Abstract
The present study tested the hypotheses that 1) short-term (ST) dietary deficiency of magnesium (MgD; 21 days) in rats would result in the upregulation of neutral-, acid-, and alkaline- sphingomyelinases SMases) in cardiac and vascular smooth muscles (VSMCs), 2) ST MgD would result in an upregulation of proto-oncogenes, i.e., c-Fos and c-Jun, as well as the p65 and c-Rel components of NF-κB in cardiac and VSMCs, 3) low levels of Mg2+ added to drinking water would either prevent or greatly reduce the upregulation of the SMases and proto-oncogene expression, 4) exposure of primary cultured VSMCs to low extracellular Mg2+ concentration would lead to release of ceramide in both cerebral and aortic VSMCs, 5) specific inhibitors of neutral- and acid-SMAs would reduce the release of ceramide in cultured VSMCs exposed to low extracellular Mg2+, and 6) specific inhibitors of neutral- and acid-SMases would lead to reductions in the expression of c-fos, c-Jun, and NF-κB components. The data indicate that neutral-, acid-and alkaline-SMases exist in rat cardiac and VSMCs. ST MgD resulted in over 150% increases in SMase activity and proto-oncogene expression in left and right ventricular muscle, atrial muscle, and abdominal aortic smooth muscle; even very low levels of Mg2+ added to drinking water either prevented or ameliorated the activation of all 3-SMases as well as expression of c-Fos and c-Jun; scyphostatin and desipramine reduced the low Mg2+ - induced expression of the proto-oncogenes as well as p65 and c-Rel in VSMCs. Exposure of the VSMCs to low Mg2+ resulted in more than a 100% increase in release of ceramide; scyphostatin and desipramine reduced greatly the release of ceramide from the VSMCs. We believe when the present data are viewed in light of our previous, recent findings on the effects of Mg deficiency on most of the major enzymes in the sphingomyelin-ceramide pathway, that they could provide a rational basis for the treatment and prevention of drug-resistant hypertension, atherogenesis, and difficult-to-treat forms of cardiac failure.
Keywords: Neutral-sphingomyelinase, acid-sphingomyelinase, alkaline-sphingomyelinase, water-borne magnesium, cardiac muscle, vascular muscle
Introduction
Disturbances in diet are known to promote lipid deposition and accelerated growth and transformation of the smooth muscle cells in the vascular walls. Reduction in dietary Mg intake has been demonstrated, experimentally, to result in hypertension, atherogenesis, cardiac failure, and strokes by ill-defined mechanisms [1-21]. Hypermagnesemic diets have been shown to ameliorate hypertension, both experimentally and clinically [1-4,11,16,17,22].
Low Mg content in drinking water, found in areas of soft water and Mg-poor soil, is associated with high incidences of ischemic heart disease, coronary vasospasm, and sudden cardiac death [1,4,12,19,23-26]. At present, the average dietary intake of Mg has declined from about 450-485 mg/day in 1900 to about 185-235 mg/day for large segments of the North American population [4,12,25,29]. Both animal and human studies have shown an inverse relationship between dietary intake of Mg and atherosclerosis [1,4,9,11,12,15,19,20,22]. Moreover, the myocardial level of Mg has consistently been observed to be lower in subjects dying from ischemic heart disease, congestive heart failure and sudden cardiac death in soft water areas than those in hard water areas [1,4,19,20,23-25].
Approximately 15 years ago, our laboratory demonstrated, using primary cerebral and peripheral vascular smooth muscle cells (VSMCs), in culture, that a variation in free magnesium ions (Mg2+) causes sustained changes in membrane phospholipids and second messengers, membrane oxidation, and truncation of several membrane fatty acids [27,28]. Decreases in extracellular free Mg ions ([Mg2+]0) produced a fall in membrane sphingomyelin (SM) and a release/synthesis of ceramide, whereas increases in [Mg2+]0 resulted in increases in SM and phosphatidylcholine [28]. The production of ceramides was suggested to be, most likely, a result of activation of at least one or more sphingomyelinases (SMases) [28]. Recently, using a short-term model of Mg deficiency (MgD) in rats (21 days), we demonstrated decreased serum levels of both SM and PC, presumably a consequence of activation of SMases [6], thus giving impetus to the studies using primary VSMCs [28]. SMases can be divided into three major isoforms according to their optimum pH (acid, alkaline, and neutral) [30]. In recent preliminary studies, using culture of primary rat aortic VSMCs, we noted that incubation of the cells with a specific inhibitor of N-SMase resulted in a marked reduction in the synthesis/release of ceramide upon exposure to low Mg2+ [8]. Since cardiovascular (CV) tissues and cells are comprised of diverse phenotypes, it is of considerable interest to determine whether all three classes of SMases exist in all CV tissues and cells.
Nuclear factor-kappa B (NF-κB) and the proto-oncogene families (e.g., c-Fos and c-Jun) are known to be principal players in regulation of growth, differentiation, vascular remodeling, cell migration, inflammatory signals, and cell death [31-33], all factors known to be involved in atherogenesis and hypertension [34-36]. It is not, however, clear as to what initiates these latter disease entities or as to what initiates expression of NF-κB or the proto-oncogenes in these molecular and cellular events. Some studies suggest that generation/release of ceramide may play an important role in activation of NF-κB and some proto-oncogenes [8,34]. NF-κB, like the proto-oncogenes, is a transcription factor and a pleiotropic regulator of numerous genes involved in inflammatory processes [35]. Recently, we have shown in preliminary studies that short-term exposure of cerebral and peripheral VSMCs to low [Mg2+]0 results in an upregulation of proto-oncogenes and several DNA-binding proteins involved in activation of NF-κB [5,8]. Very recently, using a short-term rat model of dietary Mg deficiency, we noted generation of 12 different cytokines/chemokines concomitant with an upregulation of NF-κB and p53 coupled with an upregulation of ceramide synthase and de novo synthesis of ceramide in the left ventricular (LV), right ventricular (RV), atrial, and abdominal aortic smooth muscles [8]. Whether or not the acid or alkaline SMases play any role(s) in the upregulation of NF-κB or the proto-oncogenes in Mg-deficient states of CV tissues and cells is not known.
We designed experiments to determine whether 1) a short-term model of MgD (21 days) would lead to activation of all three SMase isoforms in diverse CV tissue and cell phenotypes, 2) imbibing low levels of a water-soluble Mg salt in drinking water would inhibit or reverse these predicted effects of dietary deficiency of Mg on activation of the SMases, 3) a short-term model of MgD would upregulate proto-oncogenes and NF-κB in intact cardiac and VSMCs concomitant with release of ceramide, 4) use of specific, and selective, inhibitors of two of the three SMase isoforms would inhibit the previously demonstrated release/synthesis of ceramide as well as the activation of NF-κB and proto-oncogenes found in primary cerebral and peripheral VSMCs exposed to low [Mg2+]0 [6-8], and 5) imbibing low levels of a water-soluble Mg salt in drinking water would inhibit or reverse these predicted effects of dietary deficiency of Mg.
We believe the data generated in these studies could provide a basis for why resistant hypertension and cardiac deficiency are associated with high risks of CV complications as well as a high prevalence of target organ dysfunctions. We also believe the studies herein could provide a basis for new therapeutic approaches to prevention of resistant hypertension and cardiac failure.
Materials and methods
Animals, diets, sera, and organ-tissue collections
Mature male and female rats (200±65 g) were used for all experiments. All experiments were approved by the Animal Use and Care Committee of the State University of New York downstate Medical Center. Equal numbers of paired male and female animals were used for all nutrition experiments. Control (600 ppm Mg) and MgD (60 ppm Mg) pellet diets were obtained from DYETS (Bethlehem, PA; AIN-93G diets). All animals were given their respective diets for 21 days as previously described [6]. MgD animals were allowed to drink triply distilled water (Mg2+ < 10-6 M) containing one of four different levels of Mg aspartate-HCl (0, 15, 40, or 100 mg/l Mg, Verla-Pharm, Tutzing, Germany). All control animals received a normal Mg-containing diet (600 ppm) as well as triply distilled water to drink [6]. On the 22nd day, sera and tissues (the left and right ventricles, atria, abdominal aorta between the superior mesenteric arteries, and renal arteries, cleaned of all connective tissues) were collected quickly after anesthesia (45 mg/kg in pentobarbital sodium). Tissues were stored rapidly under liquid nitrogen (-85 °C) until use. Whole blood was collected under anaerobic conditions in red-stoppered (no anticoagulant present) tubes, allowed to clot under anaerobic conditions, and then centrifuged under anaerobic conditions in capped Vacutainer tubes [37]. The sera were then collected into additional red-stoppered Vacutainer tubes under anaerobic conditions for processing shortly thereafter, similar to previously described methods [37]. Serum samples were analyzed within 2 h after collection, as previously described [6]. Total Mg levels were measured by standard techniques in our laboratory (Kodak DT-60 Analyzer, Ektachem Colorimetric Instruments, Rochester, NY). The method favorably compares with atomic absorption techniques for total Mg [37]. An Mg2+-selective electrode with a novel neutral carrier-based membrane (NOVA 8 Analyzer, NOVA Biomedical Instruments, Waltham MA) was used to measure the free divalent cation in the sera [37]. The ion-selective electrode was used in accordance with established procedures developed in our laboratory, having an accuracy and precision of 3% [37].
Biochemical tissue measurements of acid, neutral, alkaline, and total SMase activity
For the SMase assays (0.1 g) tissues were homogenized in various buffer pH’s for the respective isoforms of SMase (acid, pH=4.7; neutral, pH=7.4; and alkaline, pH=9.0). The respective buffer compositions were: acid (50 mM sodium acetate, 1% Triton X-100, 20 mM CaCl2, 100 μM ZnCl2, pH=4.7,); neutral (100 mM Tris-HCl, 50 mM MgCl2, 5 mM DTT, and protease inhibitor, pH=7.4); alkaline (100 mM Tris-HCl, 5 mM MgCl2, 5 mM DTT, and protease inhibitor, pH=9.0). Activities of the various and total SMases were measured using the Cayman Chemical Company (Ann Arbor, MI) Sphingo-myelinase Assay Kit, employing a colormetric ELISA assay. Briefly, the reaction system consisted of the SMase reaction buffer (250 μl), NBD-C6-SM (1 mg/ml) (50 μl), tissue sample, and distilled water, incubated at 37 °C for 1 h. The reactants were then vortexed in 700 μl of chloroform/methanol (2:1 v/v) for 5-10 min at 7,000 rpm. The organic phases were transferred into new tubes, dried under N2, dissolved in 15 μl of chloroform, and TLC developed in chloroform/methanol -20% ammonium hydroxide (14:6:1). The samples were read in a microplate reader at 540 or 570 nm absorbance. Standard curves were plotted and the sample values calculated from the authentic SMase standards.
Isolation of vascular muscle and primary culture of cerebral and aortic VSMCs
Male mongrel dogs (15±3 kg, n = 10-12 dogs/group) were anesthetized with pentobarbital sodium (40 mg/kg iv) and killed by bleeding from the common carotid arteries. After a craniotomy, the brains were rapidly removed and placed in normal Krebs-Ringer bicarbonate (NKRB) solution at room temperature, and the middle cerebral and basilar cerebral arteries were excised and cleaned of arachnoid membranes and blood elements, as previously described [28,38]. Vessels were cut into segments (3-4 mm in length, [28]). Rat aortic and cerebral VSMCs were isolated according to established methods [28,38] in our laboratory (n = 8-10 animals/group) and cultured in DMEM containing 1.2 mmol/l [Mg2+]0, FCS, and antibiotics at 37 °C in a humidified atmosphere composed of 95% air - 5% CO2. After confluence had been established, VSMCs were placed in media containing either 0.3 or 1.2 mmol/l [Mg2+]0 for varying periods of time (120 min, 6 or 18 h). It should be stressed that these experiments using cell cultures and those below on primary VSMCs in culture were never part of the whole animal nutritional experiments (described above); these experiments and others were separate from the nutritional experiments.
Influence of [Mg2+]0 on ceramide levels in primary cultures of VSMCs
Cells were exposed for either 120 min or 18 h in NKRB solutions containing different concentrations of [Mg2+]0 (either 1.2 or 0.3 mM). We then extracted the lipids in the cells by first treating them with 0.1 M KOH in chloroform-methanol [1.2 (vol/vol)] at 37 °C for 1 h. The ceramide was next converted into ceramide-1- [32P] phosphate by Escherichia coli DAG kinase in the presence of [gamma-32P] ATP [7,8,51], and the lipids were then separated on high-performance TLC consisting of a chloroform-acetate-methanol-acetic acid-water [50:20: 15:10:5 (vol/vol/vol/vol/vol)] mixture. After autoradiography, spots corresponding to ceramide-1-phosphate were carefully scraped into vials, and the radioactivity was then counted in a scintillation counter (LS-6500, Beckman). Quantitation of ceramide levels was based on standard curves of known amounts of authentic ceramide [7,8,51]. The results were expressed as picomoles per 108 cells [7,8,51].
Influence of inhibitors of acid- and neutral- SMases on ceramide levels in primary VSMCs exposed to low levels of [Mg2+]0
Before the VSMCs were assayed for ceramide (via the conversion into ceramide-1- [32P] phosphate by Escherichia coli DAG kinase in the presence of [g-32P-ATP, above], either an inhibitor of acid SMase (desipramine, Sigma, St. Louis, MO, 20 μM, [49]) or N-SMase (scyphostatin, Sigma, St. Louis, MO, 75 μM, [8,49]), the cells were exposed for 6 hr to either normal (1.2) or low (0.3) [Mg2+]0 - NKRB solutions at pH 7.4. In some experiments, we added both types of SMase inhibitors to determine whether or not any residual type of SMase (i.e., alkaline SMase) could be found indirectly, as no specific inhibitor of alkaline SMase is currently available.
c-Fos and c-Jun expression in cardiovascular tissues and primary cultured VSMCs
Briefly, for the primary aortic smooth muscle cells, we used methods described previously [5]. The VSMCs were first incubated with either vehicle (NKRB) or modified NKRB containing 0.3 mM/L [Mg2+]0 for 90 min. Cells were then lysed, and total cellular RNA was extracted using the lithium-urea method. Northern blot analyses were then performed as described previously [9]. Blots were hybridized to a randomly, primed specific cDNA probe for c-Fos or c-Jun and then exposed to a Kodak Y-Omat film (Rochester, NY) for 2 to 5 days at -70 °C [5]. Relative amounts of c-Fos and c-Jun expression were determined by a densitometric scanner [5].
For measurement of c-Fos and c-Jun in LV, RV, atrial and aortic smooth muscle obtained from MgD animals, we utilized a highly sensitive ELISA kit recently developed for numerous high-through-put sampling (TransAM AP-1 Family Transcription Factor Assay Kit; Active Motif North America, Carlsbad, CA).
Influence of inhibitors of acid- and neutral-SMases on c-Fos and c-Jun expression in primary VSMCs exposed to low [Mg2+]0
Before the VSMCs cells were assayed for expression of c-Fos or c-Jun, they were exposed to either NKRB or NKRB containing 0.3 mM/L [Mg2+]0 with either desipramine (20 μM, above) or scyphostatin 75 μM, as above) at pH 7.4 for 6 hr. In some experiments, both types of SMase inhibitors were added together to determine whether or not any residual type of SMase (i.e., alkaline SMase could be found indirectly to influence the expression of either c-Fos or c-Jun).
Influence of inhibitors of acid- and N-SMase on NF-κB expression in primary VSMCs
For these studies, we employed EMSA assays similar that we reported previously [5,42]. For the supershift assays, the extracted nuclear proteins were incubated with antibodies specific for the p65 and c-Rel components (Santa Cruz Biotechnology, Santa Cruz, CA) before gamma-32P labeling [13]. NF-κB oligonucleotides were then added as described previously [42]. Before the VSMCs were assayed for the p65 and c-Rel components, the cells were exposed either to NKRB or NKRB containing 0.3 mM/L [Mg2+]0 with either desipramine (20 μM), scyphostatin (75 μM), or both inhibitors.
Statistical analyses
Where appropriate, means and means ± SE were calculated. Differences between means were assessed for statistical significance by Student’s t-test and ANOVA followed by a Newman-Keuls test. In some cases, linear correlation coefficients were calculated by the method of least squares. P values of <0.05 were considered significant.
Results
Influence of diet on water consumption, food intake, and overall physiological condition
As shown recently, using an identical dietary regimen of Mg in controls and MgD animals [6-8,51], there were no significant differences in either water consumption or food intake between the diverse subgroups of rats (i.e., controls = 600 ppm Mg, Mg-deficient-MgD, MgD + 15 mg/l Mg/day, MgD + 40 mg/l Mg/day, or MgD + 100 mg/l Mg/day). All of the MgD subgroups (n = 16-28 animals per group), irrespective of the amount of Mg in the diets or in the drinking water, showed no loss in gait or any other outward signs of pathology or behavior.
Serum total and ionized Mg levels
Feeding the animals the synthetic AIN-93G MgD pellet diet (n = 16-28/group) resulted in a total serum Mg level of 1.05 mM/L, whereas the animals receiving the MgD diet exhibited a serum total Mg level of 0.36±0.006 mM/L (p<0.01). The serum level of ionized Mg in the normal, control group was 0.62±0.004 mM/L, whereas in the MgD group the serum ionized level was reduced to 0.28±0.004 mM/L (p<0.01).
Feeding the MgD animals various levels of Mg in their drinking water (as seen previously, [6-8]) resulted in concentration-dependent rises in both the total and ionized levels of serum Mg. 100 mg/day of Mg2+ elevated the total Mg level to normal, i.e., 1.02±0.004 mM/L, whereas feeding 15 and 40 mg/day of Mg2+ in the drinking water raised the total Mg levels to 68 (0.68±0.005 mM/L) and 82% (0.83±0.005 mM/L), respectively, of normal (n = 16-26, p<0.010. With respect to the serum ionized levels, feeding the animals 15 and 40 mg/l/day of Mg2+ to the rats raised the serum ionized levels to 62% (0.42±0.004 mM/L) and 66% (0.46±0.006 mM/L), respectively, of normal (n = 16-26, p<0.01).
Influence of dietary Mg intake on total SMase and acid, alkaline, and N-SMase levels in cardiac and vascular smooth muscles: relationship to ionized Mg
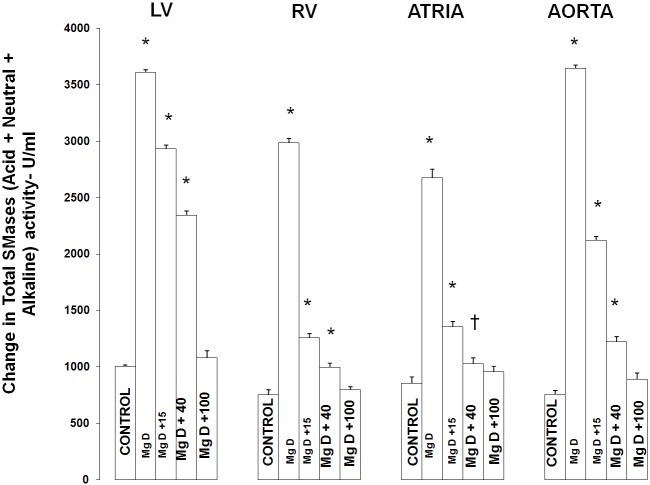
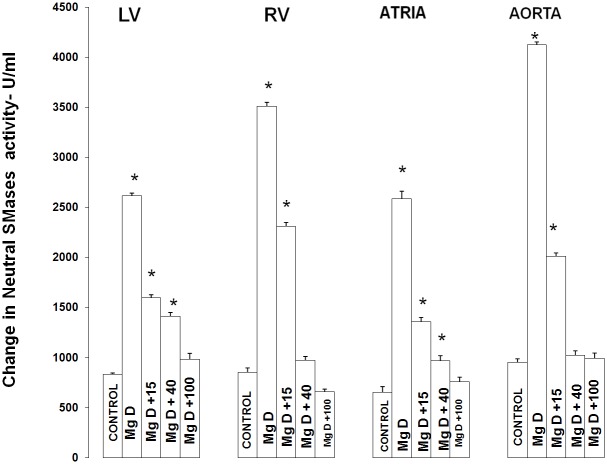
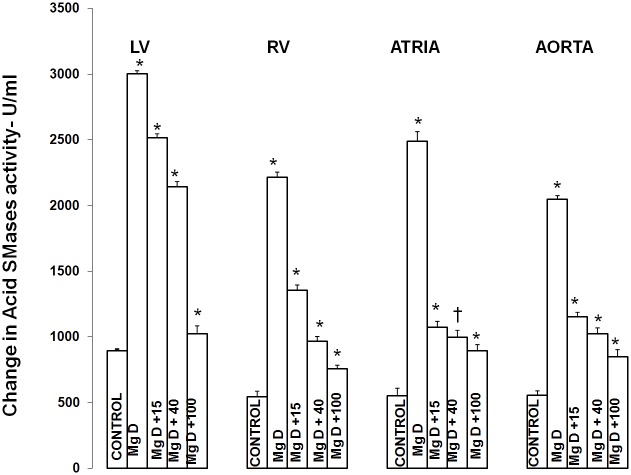
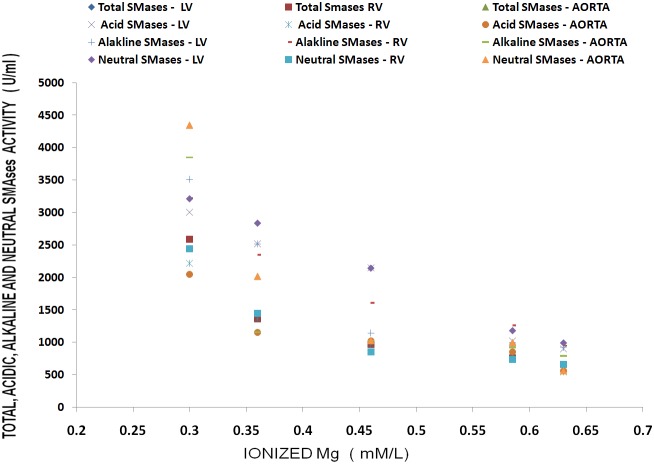
Figure 1 shows that feeding rats a MgD for 21 days resulted in an approximate 275% rise in total SMase activity in both the LV and aortic smooth muscle and an approximately 200% elevation in total SMase activity in both the RV and atria (ANOVA<p<0.01). Feeding the MgD animals as little as 15 mg/l/day Mg2+ in drinking water completely prevented the rises in total SMase activities in the RV and atria. However, 40 mg/l/day Mg2+ in drinking water was required to inhibit the elevation in total SMases in both the LV and aortic tissues (ANOVA, P<0.01). Although an almost identical pattern was found for at least one of the SMases (i.e., N-SMase activity) for the LV, RA, atria and aorta (Figure 2), the patterns of response of the tissues in MgD animals for alkaline- and acid-SMases were somewhat different and distinct for these two SMases (Figures 3 and 4). Unlike that observed for N-SMase, 40 mg/l/day Mg2+ in drinking water was required to inhibit the elevations in both alkaline-and acid-SMase (ANOVA, p<0.01). Linear regression analysis demonstrated strong correlations of increases in activities of all three SMases to the measured levels of serum ionized Mg (i.e., r = -0.6 to -0.98) (see Figure 5). When all three SMases (i.e., total SMase activity) are examined together for correlation to serum ionized Mg, the r value is -0.99 (p<0.001).
Figure 1.
Total sphingomyelinase levels in left ventricular (LV) muscle, right ventricular (RV) muscle, atria, and aortic smooth muscle in normal and Mg-deficient (MgD) rats with and without Mg added to the drinking water. N = 10-12 animals per group. Mean values for MgD animals are significantly different from all other groups (ANOVA, P<0.01). An asterisk signifies that the mean values ± SE are significantly different from all other groups (ANOVA, p<0.01), whereas a dagger signifies that the mean value is significantly different from all other mean values (in the group) except for MgD + 40 (p<0.05).
Figure 2.
Neutral sphingomyelin levels in LV muscle, RV muscle, atria, and abdominal aortic smooth muscle in normal and MgD rats with and without Mg added to the drinking water. N = 10-12 animals per group. Mean values for MgD are significantly different from all other groups (ANOVA, P<0.01). An asterisk signifies that the mean value ± SE is significantly different from all other groups (ANOVA, p<0.01).
Figure 3.
Acid sphingomyelinase levels in LV muscle, RV muscle, atria, and abdominal smooth muscle in normal and MgD rats with and without Mg added to the drinking water. N = 10-12 animals per group. Mean values for MgD are significantly different from all other groups (ANOVA, P<0.01). An asterisk signifies that the mean value ± SE is significantly different from all other groups (p<0.01), whereas the dagger signifies the mean value ± SE is significantly different from all other groups except for MgD + 15 (p<0.01).
Figure 4.
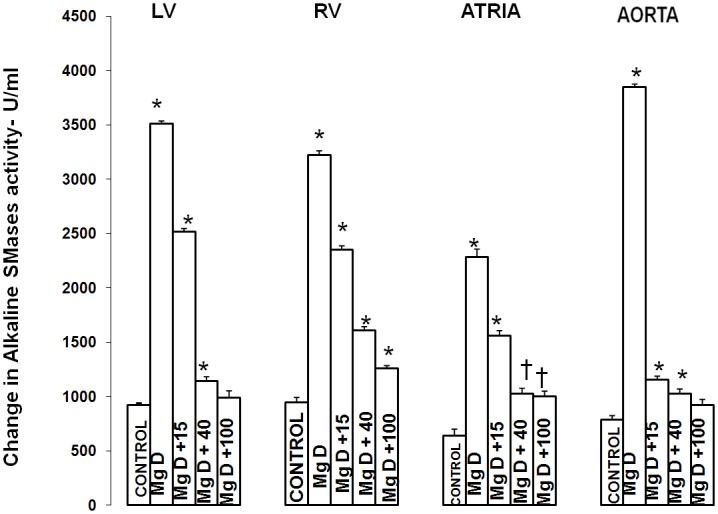
Alkaline sphingomyelin levels in LV muscle, RV muscle, atria, and abdominal smooth muscle in normal and MgD rats with and without Mg added to the drinking water. N = 10-12 animals per group. Mean values for MgD are significantly different from other groups (ANOVA, P<0.01). An asterisk signifies that the mean value ± SE is significantly different from other groups except each other (within the same group, p<0.05).
Figure 5.
Linear correlation between total, acid, alkaline, and N-SMases and ionized Mg levels in LV, RV, atria and aorta in MgD animals.
Influence of low [Mg2+]0 with and without inhibitors of N-SMase and acid-SMase on ceramide levels in primary cerebral and aortic SMCs
The data presented in Tables 1 and 2 indicate that incubation of the SMCs in 0.3 mM Mg2+ for 6 hr results in marked rises (in excess of 125-150%) in the ceramide levels in both cerebral and aortic VSMCs, very similar to results seen previously [7,8,51]. However, pre-incubation with scyphostatin in low [Mg2+]0 resulted in a considerable inhibition (e.g., 25-35%) of enhanced release of ceramide in the VSMCs (Tables 1 and 2), similar to results seen previously [8]. Surprisingly, incubation of the VSMCs with the putative acid-SMase inhibitor, desipramine, resulted in a significant 15-20% inhibition of the rise in ceramide seen in low [Mg2+]0 alone. It should be noted that this effect was observed at a normal physiological pH 7.4.
Table 1.
Influence of scyphostatin on low [Mg2+]0-induced release of ceramide in primary cultured cerebral and aortic VSMCs
| Group, [Mg2+]0 | Ceramide (pmol/108 cells) | |
|---|---|---|
|
|
||
| Cerebral VSMCs | Aortic VSMCs | |
| Controls, 1.2 mM | 28±3.2 | 38±4.6 |
| 0.3 | 59±4.1* | 86±6.4* |
| Scyphos, 0.3 | 42±3.6** | 58±5.2** |
Values are means ± SE. Scyphos = scyphostatin (75 uM).
Mean values significantly different from controls (P<0.01).
Mean values significantly different from controls and 0.3 mM [Mg2+]0 (ANOVA, P<0.05).
Table 2.
Influence of desipramine on low [Mg2+]0-induced ceramide levels in primary cultured cerebral and aortic VSMCs
| Group, [Mg2+]0 | Ceramide (pmol/108 cells) | |
|---|---|---|
|
|
||
| Cerebral VSMCs | Aortic VSMCs | |
| Controls, 1.2 mM | 34±3.6 | 48±4.8 |
| 0.3 | 62±5.4* | 82±7.8* |
| Desipra, 0.3 | 49±4.5** | 66±5.6** |
Values are means ± SE. Desipra = desipramine (20 μM).
Mean values are significantly different from controls (P<0.01).
Mean values are significantly different from controls and 0.3 mM [Mg2+]0 (p<0.05).
C-Fos and c-Jun expression in cardiovascular tissues and primary cultured VSMCs: relationship to serum ionized Mg
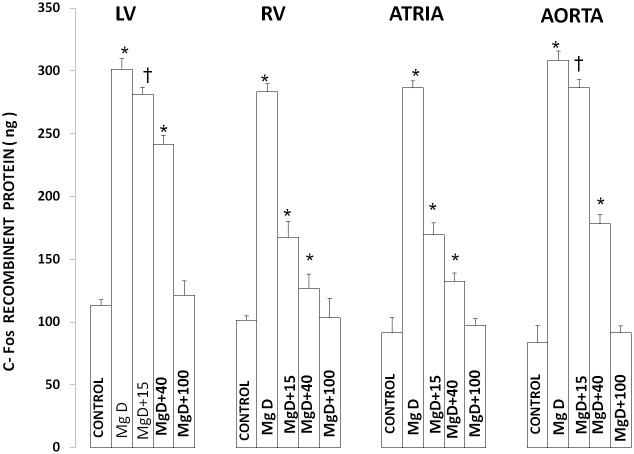
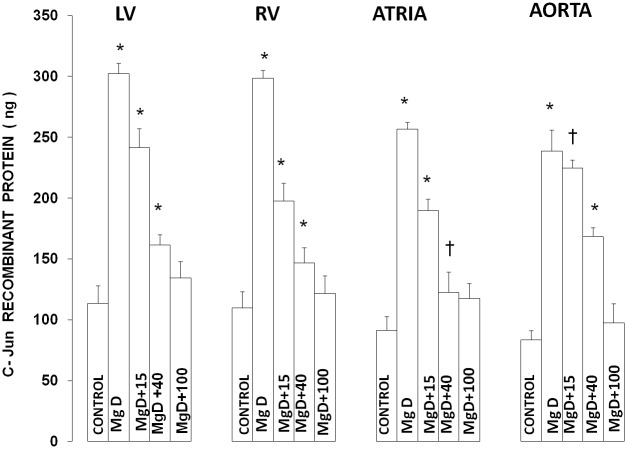
Figures 6 and 7 show that feeding rats a MgD diet for 21 days resulted in approximately 150-200% rises in c-Fos and c-Jun levels in LV, RV, atrial and aortic smooth muscles (P<0.0001). Feeding these MgD animals various levels of Mg2+ in drinking water demonstrated a range of sensitivities in the tissues. For example, although RV and atrial muscles showed that as little as 15 mg/l Mg2+ in drinking water could significantly inhibit by approximately 50% the rises in c-Fos and c-Jun in these tissues (P<0.01), much more of a Mg2+ intake in the drinking water is needed (e.g., 40-100 mg/l Mg2+) to significantly inhibit the rises observed in LV and aortic smooth muscles. Linear Regression analysis demonstrated strong correlations of increases in c-Fos and c-Jun to the measured levels of serum ionized Mg (i.e., r = 0.76 to 0.96) (see Figures 8 and 9).
Figure 6.
c-Fos expression in left ventricle(LV), right ventricle(RV), atria and aortic smooth muscle in normal and Mg-deficient (MgD) rats with and without Mg2+ added to their drinking water. Concentrations of Mg2+ added to the drinking water are identical to those given in Figure 1. All values are means ± SE; n = 10-12 animals per group. All MgD mean values are highly significantly different from mean control values (P<0.001).Asterisk signifies mean values ± SE that are significantly different from control mean value ± SE (ANOVA, p0.01). Single dagger signifies mean values ± SE which are significantly different from controls (p<0.01) Double asterisk signifies mean values ± SE which are significantly different from all other mean values (ANOVA, p<0.01).
Figure 7.
c-Jun expression in LV, RV, atria and aortic smooth muscle in normal and Mg-deficient rats with and without Mg2+ added to their drinking water. Concentrations of Mg2+ added to the drinking water are identical to those given in Figure 1. All values are means ± SE; n = 10-12 animals per group. All MgD mean values are highly significantly different from mean control values (P<0.001). Mean values for MgD +15 mg/l Mg2+ are significantly different from MgD in all treated groups, except for aorta (P<0.01). All mean values for MgD +40-100 mg/l Mg2+ are highly significantly different from their respective MgD mean values for all treated groups (P<0.001). Designation of symbols similar to those used in Figure 6.
Figure 8.
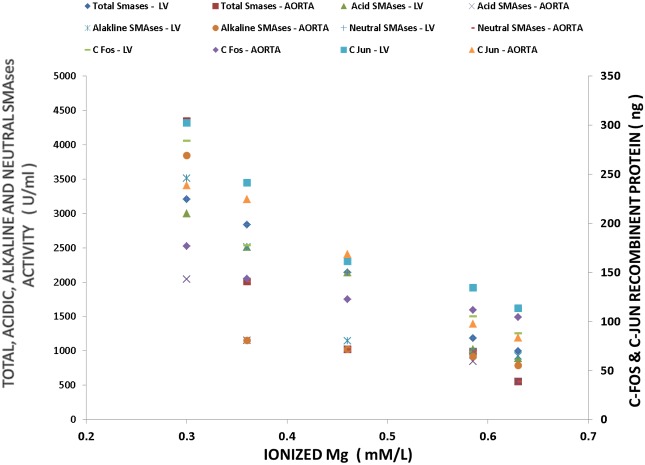
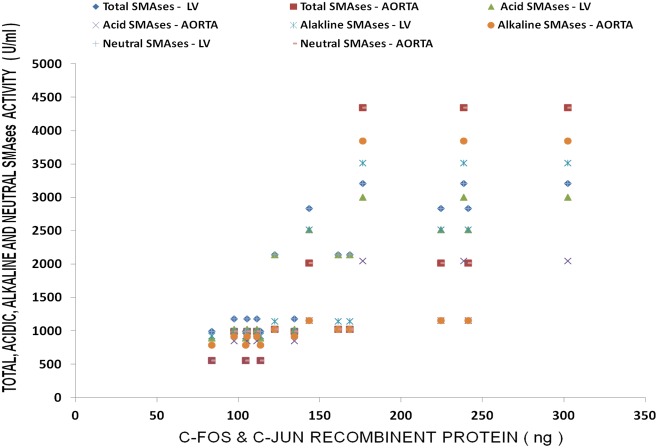
Multiple regression analysis of SMases with serum ionized Mg and proto-oncogenes (c-fos, c-Jun) in LV and aorta of MgD animals.
Figure 9.
Multiple regression analyses of SMases with c-Fos and c-Jun in CV tissues of Mg-deficient rats.
With respect to the VSMCs in culture, a MgD environment also caused clear rises in the levels of both c-Fos and c-Jun (P<0.01) (Figures 10 and 11).
Figure 10.
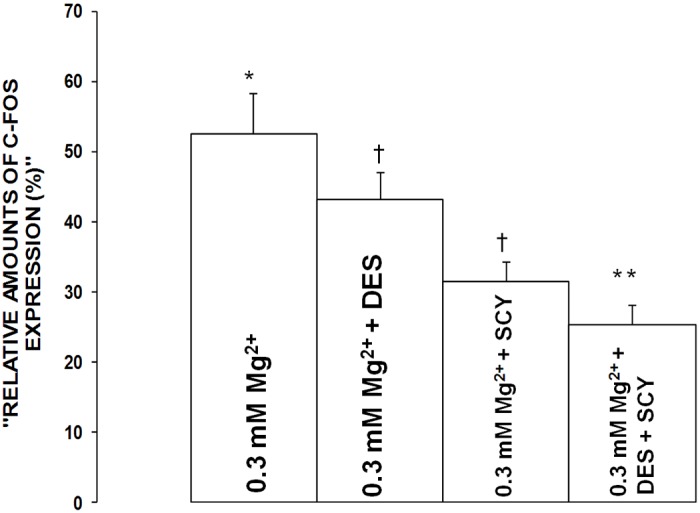
Influence of desipramine and scyphostatin on c-Fos expression in aortic VSMCs exposed to low Mg2+ (0.3 mM) in primary culture. Mean values ± SE are given in densitometric arbitrary units (see Methods). Mean values for desipramine and scyphostatin are significantly different from low Mg2+ control values (P<0.01). An asterisk signifies mean values ± SE which are significantly different from all other mean values ± SE (ANOVA, p<0.01). Dagger signifies mean values ± SE which are significantly different from all other mean values (p<0.05).
Figure 11.
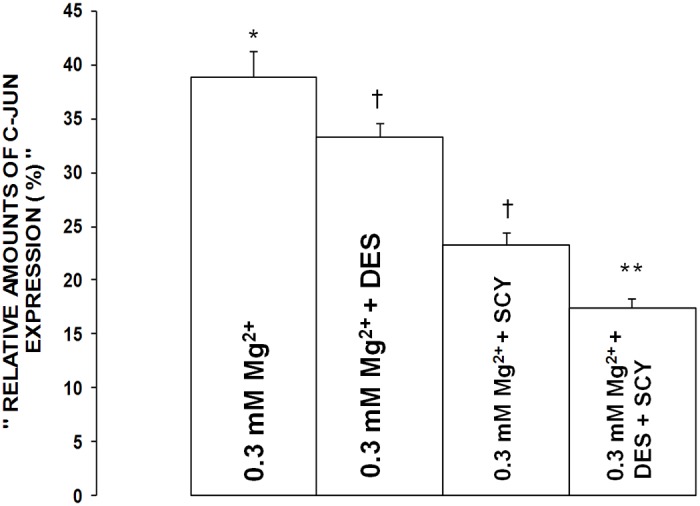
Influence of desipramine and scyphostatin on c-Jun expression in aortic VSMCs exposed to low Mg2+ (0.3 mM) in primary culture. Mean values ± SE are given in arbitrary units (see Methods). Mean values for desipramine and scyphostatin are significantly different from low Mg2+ control values (P<0.01). Symbols identical to those used in Figure 10.
Influence of inhibitors of acid- and neutral-SMases on c-Fos and c-Jun expression in primary VSMCs exposed to low [Mg2+]0
The data presented in Figures 10 and 11 show that incubation of the low [Mg2+]0-exposed VSMCs to desipramine inhibited the elevations in c-Fos and c-Jun by approximately 15-20% (P<0.01), whereas incubation of the low [Mg2+]0-exposed VSMCs with scyphostatin resulted in 35-40% inhibition of the rises in c-Fos and c-Jun (P<0.01). Incubation of the low [Mg2+]0-exposed VSMCs with both desipramine and scyphostatin resulted in an approximate additive effect on the rises in c-Fos and c-Jun, i.e., inhibitions of approximately 45-55%, suggesting that activation of alkaline-SMases might not be involved in the low [Mg2+]0-induced elevations in c-Fos and c-Jun. However, multiple regression analyses seem to indicate that alkaline-SMases may play a role in activation of c-Fos and c-Jun in CVtissues of Mg-deficient animals (see Figure 9).
Influence of inhibitors of acid- and N-SMase on NF-κB expression in primary VSMCs
Figures 12 and 13 demonstrate that incubation of the low [Mg2+]0-exposed VSMCs to desipramine resulted in an approximate 15-20% reduction in expression and activation of the p65 and c-Rel subunits of NF-κB, whereas use of scyphostatin resulted in an approximate 25-35% reduction in generation of the p65 and c-Rel subunits of NF-κB (P<0.01).
Figure 12.
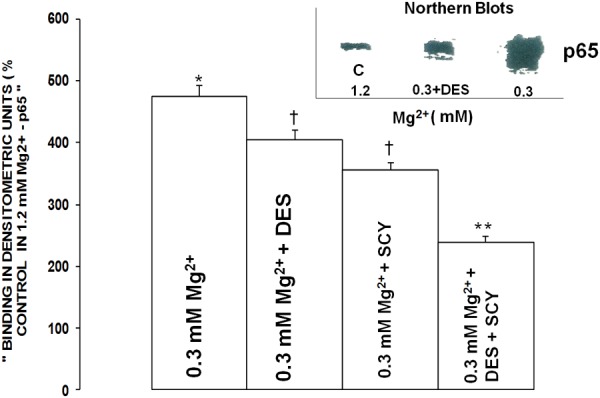
Influence of desipramine on p65 and c-Rel components of NF-κB in aortic VSMCs exposed to low Mg2+. Mean values ± SE for exposure to desipramine are significantly different from respective control p65 and c-Rel values (P<0.01). VSMCs were assayed for the p65 and c-Rel components 90 min after incubation in low Mg2+ (see Methods).
Figure 13.
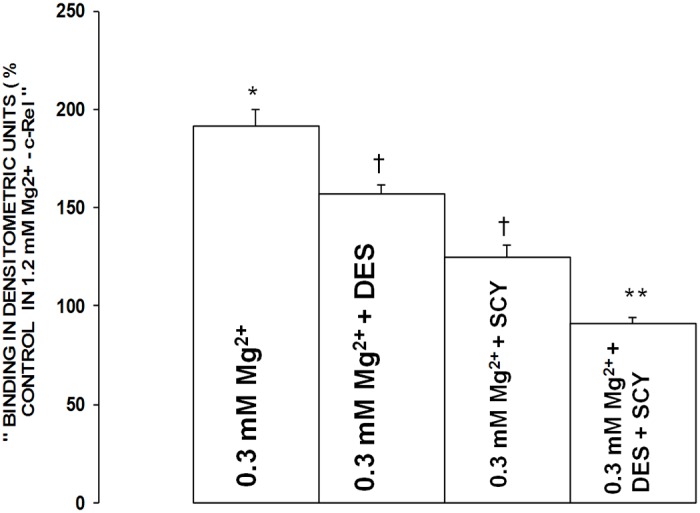
Influence of scyphostatin on p65 and c-Rel components of NF-κB in aortic VSMCs exposed to low Mg2+. Mean values ± SE for exposure to scyphostatin are significantly different from respective control p65 and c-Rel values (P<0.01). VSMCs were assayed for the p65 and c-Rel components 90 min after incubation in low Mg2+ (see Methods).
Discussion
The results reported here are the first demonstrations that all three major types of SMases are present in diverse cardiac and vascular muscles and cells in the cardiovascular system of at least in rats. We also demonstrate that all three types of SMases are activated in cardiovascular tissues and cells in MgD animals. In addition, we demonstrate for the first time that short-term dietary deficiency of Mg results in an upregulation of two important proto-oncogenes, viz., c-Fos and c-Jun. Based on the data, herein, we hypothesize that activation of SMases in a MgD state can lead to production of these proto-oncogenes and activation of DNA-binding subunits of NF-κB, pathways that are vital in atherogenesis and hypertensive disease states. To our knowledge, this is the first time anyone has shown an upregulation of all three types of SMases by Mg deficiency of any cell type in any species. The potential role(s) of SMases in several aspects of cardiovascular pathophysiology is gaining considerable attention (e.g., see [6,8,27,28,39,43,46,49,51,56,57,63,79]).
It has been suggested, repeatedly, that alkaline-SMases are only found in intestinal and lung tissues [30,49,50]. These speculations, however, for the most part, have not been undertaken on different tissues-organs or VSMCs, as is the case with the present study. We clearly show, herein, that activation of alkaline-SMases can be observed in cardiovascular tissues, of at least the rat, under proper environmental conditions. Whether, or not, the identification of alkaline-SMases can be observed at physiological pH’s remains, however, an open question.
The present experiments confirm and add further support to the concept that lowered levels of Mg can lead to the formation of ceramide in cardiovascular tissues and cells [6-8,51]. Recently, we have shown, using similar models of MgD that low [Mg2+]0 leads to the de novo synthesis of ceramide in cardiovascular tissues and cells via activation of at least three enzymes in the sphingolipid pathway, namely, sphingomyelin synthase (SMS), sphingomyelin palmitoyl-CoA transferase (SPT), and ceramide synthase (CS) [6-8,51]. SPT catalyzes the first step in the biosynthesis of sphingolipids, namely, the condensation of L-serine with palmitoyl-CoA, giving rise to 3-keto-sphinganine (leading to the formation of ceramides) [44,49,52,56,60,61,78]. 3-Keto-sphinganine is reduced to form sphinganine, which is then acylated by CS to produce dihydroceramide and desaturated to generate ceramide [52,60]. We show in the present study that use of inhibitors of both N- and acid-SMases inhibit about 50-60% of the ceramide formation in VSMCs exposed to low [Mg2+]0, which suggests, to us, that the other 40-50% of the ceramide formed in the VSMCs must be attributed to the activation of SMS, SPT, and CS. We believe this hypothesis merits attention in the future.
Ceramides are now known to be produced in many types of cells and tissues when they are exposed to ultraviolet radiation, ionizing radiation, endotoxins, cytokines, retinoic acid, balloon injury of carotid arteries, phorbol esters, serum deprivation, and daunorubicin as well as in etoposide-induced apoptosis, among other agents [41,46,48,54,56,57,59,62,64,74]. Many of these agents activate SMases as well as SPT, CS, and SMS to produce ceramides in many cell types [46,48,49,54,57,59,62]. The synthesis and release of ceramide appears to be the active messenger in most of these events and agencies. The present study suggests that Mg deficiency should be added to the list of stimuli known to activate the diverse family of SMases, at least in rats. Since the present studies indicate that sizeable quantities of ceramide can be produced in VSMCs exposed to low [Mg2+]0, via activation of N- and acid- SMases, generation of ceramide by these pathways could, in large measure, be responsible for apoptotic events. We have reported that several different ceramides (i.e., C2-, C6-, and C16-ceramide) can acutely induce apoptosis in primary rat peripheral arterial muscle cells (e.g., mesenteric artery and aorta) and canine cerebral VSMCs, as verified by several types of specific assays (e.g., TUNEL, acridine orange, propidium iodide, annexin V, and caspase-3 [6]; and unpublished observations).
Our recent studies demonstrate that several pro-inflammatory cytokines are intimately correlated to a de novo synthesis and release of ventricular, atrial and VSMC ceramides [8]. It is known that pro-inflammatory cytokines can trigger the secretion of acid-SMases in endothelial cells [47,48,62]. It is, therefore, of considerable interest to point out that Doehner et al have recently shown that patients in chronic (congestive) heart failure show elevated levels of acid-SMase in the plasma of these patients [44]. In view of our present findings, it is tempting to speculate that the upregulation of acid-SMase noted in all four chambers of the heart (and VSMC) in MgD animals may be triggered in whole, or at least in part, by the upregulation of pro-inflammatory cytokines seen recently in these cardiac tissues obtained from MgD animals [8]. This suggestion certainly merits further study.
With respect to our present findings on N-SMases and potential cardiac ischemia in MgD [6,51,67], it should be noted that early activation of N-SMases have been reported in cardiac myocytes subjected to hypoxia/reoxygenation [45]. The latter is also associated with cardiac deficiency of cellular glutathione [69-71]; recall that cardiac glutathione was clearly depleted in rats subjected to short-term MgD [51]. Interestingly, TNF-alpha and IL-1 beta, cytokines which we have recently shown to be upregulated in cardiac tissues from MgD animals [8], have been shown to be associated with activation of N-SMases concomitant with negative inotropic actions on human and rat [47,48,56] cardiomyocytes. In this context, our recent studies have clearly shown a strong correlation (i.e., r = 0.88-0.95) of cardiac levels of TNF-alpha and IL-1beta to activation of N-SMases in the MgD-animals [8], suggesting an intimate cross-talk relationship between the cytokines and N-SMase levels in the ventricular and atrial muscle cells Furthermore, the apoptosis observed in cardiac myocytes subjected to hypoxia/reoxygenation has been demonstrated to be associated with activation of N-SMases [45-48] and deterioration of mitochondrial function [57,71]. Since we have demonstrated that short-term MgD in rats results in loss of cardiac mitochondrial cytochrome c, lipid peroxidation, activation of caspase-3, apoptosis [6-8,51], and upregulation of N-SMases, we believe that some of the hemodynamic deficiencies seen in rat hearts in MgD [21,67,68] may be attributed to activation of N-SMases, as observed in the present study.
It is now widely accepted that apoptotic events play major roles in the development of atherosclerosis and hypertension [24,33,36,73]. Although it has been suggested that sphingolipids might play important roles in the pathophysiology of these cardiovascular pathogenic events, by unknown effects on vascular smooth muscles [1,4,6-8,28,29,51], up until our studies began (>15 years ago [1,28,29,39,66,79] the importance of Mg deficiency and its relation to sphingolipid metabolism were not known.
Apoptosis is now known to be a critical factor in both atherogenesis and hypertension [24,33,36,73]. Apoptosis clearly plays an important role in the rupture of atherosclerotic plaques, which often leads to thrombosis, myocardial ischemia and infarctions, and possibly death. Such events have been suggested to play important roles in resistant hypertension and congestive heart failure. Atherosclerotic plaques in vascular walls in hypertensive subjects and experimental animals have been shown to demonstrate considerable DNA damage, activation of DNA repair pathways, increased expression of p53 [24,33,36], apoptosis [24,33,36,73], and increased levels of ceramide [56]. It is of interest to point out, here, that evidence is accumulating to indicate that there appears to be a relationship between SMases, cholesterol homeostasis and atherogenesis. Kolmakova et al [46] reported apoC-1 enriched HDLs induce death in VSMCs via an activation of N-SMase. Others using a Watanabe hyperlipemic rabbit model of plaque rupture found a co-localization of apoC-1 and ceramide (Steen et al; see [75]). With respect to acid-SMases, Schissel et al found that secretory acid SMase could hydrolyze sphingomyelin on the surface of atherogenic particles [63], In this context, we have shown that MgD can: 1) accelerate atherogenesis in rabbits [9], which is associated with increased levels of p53 in the thickened atherosclerotic walls ([81], unpublished findings), and 2) produce hypertension [4,5] which is associated with DNA fragmentation, activation of caspase-3, apoptosis, and increased levels of p53 in arterial walls of rats [6-8,81]. In addition, we found in these studies that SMase inhibitors reduced p53 levels in the walls of the MgD animals. We thus hypothesize that Mg deficiency, most-likely, plays key roles in the generation of both hypertension and atherogenesis via the upregulation of N- and acid-SMases and p53, particularly as the majority of individuals who consume Western types of diets have 30-65% short falls in daily dietary intake of Mg [4,13,19,20,29,76].
Proto-oncogenes, such as c-Fos and c-Jun, along with activation of NF-κB are known to play critical roles in cell transformation, growth, and apoptotic events [5,31-35,37,46]. It should be noted here that ceramide, activation of NF-κB, and p53 can induce cell cycle arrest (and senescence), induce programmed cell death, and are associated with DNA damage (genotoxic events), all agencies we have shown here and elsewhere [4,6-8,51,81] are found in animals exposed to short-term MgD states. Our present experiments demonstrate that inhibition of low [Mg2+]0-induced release of ceramides (via activation of acid-and N-SMases) in VSMCs results in inhibition of c-fos, c-Jun, and two DNA-binding subunits of NF-κB. It is of more “than passing interest” to note that activated forms of NF-κB have been reported in VSMCs, macrophages, and endothelial cells of human atherosclerotic lesions [33] as well as in atherosclerotic lesions of MgD animals [81]. We do not believe this is a coincidence. At this point, it appears to us logical to inquire if these events are set into motion by the generation (and release) of ceramides (and possibly other sphingolipids).
Some additional discussion of the potential relevance of the present findings, with the proto-oncogenes and with the p65 and c-Rel proteins, to atherogenesis, are in order. The proto-oncogenes c-Fos and c-Jun are known to participate in numerous pathophysiological processes including signal transduction, cell growth, differentiation, and cell transformation [5,24,33,36]. Both c-Fos and c-Jun are thought to play roles as potent inducers of apoptosis in several different cell types [36]. The proto-oncogenes, c-Fos and c-Jun, encode nuclear phosphoproteins. c-Fos in a complex with c-Jun (AP-1) regulates the expression of AP-1 binding genes at the transcriptional level. Members of the Fos and Jun protein families dimerize to preferentially bind AP-1 sites with a very high affinity. The observed increased levels of c-Fos and c-Jun seen in the current study, and previously [5], could imply an upregulation of genes controlling cell growth, differentiation, and cell transformation via AP-1 binding genes, all factors essential in atherogenesis and hypertension. In normal cells, the expression of growth factors and their receptors is carefully and tightly controlled. Over-activity of the c-Fos and c-Jun proto-oncogenes may result in unregulated cell proliferation like that observed in atherogenesis and hypertension. We hypothesize that overexpression of c-Fos and c-Jun in the cardiovascular system of MgD animals and humans may play an important role in the course of tissue inflammation and the inflammatory response observed in atherogenesis we have noted in MgD animals [4,5,8]. From our new observations in the current study, it appears that generation of ceramides are needed to potentially set the latter into motion. Infiltration of inflammatory cells and necrotic areas are seen in the arterial walls of human subjects as well as MgD rabbits in the atherogenic process [24,36].
Activation of the p65 and c-Rel proteins found in the present, and our previous studies [5,8], induced by low [Mg2+]0 is pivotal in the recruitment of leukocytes [33,35] and in several arms of the innate and adaptive immune systems activated in atherogenesis [33,35]. Since MgD has been demonstrated to result in accelerated atherogenesis in rabbits [22], which was shown to be associated with increased levels of leukocytes and p53 in the thickened atherosclerotic plaques (unpublished findings), we hypothesize that p65 and c-Rel were first, more than likely, activated, at least, in part by synthesis and release of ceramides (via acid-and N-SMases, sphingomyelin synthase, SPT-1, SPT-2, and ceramide synthase) [6-8,51,81] in the MgD environment which would have acted to activate and release cytokines/chemokines [8] followed by activation of c-Fos and c-Jun leading to an AP-1 complex thus leading to the events suggested above.
At this juncture, considerable evidence has been brought forth that prolonged administration of Mg2+ (oral and intravenous) can lower blood pressure in both experimental and clinical forms of hypertension [1,4,10,11,16,19,77,80]. Likewise, administration of Mg2+ (oral and intravenous) have been shown to inhibit/attenuate cardiac arrhythmias of diverse origins, to reverse coronary arterial vasospasm, and to ameliorate congestive heart failure in humans and animals [4,19,20,77,80]. However, the precise mechanisms are not known in amelioration of hypertension or in the diverse cardiac pathophysiologies. A leading hypothesis, is Mg2+ lowers blood pressure by promoting vasodilation and decreasing work load on the myocardium by direct actions on Ca2+ channels (and cellular redistribution) in both cardiac and vascular smooth muscle cells [1,3,4,16,19,20,67,80]. In view of our present findings and those previously published [5-8,28,29,43,51,66,79], we believe that Mg’s effects on ceramide and sphingolipid metabolism must be taken into serious consideration in helping to explain the blood pressure-lowering and cardiac beneficial actions of this divalent cation. In this context, evidence has accumulated to indicate that certain ceramides can induce powerful vasodilator actions on different types of blood vessels [66,79]. Such actions could unload the myocardium and lower blood pressure, thereby ameliorating high blood pressure and left ventricular hypertrophy often observed in resistant hypertensive individuals.
More than 50 years ago, it was first suggested that in areas where water hardness is elevated, the rate of death from cardiovascular diseases is decreased [82]. In the intervening years, up to the present time, this hypothesis has gathered considerable evidence from numerous epidemiological studies from around the globe [2,3,11-13,23-26,76]; that is cardiovascular death rates are lower in hard-water areas than in soft-water areas. Despite the fact that the hardness of water is due to the concentrations of Ca2+ and Mg2+, the overwhelming evidence, to date, supports the idea that it is the Mg content that is responsible for most of the protective effects of hard water [11,12,24,76]. Twenty-five years ago, Marier [76] and Leary [24] hypothesized that as little as 15-30 mg/l/day of Mg2+ in drinking water should be cardioprotective. Recently, using the same model of dietary deficiency of Mg as in the present study, we showed, for the first time, in well-controlled experiments that as little as 15 mg/l/day of Mg2+ in drinking water either prevented or ameliorated the formation of reactive-oxygen species, lipid peroxidation, generation of cytokines and chemokines, p53, DNA fragmentation, caspase-3 activation, mitochondrial release of cytochrome c, activation of apoptosis, hydrolysis of SM, upregulation of SPT-1, SPT-2, ceramide synthase, and sphingomyelin synthase, loss of cardiac and VSMC glutathione, activation of e-NOS and n-NOS, elevation of plasma lipids, release of lactic acid dehydrogenase and creatine kinase from the myocardium, and elevation of ionized Ca [6-8,51,81]. Although the present work indicates that as little as 15 mg/l/day of Mg2+ in drinking water prevents the upregulation of three classes of SMases as well as activation of proto-oncogenes, in cardiac and vascular muscles, something in excess of 40, but <100 mg/l/day of Mg2+ in water must be imbibed to prevent the activation of both acid- and N-SMases in left ventricular cardiac muscle cells, at least in rats. We believe that when the present findings are viewed in light of our previously published results [6-8,51,81], it could be speculated that between 15 and 50 mg/l/day of water-borne Mg2+ should be both cardioprotective and vascular protective. Until our experiments, such an insight has not been possible.
While the activation of both acid- and N-SMases most likely play important roles in the biological synthesis of ceramide in Mg deficiency, we believe that until further experiments are done, activation of these enzymes is but one of many ways in which Mg deficiency is a cardiovascular risk factor.
At the very least, the present study, when taken together with our previous studies, strengthens considerably the hypothesis suggested more than two decades ago [24,76], that water intake (from tap waters, well waters, bottled waters, and beverages using tap/well waters) in humans varying between 1 and 2 liters/day, with Mg2+ intakes varying from <5 to >100 mg/l, may, as we have suggested recently [6-8,51,81], represent an excellent way to overcome and control the marginal intakes of Mg obtained with most Western diets. In addition, in view of our previous findings and those presented here, it is probably propitious to suggest that all desalinated-purified recovered/recycled waters, harvested rainwaters, well waters, tap waters, and all bottled waters given to humans should be supplemented with bioavailable Mg2+ to ameliorate/prevent the induction of cardiovascular risk factors and disease processes worldwide.
Acknowledgements
The authors appreciate the gratis supply of magnesium aspartate-HCl, which was provided by Dr. Angela Weigert of Verla Pharm (Tutzing, Germany). The authors also appreciate the invaluable technical assistance of Lewis Gersten. This work was supported, in part, by a Regalware worldwide research grant (to B.M. Altura) and an NIH Grant (to B.M. Altura).
Disclosure of conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between 1996: cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–359. [PubMed] [Google Scholar]
- 2.Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Magnesium deficiency and hypertension: correlation between magnesium deficient diets and microcirculatory changes in situ. Science. 1984;223:1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- 3.Altura BM, Altura BT, Gebrewold A, Gunther T, Ising H. Noise-induced hypertension and magnesium: Relationship to microcirculation and magnesium. J Appl Physiol. 1992;72:194–202. doi: 10.1152/jappl.1992.72.1.194. [DOI] [PubMed] [Google Scholar]
- 4.Altura BM, Altura BT. Magnesium: forgotten mineral in cardiovascular biology and atherogenesis. In: Nishizawa N, Morii H, Durlach J, editors. New Perspectives in Magnesium Research. 2007. pp. 239–260. [Google Scholar]
- 5.Altura BM, Kostellow B, Zhang A, Li W, Morrill GA, Gupta RK, Altura BT. Expression of the nuclear factor- kappa B and proto-oncogenes c-Fos and c-Jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: possible links to hypertension, atherogenesis and stroke. Am J Hypertens. 2003;16:701–707. doi: 10.1016/s0895-7061(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 6.Altura BM, Shah NC, Jiang XC, Li Z, Perez-Albela JL, Sica AC, Altura BT. Short-term magnesium deficiency results in decreased levels of serum sphingomyelin, lipid peroxidation, and apoptosis in cardiovascular tissues. Am J Physiol Heart Circ Physiol. 2009;297:H86–92. doi: 10.1152/ajpheart.01154.2008. [DOI] [PubMed] [Google Scholar]
- 7.Altura BM, Shah NC, Li Z, Jiang XC, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates serine palmitoyltransferase (SPT 1 and SPT 2) in cardiovascular tissues: relationship to serum ionized Mg and cytochrome c. Am J Physiol Heart Circ Physiol. 2010;299:H932–8. doi: 10.1152/ajpheart.01076.2009. [DOI] [PubMed] [Google Scholar]
- 8.Altura BM, Shah NC, Shah G, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells: cross-talk between cytokines, Mg2+, NF-κB and de novo ceramide. Am J Physiol Heart Circ Physiol. 2012;302:H319–32. doi: 10.1152/ajpheart.00453.2011. [DOI] [PubMed] [Google Scholar]
- 9.Altura BT, Memon ZI, Zhang A, Cracco RQ, Altura BM. Low levels of serum ionized magnesium are found in patients early after stroke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular smooth muscle cells. Neurosci Lett. 1997;230:37–40. doi: 10.1016/s0304-3940(97)00471-0. [DOI] [PubMed] [Google Scholar]
- 10.Berthelot A, Luthringer C, Myers E, Exinger A. Disturbances of magnesium metabolism in the spontaneously hypertensive rat. J Am Coll Nutr. 1987;6:329–332. doi: 10.1080/07315724.1987.10720195. [DOI] [PubMed] [Google Scholar]
- 11.Durlach J, Bara M, Guiet-Bara A. Magnesium level in drinking water and cardiovascular risk factor: a hypothesis. Magnesium. 1985;4:5–15. [PubMed] [Google Scholar]
- 12.Eisenberg MJ. Magnesium deficiency and sudden death. Am Heart J. 1992;124:544–549. doi: 10.1016/0002-8703(92)90633-7. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;121:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 14.Handwerker SM, Altura BT, Royo B, Altura BM. Ionized magnnesium and calcium levels in umbilical cord serum of pregnant women with transient hypertension during labor. Am J Hypertens. 1993;6:542–545. doi: 10.1093/ajh/6.6.542. [DOI] [PubMed] [Google Scholar]
- 15.Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: the Honolulu hear study. Am J Clin Nutr. 1987;45:469–475. doi: 10.1093/ajcn/45.2.469. [DOI] [PubMed] [Google Scholar]
- 16.Laurant P, Dalle M, Berthelot A, Rayssigiguier Y. Time-course of the change in blood pressure level in magnesium-deficient Wistar rats. Br J Nutr. 1999;82:243–251. [PubMed] [Google Scholar]
- 17.Luthringer C, Rayssguier Y, Gueux E, Berthelot A. Effect of moderate magnesium deficiency on serum lipids, blood pressure and cardiovascular reactivity in normotensive rats. Br J Nutr. 1988;59:243–250. doi: 10.1079/bjn19880031. [DOI] [PubMed] [Google Scholar]
- 18.Rayssiguier Y, Gueux E, Bussiere I, Durlach J, Mazur A. Dietary magnesium affects susceptibility of lipoproteins and tissues to peroxidation in rats. J Am Coll Nutr. 1993;12:133–137. doi: 10.1080/07315724.1993.10718293. [DOI] [PubMed] [Google Scholar]
- 19.Saris NE, Mervaala E, Karppanen H, Khawja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 20.Turlapaty PD, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980;208:198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- 21.Weglicki WB, Mak IT, Dickens BF, Stafford RE, Komarov AM, Gibson B, Cassidy MM, Phillips TM, Kramer JH. Neuropeptides, free radical stress and antioxidants in models of Mg-deficient cardiomyopathy. In: Theophanides T, Anastassopolus J, editors. Magnesium: Current Status and New Developments. 1997. pp. 169–178. [Google Scholar]
- 22.Altura BT, Brust M, Barbour RL, Stempak J, Bloom S, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci U S A. 1990;87:1840–1844. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chipperfield B, Chipperfield JR. Relation of myocardial metal concentrations to water hardness and death from ischemic heart disease. Lancet. 1979;2:709–712. doi: 10.1016/s0140-6736(79)90641-x. [DOI] [PubMed] [Google Scholar]
- 24.Leary WP. Content of magnesium in drinking water and deaths from ischemic heart disease in White South Africans. Magnesium. 1986;5:150–153. [PubMed] [Google Scholar]
- 25.Marx A, Neutra RR. Magnesium in drinking water and deaths from ischemic heart disease. Epidemiol Rev. 1997;19:258–272. doi: 10.1093/oxfordjournals.epirev.a017957. [DOI] [PubMed] [Google Scholar]
- 26.Rubenowitz E, Molin L, Axellson G, Rylander R. Magnesium in drinking water in relation to morbidity and mortality from acute myocardial infarction. Epidemiology. 2000;11:416–421. doi: 10.1097/00001648-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997;408:191–197. doi: 10.1016/s0014-5793(97)00420-1. [DOI] [PubMed] [Google Scholar]
- 28.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane sphingolipids and lipid messengers in vascular smooth muscle cells. FEBS Lett. 1998;440:167–171. doi: 10.1016/s0014-5793(98)01446-x. [DOI] [PubMed] [Google Scholar]
- 29.Mosfegh A, Goldman J, Ahyja J, Rhodes D, LaComb P. What we eat in America. NHANES 2005-2006. Usual Intakes From Food and Water Compared to 1997 Dietary Reference Index for Vitamin D, Calcium, Phosphorous and Magnesium. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2009. [Google Scholar]
- 30.Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 31.Barnes PJ, Karim M. Nuclear factor kB-A pivotal transcription factor in chronic inflammatory disease. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 32.Baeurerle PA, Baltimore D. NF-κB: ten years later. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 33.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 34.Bourcier T, Sukhova G, Libby P. The nuclear factor kappa B signaling pathway participates in dysregulation of vascular smooth muscle in vitro and in human atherosclerosis. J Biol Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 35.Hayden MS, Ghosh S. NF-κB in immunology. Cell Res. 2011;21:233–244. [Google Scholar]
- 36.Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. Eighth ed. Philadelphia: Saunders; 2010. pp. 487–506. [Google Scholar]
- 37.Altura BT, Altura BM. Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem. 1991;10:90–98. [PubMed] [Google Scholar]
- 38.Zhang A, Cheng TP, Altura BM. Magnesium regulates intracellular free ionized magnesium and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta. 1992;1134:25–9. doi: 10.1016/0167-4889(92)90024-6. [DOI] [PubMed] [Google Scholar]
- 39.Zheng T, Li W, Wang J, Altura BT, Altura BM. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca(2+)] (i) in canine cerebral vascular muscle. Am J Physiol Heart Circ Physiol. 2000;278:H1421–8. doi: 10.1152/ajpheart.2000.278.5.H1421. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki T, Bielawaska AE, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1 alpha, 25-dihydroxyvitain D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–31. [PubMed] [Google Scholar]
- 41.Meng A, Luberto C, Meier P, Nal A, Yang X, Hannun YA, Zhou D. Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 human monocytic leukemia cells. Exp Cell Res. 2004;292:385–392. doi: 10.1016/j.yexcr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Altura BM, Gebrewold A, Zhang A, Altura BT. Low extracellular magnesium ions induce lipid peroxidation and activation of nuclear factor kappa B in canine cerebral vascular smooth muscle: possible relation to traumatic brain injury and strokes. Neurosci Lett. 2003;341:189–192. doi: 10.1016/s0304-3940(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 43.Altura BM, Gebrewold A, Zheng T, Altura BT. Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyte-endothelial interactions in cerebral venules in the intact brain: insight into mechanisms and possible relation to brain injury and stroke. Brain Res Bull. 2002;58:271–8. doi: 10.1016/s0361-9230(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 44.Doehner W, Bunck AC, Bockmeyer CL, Brunkhorst FM, Losche W, Kinschef R. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J. 2007;28:821–8. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez OM, Discher DJ, Bishopric NH, Webster KA. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ Res. 2000;86:198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- 46.Kolmakova A, Kwiterovich P, Virgil D, Alaupovic P, Knight-Gibson C, Martin SF. Apolipoprotein C-1 induces apoptosis in human aortic smooth muscle cells via recruiting neutral sphingomyelinase. Arterioscler Thromb Vasc Biol. 2002;22:1990–1995. doi: 10.1161/01.ATV.0000112036.72200.ac. [DOI] [PubMed] [Google Scholar]
- 47.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–65. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien NW, Geillings NM, Guo M, Barlow SB, Glembotski CC, Sabbadini RA. Factor associated with neutral sphingomyelinase activation and its role in cardiac cell death. Circ Res. 2003;92:598–91. doi: 10.1161/01.RES.0000066290.29715.67. [DOI] [PubMed] [Google Scholar]
- 49.Pavoline C, Pecker F. Sphingomyelinases: their regulation and roles in cardiovascular pathophysiology. Cardiovasc Res. 2009;82:175–183. doi: 10.1093/cvr/cvp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan RD, Nilsson A. Sphingolipid hydrolyzing enzymes in the gastrointestinal tract: a hypothesis. Methods Enzymol. 2000;311:276–286. doi: 10.1016/s0076-6879(00)11089-4. [DOI] [PubMed] [Google Scholar]
- 51.Shah NC, Liu JP, Iqbal J, Hussain M, Jiang XC, Li Z, Li Y, Zheng T, Li W, Sica AC, Perez-Albela JL, Altura BT, Altura BM. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: relevance to de novo synthesis of ceramide, serum Mg2+ and atherogenesis. Int J Clin Exp Med. 2011;4:103–18. [PMC free article] [PubMed] [Google Scholar]
- 52.Kolesnick R. Signal transduction through the sphingomyelin pathway. Mol Chem Neuropathol. 1994;21:287–97. doi: 10.1007/BF02815356. [DOI] [PubMed] [Google Scholar]
- 53.Luberto C, Hannun YA. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J Biol Chem. 1998;273:14550–9. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 54.Perry DK, Carton J, Shah AK, Meredith F, Uhlingeri DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 55.Tafesse FG, Huitema K, Hermanson M, van der Poel s, van den Dikkenberg A, Uphoff A, Somerharju P, Holtuis JC. Both sphingomyelin synthases SMS 1 and 2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–17547. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 56.Auge N, Andrieu N, Negre-Salvayre R, Thiers JC, Levade T, Salvayre R. Sphingomyelin metabolites in vascular signaling and atherogenesis. Prog Lipid Res. 2000;39:207–239. doi: 10.1016/s0163-7827(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 57.Birbes H, Bawab SE, Hannun YA, Obid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;14:2669–79. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 58.Ding T, Li Z, Hailemarian TK, Mukherjee S, Maxfield FR, Wu MP, Jiang XC. SMS overexpression and knockdown: impact cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J Lipid Res. 2008;49:376–85. doi: 10.1194/jlr.M700401-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997;53:539–53. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 60.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–50. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 61.Hassoun SM, Lancel S, Petillot P, Decoster B, Favory R, Marchetti P. Sphingosine impairs mitochondrial function by opening permeability transition pore. Mitochondrion. 2006;6:149–154. doi: 10.1016/j.mito.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Pandey S, Murphy RF, Agawal DK. Recent advances in the immunobiology of ceramide. Exp Mol Pathol. 2007;82:298–309. doi: 10.1016/j.yexmp.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. J Biol Chem. 1998;273:2738–46. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 64.Vandreger AB, Houweling M. Effect of ceramides on phospholipid biosynthesis and its implications for apoptosis. In: Quinn PJ, Kagan VE, editors. Phospholipid Metabolism in Apoptosis. 2002. pp. 207–227. [DOI] [PubMed] [Google Scholar]
- 65.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylgycerol at the Gogi. Biochem J. 2008;414:31–41. doi: 10.1042/BJ20071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wyman M, Schneiter R. Lipid signaling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 67.Altura BM, Barbour RL, Dowd TL, Wu F, Altura BT, Gupta RK. Low extracellular magnesium induces intracellular free Mg deficits, ischemia, depletion of high-energy phosphates and cardiac failure in intact working rat hearts: a 31P-NMR study. Biochim Biophys Acta. 1993;1182:329–32. doi: 10.1016/0925-4439(93)90077-e. [DOI] [PubMed] [Google Scholar]
- 68.Wu F, Altura BT, Gao J, Barbour RL, Altura BM. Ferrylmyoglobin formation induced by acute magnesium deficiency in perfused rat heart causes cardiac failure. Biochim Biophys Acta. 1994;1225:158–64. doi: 10.1016/0925-4439(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 69.Blaustein A, Deneke SM, Stolz RI, Baxter D, Healey N, Fanburg BL. Myocardial glutathione depletion impairs recovery after short periods of ischemia. Circulation. 1989;80:1449–1457. doi: 10.1161/01.cir.80.5.1449. [DOI] [PubMed] [Google Scholar]
- 70.Ozer MK, Parlakpinar H, Cigremis Y, Ulcar M, Vardi N, Acet A. Ischemia-repefusion leads to depletion of glutathione content and augmentation of malondialdehyde production in the rat heart from over-production of oxidants; can caffeic acid phenethyl ester (CAPE) protect the heart? Mol Cell Biochem. 2005;273:169–175. doi: 10.1007/s11010-005-0551-8. [DOI] [PubMed] [Google Scholar]
- 71.Serviddio G, Di Venosa N, Federici A, D’Austino D, Rollo T, Prigigallo F. Brief hypoxia before normoxic reperfusion (postconditioning) protects the heart against ischemia-reperfusion injury by preventing mitochondria peroxide production and glutathione depletion. FASEB J. 2005;19:354–361. doi: 10.1096/fj.04-2338com. [DOI] [PubMed] [Google Scholar]
- 72.Radin MJ, Holycross BJ, Dumitrescu C, Kelley R, Altschuld RA. Leptin modulates the negative inotropic effect of interleukin-1 beta in cardiac myocytes. Mol Cell Biochem. 2008;315:179–184. doi: 10.1007/s11010-008-9805-6. [DOI] [PubMed] [Google Scholar]
- 73.Oral H, Dorn GW 2nd, Mann DL. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian cardiac myocyte. J Biol Chem. 1990;265:15823–15831. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- 74.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 75.Steen H, Kolmakova A, Stuber M, Rodriguez ER, Gao F, Chatterjee S, Lima JA. MRI visualized neo-intimal dissection and co-localization of novel apoptotic markers apolipoprotein C-1, ceramide and caspase-3 in a Watanabe hyperlipemic rabbit model. Atheroscl. 2007;191:82–89. doi: 10.1016/j.atherosclerosis.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 76.Marier JR, Neri LC. Quantifying the role of magnesium in the relationship between human mortality/morbidity and water hardness. Magnesium. 1985;4:53–59. [PubMed] [Google Scholar]
- 77.Rosanoff A. Magnesium supplements may enhance the effect of antihypertensive medications in stage i hypertensive subjects. Magnes Res. 2010;23:27–40. doi: 10.1684/mrh.2010.0198. [DOI] [PubMed] [Google Scholar]
- 78.Tejero-Taldo MI, Chmielinska JJ, Weglicki WB. Chronic dietary Mg2+ deficiency induces cardiac apoptosis in the rat heart. Magnes Res. 2007;20:208–12. [PubMed] [Google Scholar]
- 79.Zheng T, Li W, Wang J, Altura BT, Altura BM. Effects of neutral-sphingomyelinase on phenylephrine-induced vasoconstriction and Ca2+ mobilization in rat aortic smooth muscle. Eur J Pharmacol. 2000;391:127–135. doi: 10.1016/s0014-2999(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 80.Yang ZW, Gebrewold A, Nowakoski M, Altura BT, Altura BM. Mg2+-induced endothelium-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol. 2000;278:R628–39. doi: 10.1152/ajpregu.2000.278.3.R628. [DOI] [PubMed] [Google Scholar]
- 81.Altura BM, Shah NC, Li Z, Jiang XC, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates sphingomyelin synthase and p53 in cardiovascular tissues and cells: relevance to de novo synthesis of ceramide. Am J Physiol Heart Circ Physiol. 2010;299:H2046–55. doi: 10.1152/ajpheart.00671.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobayashi J. On geographical relationship between the chemical nature of river water and death from apoplexy. Ber Ohara Inst Landwirtsch Biol. 1957;11:12–21. [Google Scholar]