Abstract
Naringenin is present abundantly in citrus fruits and is one of the natural alternatives to synthetic estrogen, but the mechanism of how naringenin functions is not well known. Our study revealed that the relative estrogenic potency of the substances was E2 > genistein > naringenin. Naringenin (at 5 μM) was found to repress both luciferase activity and pS2 mRNA expression, which was induced by E2 (at 0.1 μM) or genistein (at 5 μM). Naringenin, as well as E2 and genistein, was found to modulate the transcription of pS2 and TGFβ3 in T47D-KBluc cells through an estrogen receptor-dependent mechanism. Results of our study indicated that naringenin was a weak estrogen agonist that exhibits anti-estrogenic effect in estrogen-rich states and estrogenic activity in estrogen-deficient states in T47D-KBluc breast cancer cells.
Keywords: pS2, TGFβ3, genistein, selective estrogen receptor modulator
Introduction
Estrogen is an important regulating hormone associated with physiological processes of female reproductive system. Synthetic estrogen is administered to menopausal women who are deficient in estrogen to prevent osteoporosis or to relieve vaginal dryness and hot flashes [1,2]. However, long-term administration of synthetic estrogen places women at risk for several health disorders including endometrial cancer, breast cancer, cardiovascular disorders, and so on [3]. Various attempts have been made to find natural replacements for estrogen to minimize the shortcomings of synthetic estrogen as its administration carries both benefits and risks [4,5].
Flavonoids, the so-called phytoestrogens, are well known as natural estrogen analogues and are found in abundance in roots, flowers, fruits, and stems of plants [1,6-8]. Many studies aimed at using flavonoids commercially as drugs are in progress. Naringenin, which belongs to the flavanone family, is present abundantly in citrus fruits. Modern research has found naringenin to possess anti-carcinogenic activity, anti-atherogenic effect, anti-inflammatory effect, anti-oxidant effect, and so on [9-12].
A recent paper suggested that naringenin is capable of both estrogenic and anti-estrogenic activities [13]. However, the mechanism of how naringenin functions and its interaction with other substances are poorly known. We investigated the effect of naringenin on estrogen receptor activity in T47D-KBluc breast cancer cells to understand naringenin’s interaction with potent estrogen agonists and to confirm the mixed antagonist/agonist effect of naringenin.
Materials and methods
Materials
Hormones and chemicals
17β-Estradiol (E2), genistein, naringenin, and MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide) were purchased from Sigma (St. Louis, MO, USA). ICI 182 780 was obtained from Calbiochem (San Diego, CA, USA). E2 was dissolved in ethanol prior to application and both genistein and naringenin were dissolved in dimethyl sulfoxide (DMSO). The luciferase assay system was purchased from Promega (Madison, WI, USA). PRMI1640 media and dextran charcoal-stripped fetal bovine serum (cs-FBS) were purchased from Hyclone (Logan, Utah, USA). Plastic wares used for cell culture were purchased from Corning (NY, USA). The First Strand cDNA Synthesis Kit and the 2× SYBR Green PCR Master Mix were purchased from MBI Fermentas (Hanover, MD, USA) and Applied Biosystems (Foster, CA, USA), respectively.
Breast cancer cells and cultures
The T47D-KBluc human breast cancer cell line and the ERα-negative MDA-MB-231 cell line were purchased from the American Type Culture Collection (Rockville, MD, USA). The T47D-KBluc cells, which are derived from T47D cells, express the estrogen-responsive construct estrogen response elements (ERE) (β-globin)-luciferase. T47D-KBluc cells express both ERα and ERβ and exhibit an estrogen-responsive pattern of growth. These cells were routinely maintained in RPMI 1640 medium supplemented with 10% FBS and 100 units/m (1%) penicillin/streptomycin; the medium was changed every 3 days.
Methods
Determination of cell viability (MTT assay)
Cells were cultured in 96-well plates (3 × 103 cells/well) in RPMI 1640 media containing 10% cs-FBS for a period of 24 hours, during which they were treated with the vehicle and the respective substances (such as E2, genistein, and/or naringenin). The MTT solution (2 mg/ml) was then added to each well, and the cells were then further incubated for 4 hours at 37 °C. Further, 150 μL of DMSO were added, and the absorbance of the solution at 570 nm was measured. Values were expressed as (absorbance as a percentage of the value of vehicle controls of the six replicate assays) ± (standard error of the mean).
Luciferase assay
Cells were seeded in 24-well plates at an initial concentration of 1 × 105 cells per well in a 5% CO2 atmosphere at 37 °C. Prior to this treatment, cells were cultured in RPMI 1640 medium supplemented with 10% cs-FBS for 1 day. To investigate the effects of the added substances for estrogenic activity in T47D-KBluc cells, E2 (at concentrations of 0.001, 0.01, 0.1, and 1 μM), genistein (at concentrations of 0.01, 0.1, 1, and 10 μM), naringenin (at concentrations of 0.01, 0.1, 1, and 10 μM), and ICI 182 780 (at concentrations of 0.01, 0.1, 1, and 10 μM) were each administered to cells, which were then incubated for a period of 24 hours. To investigate the interaction between naringenin and E2 or genistein, cells were treated for 24 hours with E2 (at a concentration of 0.1 μM) or genistein (at a concentration of 5 μM) in combination with naringenin (at concentrations of 5 and 10 μM). To demonstrate that E2, genistein, and naringenin modulate luciferase activity through an estrogen receptor-dependent mechanism, cells were treated with E2 (at a concentration of 0.1 μM), genistein (at a concentration of 5 μM), or naringenin (at a concentration of 5 μM) in conjunction with ICI 182 780 (at concentrations of 0.1, 1, and 10 μM) for 24 hours.
Before harvesting, the cells were washed twice with PBS, after which 100 μL of the lysis buffer was added to each well; the plates were then stored at a temperature of -70 °C for 3 hours. The cell lysates were then transferred to 1.5 mL Eppendorf tubes and centrifuged at 12,000 rpm for 10 min. From this solution, 60 μL of the supernatant was taken to assess luciferase activity with a Berthold luminometer, and another 10 μL was used for protein determination. Luciferase activity was normalized according to protein concentration. Values were expressed as (mean value of induction as a multiple of the value of vehicle controls of four replicate assays) ± (standard error of the mean).
RNA extraction and RT-PCR analysis
Cells (5 × 105) were seeded onto each 60-mm culture dish with RPMI 1640 medium supplemented with 10% CS-FBS. After one day of incubation, T47D-KBluc cells were treated with each of the substances at their respective concentrations; E2 (at concentrations of 0.001, 0.01, 0.1, and 1, μM), genistein (at concentrations of 0.001, 0.1, 1, and 10 μM), and naringenin (at concentrations of 0.001, 0.1, 1, and 10 μM) to determine whether the expression of endogenous pS2 and TGFβ3 transcripts is modulated by E2, genistein, or naringenin for one day. To investigate the effect of the treatment with ICI 182 780 on endogenous pS2 transactivation by E2, genistein, and naringenin and the effect of treatment of naringenin on endogenous pS2 mRNA expression induced by E2 and genistein in T47D-KBluc cells, cells were treated for 24 hours with E2, genistein, naringenin, and ICI 182 780 in the following combinations and concentrations: E2 (at 0.1 μM) with naringenin (at 5 μM), genistein (at 5 μM) with naringenin (at 5 μM), naringenin (at 5 μM) with ICI 182 780 (at 1 μM), E2 (at 0.1 μM) with ICI 182 780 (at 1 μM), and genistein (at 5 μM) with ICI 182 780 (at 1 μM). MDA-MB231 cells were treated with naringenin (at concentrations of 0.01, 0.1, 1, and 10 μM) for 24 hours to investigate the effect of naringenin on the expression of pS2 and TGFβ3 mRNA.
Total RNA was isolated from the T47D-KBluc and MDA-MB231 cells with TRIzol as per the manufacturer’s instructions. Total RNA (3-5 μg) from three independent experiments in 20 μL of reaction mixture was used for reverse-transcription into cDNA using the First-Strand cDNA Synthesis Kit as per the manufacturer’s instructions. The resulting cDNA was diluted 10-fold and stored at a temperature of -70 °C.
Real-time quantitative RT-PCR was carried out using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, USA). SYBR Green PCR Master Mix (10 μL), diluted cDNA (4 μL), and 200 nM of the primer set were used for amplification in a final reaction mixture volume of 20 μL. All the samples were amplified in triplicates in a 96-well plate, and the cycling conditions were as follows: incubation for 2 minutes at 50 °C, incubation for 10 minutes at 95 °C, and 40 cycles at 95 °C for 15 seconds, followed by incubation for 1 minute at 60 °C. A comparison of the effect of each substance was done by calculating the values of the threshold cycle by normalizing the average Ct value for each substance according to the value of the endogenous control (GAPDH). The respective 2-Ct values were then calculated, followed by a statistical analysis of the data as described previously [14]. Primers for real-time quantitative analysis were used as shown in Table 1.
Table 1.
Oligonucleotide primer sequences used in the study
| Gene | oligonucleotides | Sequence |
|---|---|---|
| GAPDH | sense | 5’-TGG GCT ACA CTG AGC ACC AG-3’ |
| anti-sense | 5’-GGG TGT CGC TGT TGA AGT CA-3’ | |
| pS2 | sense | 5’-CCA CCA TGG AGA ACA AGG TGA-3’ |
| anti-sense | 5’-GCA GCC CTT ATT TGC ACA CTG-3’ | |
| TGFβ3 | sense | 5’-ATGAGCACATTGCCAAACAGC-3’ |
| anti-sense | 5’-CAC TCA CGC ACA GTG TCA GTG A-3’ |
Statistical analysis
Statisti significance was determined by the Student’s t-test using Microcal Origin 5.0 software.
Results
Effects of substances on estrogenic activity in T47D-KBluc breast cancer cells expressing both ERα and ERβ
We used T47D-KBluc cells cultured in estrogen-free cs-FBS-supplemented medium to eliminate the effect of estrogen, which may be present in FBS. Our experiments showed that E2, genistein, and naringenin possessed estrogenic activity and ICI 182 780 displayed anti-estrogenic activity in T47D-KBluc cells. E2, genistein, or naringenin increased the luciferase activity, whereas ICI 182 780 decreased the luciferase activity in a concentration-dependent manner. Figure 1 shows the individual effects of E2, genistein, naringenin, and ICI 182 780 in T47D-KBluc breast cancer cells.
Figure 1.
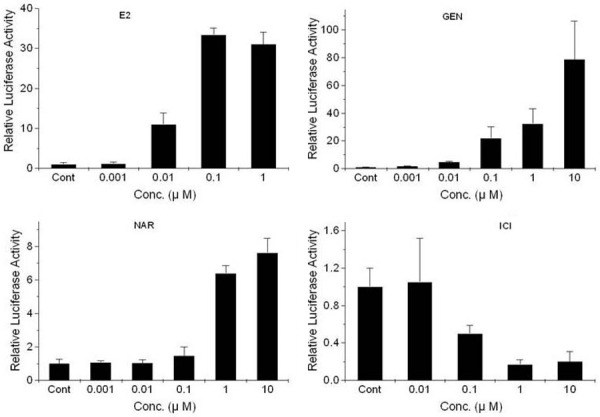
Effects of 17β-estradiol (top, left), genistein (top, right), naringenin (bottom, left), and ICI 182 780 (bottom, right) on luciferase gene expression in T47D-KBluc cells cultured in cs-FBS-supplemented medium. Values are expressed as (mean value of induction as a multiple of the value of the vehicle control of four replicate assays) ± (standard error of the mean). The level of luciferase activity was significantly different from the activity observed following treatment (P<0.05). E2, 17β-estradiol; GEN, genistein; NAR, naringenin; ICI, ICI 182 780.
Naringenin significantly repressed the activity of luciferase, which was induced by 0.1 μM E2 or 5 μM genistein. The effects of E2-naringenin or genistein-naringenin co-treatment on ER-mediated luciferase induction in T47D-KBluc cells are displayed in Figure 2. ICI 182 780 dose-dependently repressed the luciferase activity, which was induced by E2, genistein, or naringenin in T47D-KBluc cells (Figure 3).
Figure 2.
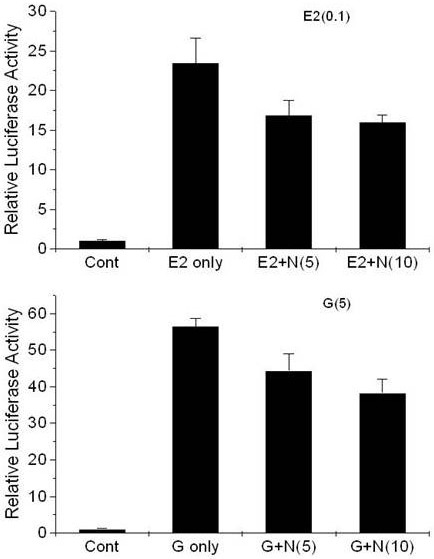
Effects of treatment in conjunction with naringenin, with E2 (top) and genistein (bottom) on luciferase gene expression in T47D-KBluc cells cultured in cs-FBS-supplemented medium. Values are expressed as (mean value of induction as a multiple of the value of the vehicle control of four replicate assays) ± (standard error of the mean). The level of luciferase activity was significantly different from the activity revealed following treatment with 0.1 μM 17β-estradiol or 5 μM genistein (P<0.05). E2 only, 0.1 μM 17β-estradiol; E2+N(5), 0.1 μM 17β-estradiol with 5 μM naringenin; E2+N(10), 0.1 μM 17β-estradiol with 10 μM naringenin; G only, 5 μM genistein; G+N(5), 5 μM genistein with 5 μM naringenin; G+N(10), 5 μM genistein with 10 μM naringenin.
Figure 3.
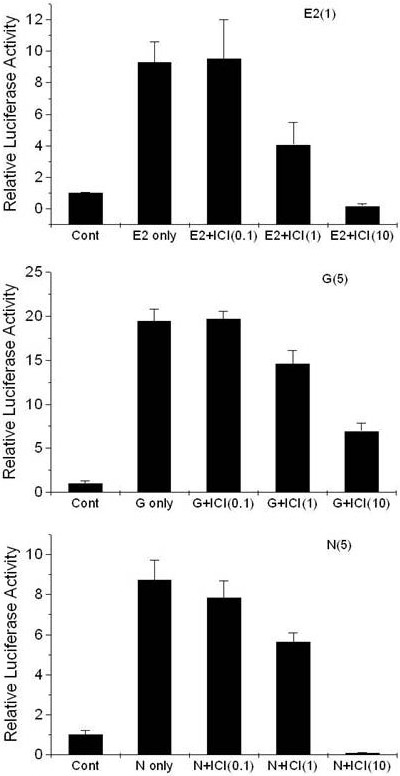
Effects of treatment, in conjunction with estrogen antagonist ICI 182 780, with E2 (top), genistein (middle), and naringenin (bottom) on luciferase gene expression in T47D-KBluc cells cultured in cs-FBS-supplemented medium. Values are expressed as (mean value of induction as a multiple of the value of the vehicle control of four replicate assays) ± (standard error of the mean). The level of luciferase activity was significantly different from the activity revealed following treatment with 1 μM 17β-estradiol, 5 μM genistein or 5 μM naringenin (P<0.05). E2, 1 μM 17β-estradiol; E2+I (0.1, 1, and 10), 1 μM 17β-estradiol with ICI 182 780 (0.1, 1, and 10 μM); G only, 5 μM genistein; G+I (0.1, 1, and 10), 5 μM genistein with ICI 182 780 (0.1, 1, and 10 μM); N only, 5 μM naringenin; N+I (0.1, 1, and 10), 5 μM naringenin with ICI 182 780 (0.1, 1, and 10 μM).
Expression of endogenous pS2 and TGFβ3 mRNA modulated by E2, genistein, or naringenin in T47D-KBluc cells
Individual effects of substances
Transcriptions of pS2 and TGFβ3 were evaluated using quantitative RT-PCR, as described in Materials and Methods. It was observed that E2, genistein, and naringenin displayed estrogenic effects on T47D-KBluc cells cultured in cs-FBS-supplemented medium. E2, genistein, and naringenin increased pS2 transcription in a concentration-dependent manner (Figure 4), whereas they decreased TGFβ3 transcription (Figure 5).
Figure 4.
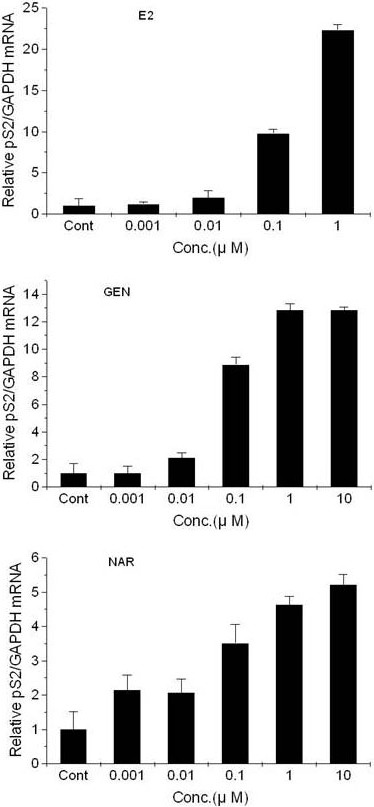
Effects of 17β-estradiol (top), genistein (middle), and naringenin (bottom) on the expression of pS2 mRNA in T47D-KBluc cells cultured in cs-FBS-supplemented medium. Results are expressed as (N-fold difference in target gene expression relative to GAPDH gene expression). E2, 17β-estradiol; GEN, genistein; NAR, naringenin; Cont, control; Conc, concentration.
Figure 5.
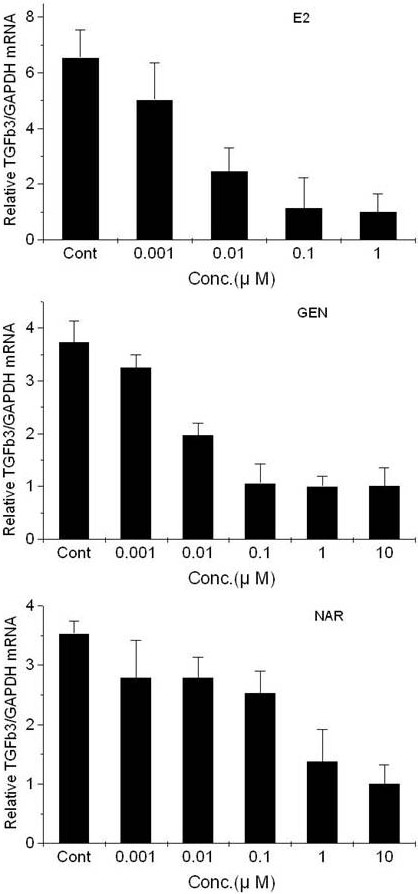
Effects of 17β-estradiol (top), genistein (middle), and naringenin (bottom) on the expression of TGFβ3 mRNA in T47D-KBluc cells cultured in cs-FBS-supplemented medium. Results are expressed as (N-fold difference in target gene expression relative to GAPDH gene expression). E2, 17β-estradiol; GEN, genistein; NAR, naringenin; Cont, control; Conc, concentration.
Effects of co-treatment of substances
The results of the co-treatment revealed that naringenin (at 5 μM) repressed pS2 mRNA expression, induced by E2 (at 0.1 μM) or genistein (at 5 μM), whereas ICI 182 780 (at 1 μM) repressed pS2 transcription, induced by E2 (at 0.1 μM), genistein (at 5 μM), or naringenin (at 5 μM) (Figure 6).
Figure 6.
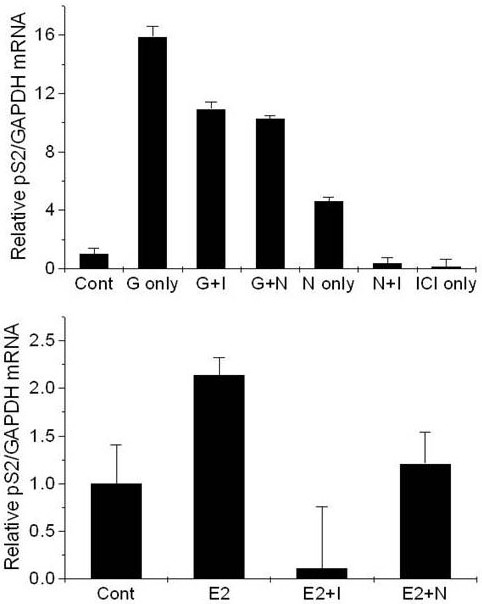
Effects of co-treatment on the expression of the mRNA in T47D-KBluc cells cultured in cs-FBS-supplemented medium. Results are expressed as (N-fold difference in target gene expression relative to GAPDH gene expression). E2, 0.1 μM 17β-estradiol; E2+I, 0.1 μM 17β-estradiol with 1 μM ICI 182 780; E2+N, 0.1 μM 17β-estradiol with 5 μM naringenin; Gen 5 μM genistein; G+I, 5 μM genistein with 1 μM ICI 182 780; G+N, 5 μM genistein with 5 μM naringenin; NAR, 5 μM naringenin; N+I, 5 μM naringenin with 1 μM ICI 182 780; ICI, 1 μM ICI 182 780; CONT, control.
Effect of naringenin on expression of pS2 and TGFβ3 mRNA in MDA-MB-231 breast cancer cells
Naringenin treatment in MDA-MB-231 cells led to no significant change in pS2 mRNA expression and a slight decrease in TGFβ3 mRNA expression (Figure 7).
Figure 7.
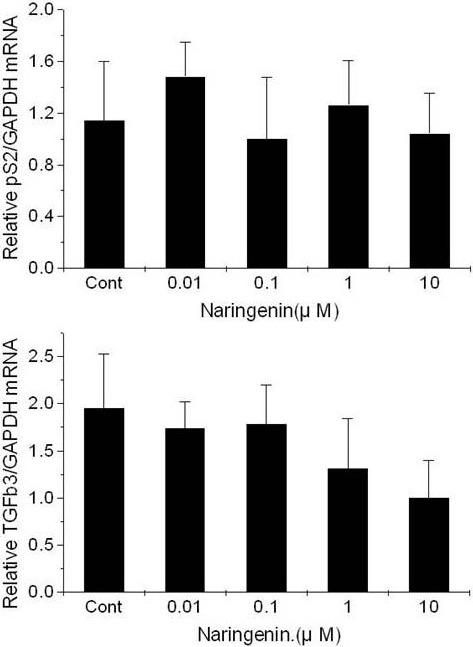
Effect of naringenin on the expression of pS2 and TGFβ3 mRNA in MDA-MB-231 breast cancer cells cultured in cs-FBS-supplemented medium. Results are expressed as (N-fold difference in target gene expression relative to GAPDH gene expression). Cont, control.
Cell viability
T47D-KBluc cells were exposed individually or in combination to the substances for 24 hours at the respective concentrations, and then MTT assay was carried out. The results of the MTT assay indicate that there was no proliferative effect of substances on the cells (Figure 8).
Figure 8.
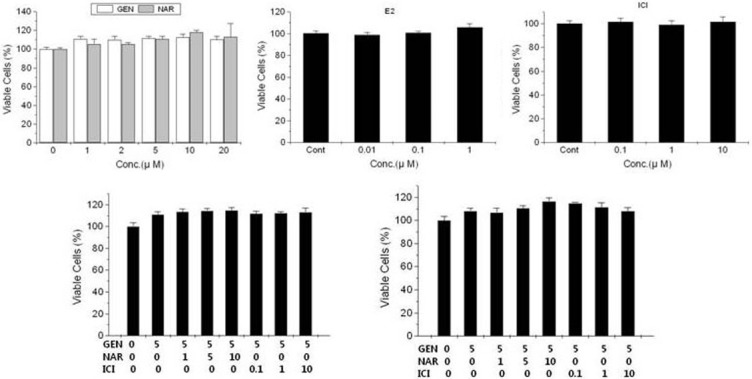
Effects of the substances individually (top) and in combination (bottom) on proliferation of T47D-KBluc cells. Values are expressed as (viable cell population as a percentage of the vehicle control population of six replicate assays) ± (standard error of the mean). Gen, genistein; Nar, naringenin; ICI, ICI 182 780; E2, 17β-estradiol; Cont, control; Conc, concentration.
Discussion
The incidence of osteoporosis rapidly increased as rapid strides in medical technology have contributed to an increase in the average life span of a human. A major complication arising out of osteoporosis is bone fracture, which may produce a high morbidity in elderly people as well as menopausal women. Synthetic estrogens are administered to women to eliminate menopausal syndrome and prevent osteoporosis, which is a common metabolic bone disorder in menopausal women. Because the long-term administration of synthetic estrogen in menopausal women can produce benefits as well as the risk of serious health problems, many studies have been carried out with the aim of finding alternatives to conventional hormone replacement therapy [1-3] and investigators needed reliable and rapid assays to identify hormone-related compounds, especially agents with estrogenic activity. The important factors to verify the estrogenic effect of substances include affinity with and activity on the estrogen receptors (ERα and ERβ), which respectively possess different tissue distribution and biological end functions [15-18]. The expression of estrogen-related genes is modulated by estrogen receptors, which bind to the ligand and then to the ERE [19]. By studying this mechanism, the affinity of a substance to estrogen receptors and its agonist activity can be examined. In an effort to create such an experimental model, Pons et al. [20] produced the MVLN cell line, which is derived from transfection of MCF-7 cells with a fusion gene combining an ERE and a luciferase gene. Using the MVLN cell line, Demirpence et al. [21] evaluated the agonistic activity of various substances on estrogen receptors. However, the method employed by Demirpence et al. was limited due to its low sensitivity. Another published method utilized the T47D-KBluc cell line, which is derived from T47D breast cancer cells after transfection with a plasmid combining three EREs with a luciferase gene [22]. By measuring substance’s influence of luciferase activity in T47D-KBluc breast cancer cells, the agonist activity of that substance on an estrogen receptor can be evaluated with high sensitivity. We investigated the effect of naringenin on estrogen receptor activity in T47D-KBluc breast cancer cells, either alone or in conjunction with E2 and genistein (via E2-naringenin and genistein-naringenin co-treatment).
Zierau et al. [23] found naringenin to be having no effect on estrogen receptors in both the assay utilizing recombinant yeast strain and the method involving MCF-7 cells expressing ERα. Rub et al. [24] suggested that naringenin might possess anti-estrogenic activity, as inferred by naringenin’s repression of E2-induced increases in uterus weight and pS2-LUC expression in rats. It was reported in studies that involved a recombinant yeast strain and HELa and COS-7 cells that naringenin possessed estrogenic activity [8,19]. In a study involving the BT-474 breast cancer cell line, Zand et al. [25] suggested that naringenin was an ER agonist; it increased the expression of the pS2 protein, which was modulated by estrogen receptor activation. Guo et al. [13] suggested that naringenin possessed a double directional adjusting function of estrogenic and anti-estrogenic activities. There is still controversy regarding the estrogenic activity of naringenin, either alone or in combination with other estrogenic substances. EC 50 values of each of substances were calculated through the use of Microcal Origin 5.0 software, based on the RLU (relative luminescence unit) data obtained transactivation experiments performed on T47D-KBLuc cells. EC50 values of E2, genistein, and naringenin were 3.05921, 3.78468, and 4.86498, respectively. Our results of transactivation experiments showed that E2, genistein, and naringenin had estrogenic effects in the T47D-KBluc cells, with the relative strengths of estrogenic activity, in descending order of E2 > genistein > naringenin. Considering the experimental results that naringenin suppressed the elevation of luciferase activity, which was induced by genistein or E2 in co-treatment experiments (E2 or genistein administered in conjunction with naringenin), naringenin was a weak estrogen agonist of the estrogen receptor.
The expression levels of two endogenous marker genes, pS2 (a well-known estrogen-regulated protein) and TGFβ3, were also analyzed along with the ERE-mediated luciferase activity for accuracy and reliability. The expression pattern of endogenous pS2 and TGFβ3 mRNA in our study supported the results of the ERE-luciferase assay and confirmed that E2, genistein, and naringenin possessed estrogenic activity in T47D-KBluc cells cultured in cs-FBS medium. The pS2 protein belongs to the trefoil family, whose members are considered to be proliferation regulators; trefoil proteins were found to interact directly with epidermal growth factor receptors and to activate the RAS/MEK/MAP-kinase signal transduction pathway [26,27]. It was well known that estrogen induces both the transcription and translation of pS2 in MCF-7 breast cancer cells. On the contrary, no non-estrogenic compound to date has been found to induce pS2 expression in MCF-7 cells. Correspondingly, the expression of pS2 mRNA is widely accepted and measured as a marker of estrogenicity. It was demonstrated that the transforming growth factor β (TGFβ) family, including TGFβ1, TGFβ2, and TGFβ3, was a pleiotropic cytokine group and that it played key roles in tissue morphogenesis and growth [27-30]. Members of the TGFβ family are abundant in reproductive tissues, whose development and cyclic remodeling continue in post-natal and adult life. Interestingly, it was previously reported that TGFβ3 expression was repressed by E2 in MCF-7 breast cancer cells. However, unlike pS2, the expression of TGFβ3 is modulated by non-estrogenic substances. Knabbe et al. also reported that TGFβ3 inhibited the growth of MCF-7 cells and that anti-estrogens induced the secretion of TGFβ3.
To verify that modulation of luciferase activity and transcription of pS2 mRNA and TGFβ3 mRNA were mediated by ER, the effect of the estrogen-specific antagonist ICI 182 780, which was previously demonstrated to block estrogen receptors in breast cancer cells, was studied. As shown in Figures 3 and 6, ICI 182 780 repressed the effect of the added substances on luciferase activity and expression of pS2 and TGFβ3 mRNA in T47D-KBluc cells. These results demonstrate that E2, genistein and naringenin modulated luciferase activity and expression of pS2 and TGFβ3 mRNA via an estrogen receptor-dependent mechanism in T47D-KBluc cells.
In addition, the effect of naringenin treatment on expression of pS2 and TGFβ3 mRNA in MDA-MB-231 breast cancer cells, which are expressed only in small amounts of ERβ and was devoid of ERα, was examined. Unlike the increase in pS2 mRNA expression and the decrease in TGFβ3 mRNA expression by following addition of naringenin in T47D-KBluc cells, no change in pS2 mRNA expression and a slight decrease in TGFβ3 mRNA expression were observed in MDA-MB-231 cells (Figure 8). These results suggested that ERα acts as a necessary mediator in the modulation of the transcription of pS2 and TGFβ3 mRNA by naringenin and that naringenin modulates, to some extent, TGFβ3 mRNA transcription through activation of ERβ regardless of the presence of ERα. To examine the effect of the substances used in the present study on cell proliferation, cells were exposed to each of the substances for 24 hours at the respective concentrations and then an MTT assay was carried out. The results of the MTT assay confirmed that these substances had no effect on cell proliferation. The studies by Han et al. and So et al. had shown a relationship between administration of flavonoids and cell proliferation in the MCF-7 cell line. According to their findings, treatment over 24 hours was necessary in order for a flavonoid to manifest its influence on cell proliferation [31,32]. Our study confirmed that estrogenic activity was irrelevant to cell proliferation within the first 24 hours of estrogen analogue treatment.
Our study has some limitations. First, we didn’t clearly reveal whether the effect of naringenin is ERα-mediated or not because the experiment using selective agonists of ERα and ERβ in combination with naringenin was not performed. Second, we used a pure form of phytoestrogens which is not circulating in the body. Result of our experiment using a pure form of phytoestrogens is insufficient to reveal clinical significance of naringenin because most of phytoestrogens do not reach internal compartments of humans in the pure (glycosides) form. Finally, we did not perform the in vivo experiment. Further experiment with animal may give more information to us than in vitro experiment and may help to reveal clinical usefulness of naringenin.
In conclusion, naringenin was a weak agonist that differed from E2 or genistein in the potency of the functional response as an estrogen receptor. A net decrease in the estrogen receptor activity was observed as compared to that observed with the E2 or genistein alone when naringenin competed with the E2 or genistein for receptor occupancy. Therefore, naringenin was found to be able to act as a competitive antagonist in the presence of a potent (or full) agonist like E2 or genistein even though naringenin is an agonist. Naringenin is not an efficient antagonist to activate estrogen receptor but a partial agonist that can act as a competitive antagonist in the presence of a potent (or full) agonist. Clinical usefulness of naringenin may be derived from its ability to augment deficient estrogen activity while simultaneously interrupting excessive estrogen activity. Our study proposes that naringenin could be an excellent agent in the treatment of disorders related to sex hormones, as a new selective estrogen receptor modulator. Further studies are still essential to confirm whether naringenin inhibits action of E2 and genistein on estrogen receptors at effective and human serum concentrations.
Acknowledgements
The authors would like to thank Kang GS, a research assistant, for his contribution to the experiment.
Disclosure of conflict of interest
None.
References
- 1.Canderelli R, Leccesse LA, Miller NL, Unruh Davidson J. Benefits of hormone replacement therapy in postmenopausal women. J Am Acad Nurse Pract. 2007;19:635–641. doi: 10.1111/j.1745-7599.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 2.Rymer J, Wilson R, Ballard K. Making decisions about hormone replacement therapy. BMJ. 2003;326:322–6. doi: 10.1136/bmj.326.7384.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lignieres B, de Vathaire F, Fournier S, Urbinelli R, Allaert F, Le MG, Kuttenn F. Combined hormone replacement therapy and risk of breast cancer in a French cohort study of 3175 women. Climacteric. 2002;5:332–340. doi: 10.1080/713605312. [DOI] [PubMed] [Google Scholar]
- 4.Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Al-Azzawi F, Wahab M. Effectiveness of phytoestrogens in climacteric medicine. Ann N Y Acad Sci. 2010;1205:262–267. doi: 10.1111/j.1749-6632.2010.05678.x. [DOI] [PubMed] [Google Scholar]
- 6.Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10:236–242. doi: 10.1016/j.tplants.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Le Bail JC, Varnat F, Nicolas JC, Habrioux G. Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids. Cancer Lett. 1998;130:209–216. doi: 10.1016/s0304-3835(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 8.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 9.Meiyanto E, Hermawan A, Anindyajati Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pac J Cancer Prev. 2012;13:427–436. doi: 10.7314/apjcp.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- 10.Bodet C, La VD, Epifano F, Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodontal Res. 2008;43:400–7. doi: 10.1111/j.1600-0765.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Jeong TS, Choi YK, Hyun BH, Oh GT, Kim EH, Kim JR, Han JI, Bok SH. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun. 2001;284:681–8. doi: 10.1006/bbrc.2001.5001. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective Effect of Naringenin Against Lead-Induced Oxidative Stress in Rats. Biol Trace Elem Res. 2012;146:354–9. doi: 10.1007/s12011-011-9268-6. [DOI] [PubMed] [Google Scholar]
- 13.Guo D, Wang J, Wang X, Luo H, Zhang H, Cao D, Chen L, Huang N. Double directional adjusting estrogenic effect of naringin from Rhizoma drynariae (Gusuibu) J Ethnopharmacol. 2011;138:451–457. doi: 10.1016/j.jep.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Matthews J, Gustafsson JA. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 16.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 17.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitiative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knock mouse. Endocrinology. 1997;138:4613–21. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 18.Couse JF, Korach KS. Estrogen null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 19.Bourdeau V, Deschenes J, Laperriere D, Aid M, White JH, Mader S. Mechanisms of primary and secondary estrogen target gene regulation in breast cancer cells. Nucleic Acids Res. 2008;36:76–93. doi: 10.1093/nar/gkm945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pons M, Gagne D, Nicolas JC, Mehtali M. A new cellular model of response to estrogens: a bioluminescent test to characterize (anti) estrogen molecules. Biotechniques. 1990;9:450–9. [PubMed] [Google Scholar]
- 21.Demirpence E, Duchesne MJ, Badia E, Gagne D, Pons M. MVLN cells: a bioluminescent MCF-7 derived cell line to study the modulation of estrogen activity. J Steroid Biochem Mol Biol. 1993;46:355–64. doi: 10.1016/0960-0760(93)90225-l. [DOI] [PubMed] [Google Scholar]
- 22.Wilson VS, Bobseine K, Gray LE. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004;81:69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- 23.Zierau O, Hamann J, Tischer S, Schwab P, Metz P, Vollmer G, Gutzeit HO, Scholz S. Naringenin-type flavonoids show different estrogenic effects in mammalian and teleost test systems. Biochem Biophys Res Commun. 2005;326:909–16. doi: 10.1016/j.bbrc.2004.11.124. [DOI] [PubMed] [Google Scholar]
- 24.Ruh MF, Zacharewski T, Connor K, Howell J, Chen I, Safe S. Naringenin: a weakly estrogenic bioflavonoid that exhibits antiestrogenic activity. Biochem Pharmacol. 1995;50:1485–93. doi: 10.1016/0006-2952(95)02061-6. [DOI] [PubMed] [Google Scholar]
- 25.Zand RS, Jenkins DJ, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res Treat. 2000;62:35–49. doi: 10.1023/a:1006422302173. [DOI] [PubMed] [Google Scholar]
- 26.Taupin D, Wu DC, Jeon WK, Devaney K, Wang TC, Podolsky DK. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999;103:R31–8. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen M, Vendelbo B, Skakkebaek NE, Leffers H. Assaying estrogenicity by quantitating the expression levels of endogenous estrogen-regulated genes. Environ Health Perspect. 2000;108:403–412. doi: 10.1289/ehp.108-1638061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987;48:417–28. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- 29.Dannecker C, Possinger K, Classen S. Induction of TGF-beta by an antiprogestin in the human breast cancer cell line T-47D. Ann Oncol. 1996;7:391–395. doi: 10.1093/oxfordjournals.annonc.a010606. [DOI] [PubMed] [Google Scholar]
- 30.Bates SE, Davidson NE, Valverius EM, Freter CE, Dickson RB, Tam JP, Kudlow JE, Lippman ME, Salomon DS. Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol Endocrinol. 1988;2:543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- 31.So FV, Guthrie N, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997;112:127–133. doi: 10.1016/s0304-3835(96)04557-0. [DOI] [PubMed] [Google Scholar]
- 32.Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppuression by flavonoids. Biosci Biotechnol Biochem. 2002;66:1479–87. doi: 10.1271/bbb.66.1479. [DOI] [PubMed] [Google Scholar]


