [73] |
BRCA1 or BRCA 2mutation carriers (Canada)c |
1995 | Cases: mean 56.2 Controls: mean 50.9 |
137 | Not provided | Versus lowest tertile of alcohol intake (≤1.7 g/day): middle tertile (>1.7-7.9 g/day) OR 0.88, 95% CI 0.36-2.17 highest tertile (>7.9 g/day) OR 1.09, 95% CI 0.44-2.75 No increased risks by alcohol type (beer, wine, or spirits) |
age, maximum lifetime BMI, physical activity |
| Moorman et al. 2010 [74] |
Breast cancer cases only including 487 BRCA1 or BCRA2 mutation carriers (USA and Australia)e |
2004e |
BRCA1 carriers: mean 41.2 BRCA2 carriers: mean 44.0 Non-carriers: mean 44.1 |
1381 | 72% current users |
Versus non-carriers: BRCA1 Interaction Risk Ratiof 0.65, 95% CI 0.48-0.90 BRCA2 Interaction Risk Ratio 0.80, 95% CI 0.55-1.16 |
age, center |
| Dennis et al. 2010 [75] |
BRCA1 or BRCA2 mutation carriers (USA, Canada, Austria, Israel, Italy, Norway, Poland, and UK)g |
1992-2009 | Cases: mean 46.6 Controls: mean 47.0 |
1925 matched pairs |
59% current users |
Versus non-current users, among BRCA1 carriers: 0-3 drinks/week OR 0.77, 95% CI 0.67-0.94 4-9 drinks/week OR 0.98, 95% CI 0.73-1.32 ≥10 drinks/week OR 0.55, 95% CI 0.33-0.91 exclusive wine consumers OR 0.64, 95% CI 0.47-0.87 Versus non-current users, among BRCA2 carriers: 0-3 drinks/week OR 0.97, 95% CI 0.67-1.41 4-9 drinks/week OR 1.04, 95% CI 0.67-1.63 ≥10 drinks/week OR 1.16, 95% CI 0.55-2.45 exclusive wine consumers OR 1.01, 95% CI 0.61-1.69 |
BMI, menopausal status, parity, HRT use, OC use, smoking, ethnicity, oophorectomy Matched on age, BRCA mutation carrier status, country of residence, ethnicity (Canada only) |
| Dennis et al. 2011 [71] |
Breast cancer cases only including 10 BRCA1 and 33 BRCA2 carriers (Canada)h |
2004-2009 |
BRCA carriers: mean 48.4 Non-carriers: mean 51.0 |
857 | 85% current users |
Alcohol dichotomized at median (3 drinks/week): BRCA1 case-only ORi 0.79, 95% CI 0.22-2.83 BRCA2 case-only OR 1.99, 95% CI 0.96-4.11 Wine dichotomized at median (2 drinks/week): BRCA1 case-only OR 0.38, 95% CI 0.08-1.81 BRCA2 case-only OR 1.58, 95% CI 0.78-3.17 Other alcoholj dichotomized at median (0.33 drinks/week): BRCA1 case-only OR 2.49, 95% CI 0.64-9.73 BRCA2 case-only OR 2.15, 95% CI 1.03-4.50 |
age |
| Lecarpentier et al. 2011 [76] |
BRCA1 or BRCA2 carriers (France)k |
2000-2010 | Cases: mean 49.4 Controls: mean 41.0 |
990 | 74% ever users |
Versus never users, among BRCA1 carriersl: current users (never smokers) HR 1.02, 95% CI 0.65-1.60 current users (ever smokers) HR 0.90, 95% CI 0.49-1.68 Versus never users, among BRCA2 carriers: current users HR 1.21, 95% CI 0.68-2.15 |
menopausal status, parity, BRCA mutation carriers status, number of years of smoking interruption (when appropriate) |
Abbreviations: ADH, alcohol dehydrogenase; BMI, body mass index; CI, confidence interval; g, grams; HR, hazard ratio; HRT, hormone replacement therapy; OR, odds ratio; RR, relative risk; UK, United Kingdom; USA, United States of America
Participants were from the Minnesota Breast Cancer Family registry. The analysis included 426 multigenerational families, of which, 132 families had three or more breast and/or ovarian cancers in the family. The results shown here are for these 132 high risk families.
Participants were women from the Breast Cancer Family Registry. There were three sites included, two US sites (New York and California) and the Ontario Cancer Genetic Network in Canada.
Participants were non-Hispanic white women from the Breast Cancer Family Registry (includes six research institutions in USA, Canada, and Australia), the Kathleen Cunningham Foundation Consortium for Research into Family Breast Cancer (Australia), and the Ontario Cancer Genetic Network (Canada).
Participants were French-Canadian women who were recruited and interviewed since 1995.
Participants form the Genetic and Environmental Modifiers of BRCA1/BRCA2 Study (GEMS). The study comprised of 11 centers in USA and Australia. Recruitment for the study began in 2004 and cases were identified both prospectively and retrospectively.
Interaction risk ratio is a ratio of risk ratios and can be expressed as the risk ratio of disease given exposure among carriers to the risk ratio of disease given exposure among non-carriers. Since there was no traditional ‘control group’ of unaffected subjects, the main effects were not assessed. The exposed and the unexposed were current alcohol users and non-users, respectively.
Participants from 54 centers within 8 countries.
Participants were of French-Canadian ancestry.
Case-only odds ratio (COR) measures the extent to which the joint effect of genotype (BRCA1 / 2 status) and environment (alcohol consumption) differs from the product of their independent effects. COR>1 indicates supra-multiplicative interaction while COR<1 indicates sub-multiplicative interaction.
Includes beer, fortified wine and spirits.
Participants were from the GENEPSO study (France).
In BRCA1 mutation carriers, there was significant interaction between alcohol and tobacco consumption, so the analysis was stratified based on tobacco and alcohol use.
More recent studies of high risk women have focused on women with BRCA1 or BRCA2 mutations. With at least one exception [71], these studies also reported that alcohol consumption was not associated with breast cancer risk [72-75, 71, 76] (Table 2). For example, in a large case-control study conducted in primarily postmenopausal BRCA1 or BRCA2 mutation carriers (N=1,925 sets), alcohol intake was not associated with BRCA-related breast cancer [75•]. The Genetic and Environmental Modifiers Study (GEMS) examined the interaction risk ratio for BRCA mutation carriers and alcohol intake, which measures the risk ratio of disease given exposure among carriers to the risk ratio of disease given exposure among non-carriers. The study found that the effect of alcohol consumption (drinkers versus non-drinkers) on BRCA1 mutation carriers was weaker than in non-BRCA mutation carriers (IRR 0.65, 95% CI 0.48-0.90) [74].
The BRCA carrier studies till now have largely been retrospective and based on their sampling, may be affected by survivor bias [75, 72]. The low prevalence of BRCA mutation carriers could also limit the ability to detect modest associations between alcohol and carrier status [71, 74, 73] or result in not examining BRCA1 and BRCA2 mutation carries status independently [73]. Although the empirical evidence thus far does not suggest that high risk women have an increased risk of breast cancer from alcohol consumption, prospective confirmation of these findings across women at a higher continuum of risk, including BRCA mutation carriers, is needed.
Alcohol consumption and breast cancer-related outcomes
With an increasing population of breast cancer survivors, there has been an emphasis on examining the effect of alcohol on breast cancer recurrence and survival. Thus far, studies of recurrence and mortality have shown mixed results (reviewed in [77-79]), although this may be due to studies having few breast cancer specific mortality events and relatively low levels of alcohol consumption, making it difficult to observe a dose-response effect [80, 81]. Moreover, many studies focused on pre-diagnostic alcohol exposure rather than post-diagnostic use. The Collaborative Breast Cancer Study (N=22,890), a population based study with a median follow-up time of 11.3 years, found that moderate alcohol intake before breast cancer diagnosis was associated with lower overall breast cancer mortality (3-6 drinks/week vs. nondrinkers: HR 0.85, 95% CI 0.75-0.95), but there was no association with alcohol intake after diagnosis [82•]. Interestingly, in a prospective Danish cohort (N=1,052) that found a modest association between higher alcohol consumption and recurrence (>2 units/day vs. ≤1 unit/day: HR 1.65, 95% CI 1.02-2.67), the average alcohol intake was higher after breast cancer diagnosis as compared to pre-diagnosis use [83]. The After Breast Cancer Pooling Project (N=9,329), the largest and the longest study to date to assess recurrence and the effect of post-diagnostic alcohol consumption found that increasing levels of alcohol consumption were not associated with either recurrence or overall survival. However, in women who were postmenopausal and ER+ there was a marginal association between higher levels of alcohol use and breast cancer recurrence (≥6 g/day HR 1.19, 95% CI 0.99-1.45) [84•]. These findings have also been seen in previous reports [85-87] and provide further evidence in support of alcohol-induced breast carcinogenesis through hormonal regulation.
Conclusion
Alcohol consumption is associated with a modest increase in breast cancer risk. This association has been consistently found in case-control and cohort studies, reducing the likelihood that it could be explained by selection or information biases. Effect modification of this relationship by the ADH1C genotype, and the associations between alcohol use and higher breast density, provide further evidence in support of a causal effect. Although the majority of the studies to date do not support increased breast cancer risk with higher alcohol use in women at high risk of breast cancer, prospective studies are needed to rule out biases. Although the literature is relatively scant, studies also do not suggest that alcohol affects breast cancer recurrence or survival. Thus, until prospective data become available for women across the spectrum of breast cancer risk, the best evidence suggests that higher risk women are not at increased risk from moderate alcohol consumption. Given the prevalence of drinking in U.S. women, many could benefit from staying within the drinking guidelines of ≤1 drink a day for overall health, and consider stopping entirely to reduce breast cancer risk.
Footnotes
Conflict of Interest Jasmine A. McDonald, Abhishek Goyal, and Mary Beth Terry declare that they have no conflict of interest.
Contributor Information
Jasmine A. McDonald, 722W 168St, R719 Department of Epidemiology Mailman School of Public Health Columbia University New York, NY 10032 Phone: 212-305-9114 Fax: 212-305-9413 jam2319@columbia.edu.
Abhishek Goyal, 722W 168St, R723 Department of Epidemiology Mailman School of Public Health Columbia University New York, NY 10032 Phone: 212-305-3586 Fax: 212-305-9413 ag3078@columbia.edu.
Mary Beth Terry, 722W 168St, R724A Department of Epidemiology Mailman School of Public Health Columbia University New York, NY 10032; Herbert Irving Comprehensive Cancer Center 1130 St. Nicholas Ave. Columbia University New York, NY 10032.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol and alcoholism. 2012;47(3):204–12. doi: 10.1093/alcalc/ags011. [DOI] [PubMed] [Google Scholar]
- 2.Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35(3):213–25. doi: 10.1016/j.alcohol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Oyesanmi O, Snyder D, Sullivan N, et al. Alcohol consumption and cancer risk: understanding possible causal mechanisms for breast and colorectal cancers. Evidence report/technology assessment. 2010;(197):1–151. [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez SV. Estrogen, alcohol consumption, and breast cancer. Alcoholism, clinical and experimental research. 2011;35(3):389–91. doi: 10.1111/j.1530-0277.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 5.Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA : the journal of the American Medical Association. 2001;286(17):2143–51. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 6.Terry MB, Zhang FF, Kabat G, et al. Lifetime alcohol intake and breast cancer risk. Annals of epidemiology. 2006;16(3):230–40. doi: 10.1016/j.annepidem.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 7•.Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA : the journal of the American Medical Association. 2011;306(17):1884–90. doi: 10.1001/jama.2011.1590. The prospective Nurses’ Health Study found levels as low as 3-6 drinks/week were associated with increased breast cancer risk. Stratified analysis found that alcohol consumption at ages 18-40 years and after age 40 was independently associated with increased breast cancer risk. Alcohol consumption was also strongly associated with hormone receptor positive tumor status.
- 8.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA : the journal of the American Medical Association. 1998;279(7):535–40. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 9.Boffetta P, Hashibe M. Alcohol and cancer. The lancet oncology. 2006;7(2):149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 10.Hamajima N, Hirose K, Tajima K, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. British journal of cancer. 2002;87(11):1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosron WF, Li TK. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6(3):502–10. doi: 10.1002/hep.1840060330. [DOI] [PubMed] [Google Scholar]
- 12.Seitz HK, Oneta CM. Gastrointestinal alcohol dehydrogenase. Nutrition reviews. 1998;56(2 Pt 1):52–60. doi: 10.1111/j.1753-4887.1998.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 13.Wright RM, McManaman JL, Repine JE. Alcohol-induced breast cancer: a proposed mechanism. Free Radical Biology and Medicine. 1999;26(3–4):348–54. doi: 10.1016/s0891-5849(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 14.Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. The Proceedings of the Nutrition Society. 2004;63(1):49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 15.Druesne-Pecollo N, Tehard B, Mallet Y, et al. Alcohol and genetic polymorphisms: effect on risk of alcohol-related cancer. The lancet oncology. 2009;10(2):173–80. doi: 10.1016/S1470-2045(09)70019-1. [DOI] [PubMed] [Google Scholar]
- 16.Fan S, Meng Q, Gao B, et al. Alcohol Stimulates Estrogen Receptor Signaling in Human Breast Cancer Cell Lines. Cancer Research. 2000;60(20):5635–9. [PubMed] [Google Scholar]
- 17.Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes & nutrition. 2010;5(2):121–8. doi: 10.1007/s12263-009-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines LM, Hankinson SE, Smith-Warner SA, et al. A prospective study of the effect of alcohol consumption and ADH3 genotype on plasma steroid hormone levels and breast cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9(10):1099–105. [PubMed] [Google Scholar]
- 19.Freudenheim JL, Ambrosone CB, Moysich KB, et al. Alcohol dehydrogenase 3 genotype modification of the association of alcohol consumption with breast cancer risk. Cancer causes & control : CCC. 1999;10(5):369–77. doi: 10.1023/a:1008950717205. [DOI] [PubMed] [Google Scholar]
- 20.Coutelle C, Hohn B, Benesova M, et al. Risk factors in alcohol associated breast cancer: alcohol dehydrogenase polymorphism and estrogens. International journal of oncology. 2004;25(4):1127–32. [PubMed] [Google Scholar]
- 21.Terry MB, Gammon MD, Zhang FF, et al. ADH3 genotype, alcohol intake and breast cancer risk. Carcinogenesis. 2006;27(4):840–7. doi: 10.1093/carcin/bgi285. [DOI] [PubMed] [Google Scholar]
- 22.Terry MB, Knight JA, Zablotska L, et al. Alcohol metabolism, alcohol intake, and breast cancer risk: a sister-set analysis using the Breast Cancer Family Registry. Breast cancer research and treatment. 2007;106(2):281–8. doi: 10.1007/s10549-007-9498-7. [DOI] [PubMed] [Google Scholar]
- 23.Visvanathan K, Crum RM, Strickland PT, et al. Alcohol dehydrogenase genetic polymorphisms, low-to-moderate alcohol consumption, and risk of breast cancer. Alcoholism, clinical and experimental research. 2007;31(3):467–76. doi: 10.1111/j.1530-0277.2006.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarty CA, Reding DJ, Commins J, et al. Alcohol, genetics and risk of breast cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Breast cancer research and treatment. 2012;133(2):785–92. doi: 10.1007/s10549-012-1972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benzon Larsen S, Vogel U, Christensen J, et al. Interaction between ADH1C Arg(272)Gln and alcohol intake in relation to breast cancer risk suggests that ethanol is the causal factor in alcohol related breast cancer. Cancer letters. 2010;295(2):191–7. doi: 10.1016/j.canlet.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Sturmer T, Wang-Gohrke S, Arndt V, et al. Interaction between alcohol dehydrogenase II gene, alcohol consumption, and risk for breast cancer. British journal of cancer. 2002;87(5):519–23. doi: 10.1038/sj.bjc.6600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilla C, Koehler T, Kropp S, et al. Alcohol dehydrogenase 1B (ADH1B) genotype, alcohol consumption and breast cancer risk by age 50 years in a German case-control study. British journal of cancer. 2005;92(11):2039–41. doi: 10.1038/sj.bjc.6602608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawase T, Matsuo K, Hiraki A, et al. Interaction of the effects of alcohol drinking and polymorphisms in alcohol-metabolizing enzymes on the risk of female breast cancer in Japan. Journal of epidemiology / Japan Epidemiological Association. 2009;19(5):244–50. doi: 10.2188/jea.JE20081035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan P, Lewis S, Hashibe M, et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: a HuGE review. American journal of epidemiology. 2004;159(1):1–16. doi: 10.1093/aje/kwh003. [DOI] [PubMed] [Google Scholar]
- 30.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast cancer research : BCR. 2008;10(1):201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast cancer research : BCR. 2011;13(6):223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrinton LJ, Saftlas AF, Stanford JL, et al. Do alcohol intake and mammographic densities interact in regard to the risk of breast cancer? Cancer. 1993;71(10):3029–35. doi: 10.1002/1097-0142(19930515)71:10<3029::aid-cncr2820711024>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Byrne C, Schairer C, Wolfe J, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. Journal of the National Cancer Institute. 1995;87(21):1622–9. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 34.Conroy SM, Koga K, Woolcott CG, et al. Higher alcohol intake may modify the association between mammographic density and breast cancer: an analysis of three case-control studies. Cancer epidemiology. 2012;36(5):458–60. doi: 10.1016/j.canep.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR American journal of roentgenology. 1976;126(6):1130–7. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37(5):2486–92. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.American College of Radiology . Breast Imaging Reporting and Data System Atlas (BI-RADS) American College of Radiology; Reston, VA: 2003. [Google Scholar]
- 38.Boyd NF, Martin LJ, Yaffe M, Minkin S. Mammographic density. Breast cancer research : BCR. 2009;11(Suppl 3):S4. doi: 10.1186/bcr2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaffe M. Mammographic density. Measurement of mammographic density. Breast Cancer Research. 2008;10(3):209. doi: 10.1186/bcr2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funkhouser E, Waterbor JW, Cole P, Rubin E. Mammographic patterns and breast cancer risk factors among women having elective screening. Southern medical journal. 1993;86(2):177–80. doi: 10.1097/00007611-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Sala E, Warren R, Duffy S, et al. High risk mammographic parenchymal patterns and diet: a case-control study. British journal of cancer. 2000;83(1):121–6. doi: 10.1054/bjoc.2000.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brisson J, Verreault R, Morrison AS, et al. Diet, mammographic features of breast tissue, and breast cancer risk. American journal of epidemiology. 1989;130(1):14–24. doi: 10.1093/oxfordjournals.aje.a115305. [DOI] [PubMed] [Google Scholar]
- 43.Masala G, Ambrogetti D, Assedi M, et al. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. International journal of cancer Journal international du cancer. 2006;118(7):1782–9. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 44.Tseng M, Byrne C, Evers KA, Daly MB. Dietary intake and breast density in high-risk women: a cross-sectional study. Breast cancer research : BCR. 2007;9(5):R72. doi: 10.1186/bcr1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gapstur SM, Lopez P, Colangelo LA, et al. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003;12(10):1074–80. [PubMed] [Google Scholar]
- 46.Qureshi SA, Couto E, Hofvind S, et al. Alcohol intake and mammographic density in postmenopausal Norwegian women. Breast cancer research and treatment. 2012;131(3):993–1002. doi: 10.1007/s10549-011-1812-8. [DOI] [PubMed] [Google Scholar]
- 47•.Yaghjyan L, Mahoney MC, Succop P, et al. Relationship between breast cancer risk factors and mammographic breast density in the Fernald Community Cohort. British journal of cancer. 2012;106(5):996–1003. doi: 10.1038/bjc.2012.1. The longitudinal Fernald Community Cohort study defined low density (BIRADS I) and high density (BI-RADS IV) as sustained low or high densities throughout the cohort’s follow-up. They found that among both pre- and postmenopausal women, ever users of alcohol were at twice the risk of having high breast density (BIRADS density IV vs. I: OR 2.0; 95% CI 1.4-2.8) compared to never users.
- 48.Jeon JH, Kang JH, Kim Y, et al. Reproductive and Hormonal Factors Associated with Fatty or Dense Breast Patterns among Korean Women. Cancer research and treatment : official journal of Korean Cancer Association. 2011;43(1):42–8. doi: 10.4143/crt.2011.43.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyd NF, Connelly P, Byng J, et al. Plasma lipids, lipoproteins, and mammographic densities. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1995;4(7):727–33. [PubMed] [Google Scholar]
- 50.Vachon CM, Kuni CC, Anderson K, et al. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer causes & control : CCC. 2000;11(7):653–62. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- 51.Vachon CM, Kushi LH, Cerhan JR, et al. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9(2):151–60. [PubMed] [Google Scholar]
- 52.Flom JD, Ferris JS, Tehranifar P, Terry MB. Alcohol intake over the life course and mammographic density. Breast cancer research and treatment. 2009;117(3):643–51. doi: 10.1007/s10549-008-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabanes A, Pastor-Barriuso R, Garcia-Lopez M, et al. Alcohol, tobacco, and mammographic density: a population-based study. Breast cancer research and treatment. 2011;129(1):135–47. doi: 10.1007/s10549-011-1414-5. [DOI] [PubMed] [Google Scholar]
- 54.Maskarinec G, Takata Y, Pagano I, et al. Alcohol consumption and mammographic density in a multiethnic population. International journal of cancer Journal international du cancer. 2006;118(10):2579–83. doi: 10.1002/ijc.21705. [DOI] [PubMed] [Google Scholar]
- 55.Vachon CM, Sellers TA, Janney CA, et al. Alcohol intake in adolescence and mammographic density. International journal of cancer Journal international du cancer. 2005;117(5):837–41. doi: 10.1002/ijc.21227. [DOI] [PubMed] [Google Scholar]
- 56.Boyd NF, McGuire V, Fishell E, et al. Plasma lipids in premenopausal women with mammographic dysplasia. British journal of cancer. 1989;59(5):766–71. doi: 10.1038/bjc.1989.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyd NF, Melnichouk O, Martin LJ, et al. Mammographic density, response to hormones, and breast cancer risk. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(22):2985–92. doi: 10.1200/JCO.2010.33.7964. [DOI] [PubMed] [Google Scholar]
- 58.Vanderweele TJ, Adami HO, Tamimi RM. Mammographic density as a mediator for breast cancer risk: analytic approaches. Breast cancer research : BCR. 2012;14(4):317. doi: 10.1186/bcr3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horn-Ross PL, Canchola AJ, Bernstein L, et al. Alcohol consumption and breast cancer risk among postmenopausal women following the cessation of hormone therapy use: the California Teachers Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(11):2006–13. doi: 10.1158/1055-9965.EPI-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention [Accessed March 2013];Alcohol and Public Health. Available at http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm.
- 61.Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. Bmj. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Heart Association [Accessed March 2013];Alcoholic Beverages and Cardiovascular Disease. Available at http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Alcoholic-Beverages-and-Cardiovascular-Disease_UCM_305864_Article.jsp.
- 63.National Institute of Alcohol Abuse and Alcoholism [Accessed March 2013];Moderate & Binge Drinking. Available at http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- 64.The U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary Guidelines for Americans. 7th ed U.S. Government Printing Office; Washington, D.C.: Dec, 2010. [Google Scholar]
- 65.Centers for Disease Control and Prevention (CDC)/National Center for Health Statistics (NCHS) [Accessed February 2013];National Health and Nutrition Examination Survey Data 1999-2010. Available at http://www.cdc.gov/nchs/nhanes.htm.
- 66.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion [Accessed March 2013];Healthy People 2020. Available at http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=40#260956.
- 67.Chiung M, Chen, Hsiao-ye Yi PD, Deborah A, Dawson, et al. Alcohol Use And Alcohol Use Disorders In The United States, A 3-Year Follow-Up: Main Findings From The 2004–2005 Wave 2 National Epidemiologic Survey On Alcohol And Related Conditions (NESARC) 2. Vol. 8. National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health; Sep, 2010. [Google Scholar]
- 68.Willett WC, Stampfer MJ, Colditz GA, et al. Moderate alcohol consumption and the risk of breast cancer. The New England journal of medicine. 1987;316(19):1174–80. doi: 10.1056/NEJM198705073161902. [DOI] [PubMed] [Google Scholar]
- 69.Schatzkin A, Jones DY, Hoover RN, et al. Alcohol consumption and breast cancer in the epidemiologic follow-up study of the first National Health and Nutrition Examination Survey. The New England journal of medicine. 1987;316(19):1169–73. doi: 10.1056/NEJM198705073161901. [DOI] [PubMed] [Google Scholar]
- 70.Vachon CM, Cerhan JR, Vierkant RA, Sellers TA. Investigation of an interaction of alcohol intake and family history on breast cancer risk in the Minnesota Breast Cancer Family Study. Cancer. 2001;92(2):240–8. doi: 10.1002/1097-0142(20010715)92:2<240::aid-cncr1315>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 71.Dennis J, Krewski D, Cote FS, et al. Breast cancer risk in relation to alcohol consumption and BRCA gene mutations--a case-only study of gene-environment interaction. The breast journal. 2011;17(5):477–84. doi: 10.1111/j.1524-4741.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 72.McGuire V, John EM, Felberg A, et al. No increased risk of breast cancer associated with alcohol consumption among carriers of BRCA1 and BRCA2 mutations ages <50 years. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(8):1565–7. doi: 10.1158/1055-9965.EPI-06-0323. [DOI] [PubMed] [Google Scholar]
- 73.Nkondjock A, Robidoux A, Paredes Y, et al. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast cancer research and treatment. 2006;98(3):285–94. doi: 10.1007/s10549-006-9161-8. [DOI] [PubMed] [Google Scholar]
- 74.Moorman PG, Iversen ES, Marcom PK, et al. Evaluation of established breast cancer risk factors as modifiers of BRCA1 or BRCA2: a multi-center case-only analysis. Breast cancer research and treatment. 2010;124(2):441–51. doi: 10.1007/s10549-010-0842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Dennis J, Ghadirian P, Little J, et al. Alcohol consumption and the risk of breast cancer among BRCA1 and BRCA2 mutation carriers. Breast. 2010;19(6):479–83. doi: 10.1016/j.breast.2010.05.009. In a case-control study of 1,925 matched pairs in primarily postmenopausal BRCA1 or BRCA2 mutation carriers, alcohol intake was not associated with BRCA-related breast cancer. They also found, compared to non-drinkers, an inverse association between exclusive wine consumers and breast cancer risk in BRCA1 mutation carriers (Ptrend=0.01).
- 76.Lecarpentier J, Nogues C, Mouret-Fourme E, et al. Variation in breast cancer risk with mutation position, smoking, alcohol, and chest X-ray history, in the French National BRCA1/2 carrier cohort (GENEPSO) Breast cancer research and treatment. 2011;130(3):927–38. doi: 10.1007/s10549-011-1655-3. [DOI] [PubMed] [Google Scholar]
- 77.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: A review of the epidemiologic literature. Maturitas. 2010;66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Ligibel J. Lifestyle Factors in Cancer Survivorship. Journal of Clinical Oncology. 2012;30(30):3697–704. doi: 10.1200/JCO.2012.42.0638. [DOI] [PubMed] [Google Scholar]
- 79.Brooks PJ, Zakhari S. Moderate Alcohol Consumption and Breast Cancer in Women: From Epidemiology to Mechanisms and Interventions. Alcoholism: Clinical and Experimental Research. 2013;37(1):23–30. doi: 10.1111/j.1530-0277.2012.01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holmes MD. Challenge of Balancing Alcohol Intake. Journal of Clinical Oncology. 2010;28(29):4403–4. doi: 10.1200/JCO.2010.31.0102. [DOI] [PubMed] [Google Scholar]
- 81.Bandera EV, August DA. Alcohol Consumption and Breast Cancer Survival. Journal of Clinical Oncology. 2009;27(10):1727. doi: 10.1200/JCO.2009.21.3371. [DOI] [PubMed] [Google Scholar]
- 82.Newcomb PA, Kampman E, Trentham-Dietz A, et al. Alcohol Consumption Before and After Breast Cancer Diagnosis: Associations With Survival From Breast Cancer, Cardiovascular Disease, and Other Causes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.46.5765. The Collaborative Breast Cancer Study found that moderate alcohol intake before breast cancer diagnosis was associated with improved breast cancer survival, but there was no association with alcohol intake after diagnosis. Furthermore, compared to nondrinkers, alcohol intake before or after breast cancer diagnosis was associated with increased cardiovascular disease survival.
- 83•.Holm M, Olsen A, Christensen J, et al. Pre-diagnostic alcohol consumption and breast cancer recurrence and mortality: Results from a prospective cohort with a wide range of variation in alcohol intake. International Journal of Cancer. 2013;132(3):686–94. doi: 10.1002/ijc.27652. [DOI] [PubMed] [Google Scholar]
- 84.Kwan ML, Chen WY, Flatt SW, et al. Postdiagnosis alcohol consumption and breast cancer prognosis in the after breast cancer pooling project. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(1):32–41. doi: 10.1158/1055-9965.EPI-12-1022. This is the largest and the longest study to date to assess the effects of post-diagnostic alcohol use and recurrence. Post-diagnosis alcohol consumption (average 2 years) was not associated with breast cancer recurrence or mortality; however, in women who were postmenopausal and ER+ there was a marginal association between higher levels of alcohol use and breast cancer recurrence.
- 85.Li Y, Baer D, Friedman GD, et al. Wine, liquor, beer and risk of breast cancer in a large population. European journal of cancer. 2009;45(5):843–50. doi: 10.1016/j.ejca.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki R, Orsini N, Mignone L, et al. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis of epidemiological studies. International journal of cancer Journal international du cancer. 2008;122(8):1832–41. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 87.Lew JQ, Freedman ND, Leitzmann MF, et al. Alcohol and risk of breast cancer by histologic type and hormone receptor status in postmenopausal women: the NIH-AARP Diet and Health Study. American journal of epidemiology. 2009;170(3):308–17. doi: 10.1093/aje/kwp120. [DOI] [PMC free article] [PubMed] [Google Scholar]


