Abstract
Objective: To investigate the role of mouse nerve growth factor (mNGF) in neutral repair following hypoxic-ischemic brain damage (HIBD) in a neonatal rat model. Methods: A total of 120 neonatal rats aged 7 days were randomly divided into control group, HIBD group and mNGF group (n=40 /group). Immediately after HIBD, mNGF was intramuscularly injected into rats in the mNGF group. 7, 14, 21 and 28 days after injection, immunohistochemistry and TUNEL staining were performed. Results: In the HIBD group, Nestin expression was mainly found in the CA1, CA2 and CA3 regions of hippocampus. In the mNGF group, Nestin was mainly noted in the DG and CA3 regions. 7, 14 and 28 days after treatment, significant difference was found in the Nestin expression among three groups, but not observed 21 days after treatment. 21 and 28 days after treatment, the number of apoptotic neurons was markedly reduced when compared with that 7 and 14 days after treatment. Conclusion: mNGF can improve the neurogenesis and inhibit neural apoptosis in the hippocampus following HIBD in a neonatal rat model.
Keywords: Brain, hypoxia/ischemia, nerve growth factor, rat
Introduction
Perinatal hypoxic-ischemic encephalopathy (HIE) is a major cause of brain injury and usually has a poor long-term prognosis. Perinatal hypoxia is a key factor resulting in long-term complications of the nervous system including abnormal behavior, severe epilepsy, mental retardation, cerebral palsy and others. The specific pathophysiology of neural injury following perinatal hypoxia and the effective strategies for the treatment of HIE are poorly understood [1]. The incidence of hypoxic-ischemic brain damage (HIBD) is about 6/1000 live births, and 25-30% of survivals may present with long-term sequela. Thus, HIBD has become a major problem influence the quality of life of children worldwide [2]. To take effective measures to early interfere with the pathophysiology of HIBD is crucial to reduce the mortality and disability of neonates. Nerve growth factor (NGF) has been found to exert neuroprotective effect, anti-oxidative effect, and anti-apoptotic effect and to inhibit the cytotoxicity of excitatory amino acids, stabilize the intracellular calcium concentration, and suppress the calcium overload. These effects may effectively protect the neurons and promote the neurogenesis following injury. It has been revealed that mouse NGF (mNGF) exerts therapeutic effect on HIBD [3], but the specific mechanism remains still unclear. In the present study, HIBD was introduced to neonatal rats which then received treatment with mNGF, to investigate the mechanism of therapeutic effect of mNGF on neonatal HIBD. Our findings may provide evidence on the wide application of mNGF in the treatment of HIBD.
Materials and methods
Animals, grouping and treatment
A total of 120 neonatal rats aged 7 days and weighing 10-12 g (specific pathogen free) were purchased from the Institute of Animals of Academy of Military Medical Sciences. These rats were randomly assigned into 3 groups: control group, HIBD group and mNGF group (n=40 per group). In the control group, animals were intramuscularly injected with 0.1 M Na2HPO4-NaH2PO4 solution at 10 μl/g/d at the buttock for 5 days. In the HIBD group, HIBD was introduced to animals which did not receive any other treatment. In the mNGF group, HIBD was introduced to animals which immediately received intramuscular injection of mNGF at 20 ng/g/d (10 μl) for 5 days. 7, 14, 21 and 28 days after treatment, animals were sacrificed for further experiments (n=10 per time point).
Main reagent
Mouse anti-rat Nestin monoclonal antibody (Chemicon), DEPC, Triton X-100 (Sigma), Nestin in situ hybridization kit, cell apoptosis kit, DAB visualization kit, SABC kit (Zhongshan Biotech Co., Ltd), mNGF (Wuhan Haite Biological Pharmaceutical Co., Ltd) and other reagents (analytically pure) were used in the present study.
Establishment of HIBD animal model, sample collection
The 7-day old SD rats were anesthetized with anhydrous ether for 0.5-1 min. The animal was placed in left palm under a microscope. The forefinger and middle finger were used to fix the head of the animal, and the thumb to fix the bilateral forelimbs. A midline incision was made at the neck, and the subcutaneous fat was separated. The left common carotid artery was separated through the inner side of sternocleidomastoid, and ligated with 5-0 suture twice followed by disconnecting the common carotid artery. Hemostasis was done with gelatin sponge [4]. 2-3 h after surgery, animals were placed in a close chamber (10 L) and received hypoxic treatment (8% oxygen) at 37°C at a flow rate of 0.5 L/min for 2.5 h. In the normal control group, animals did not receive any treatment. In the hypoxia group, animals received hypoxia alone for 2.5 h. In the HIBD group, animals receive both ligation of common carotid artery and hypoxia for 2.5 h. At the predesigned time points, animals were anesthetized with ether and thoracotomy was done. The heart was exposed and a catheter was inserted via the aorta. Perfusion was done with cold normal saline at 20 ml/kg to remove blood and the brain was collected. The macroscopic features of the brain were recorded and then placed in a freezing microtome at -20°C for balance for 2 h. Coronal sections (8 μm) were collected along the suprachiasmatic midpoint into the polylysine-coated slides followed by fixation in acetone for 20 min. The slides were stored at -20°C or immediately used in the following experiments.
Immunohistochemistry for neural stem cell protein (Nestin) in hippocampus
Immunohistochemistry was carried out and visualization was done with Diaminobenzidine (DAB). Pathological examination was done under light microscope. The cells with yellow-brown granules in the cytoplasm were regarded as Nestin positive cells. The number of positive cells and total cells was counted at a magnification of 200×. A total of 100 grids were included in a field and 3 sections were examined in each group. A total of 4 fields were randomly selected for examination and thus 12 fields were employed for the detection of the positive cells. Then, the number of Nestin positive cells was calculated in each group.
Detection of apoptosis by TUNEL staining
The apoptotic cells were detected with TUNEL staining according to the manufacturer’s instructions with slight modification. Cells with yellow-brown granules in the nucleus were regarded to be apoptotic cells.
Statistical analysis
Statistical analysis was done with SPSS version 12.0. Data were expressed as mean ± standard deviation (SD) (χ̅±s). Analysis of variance was used for comparisons. A value of P<0.05 was considered statistically significant.
Results
Nestin positive cells in the hippocampus
There was difference in the distribution of Nestin positive cells in the hippocampus among three groups. In the HIBI group, Nestin expression was mainly found in the CA1, CA2 and CA3 regions of the hippocampus. In the mNGF group, Nestin was mainly noted in the DG and CA3 region (Figure 1A-F). The number of Nestin positive cells is shown in Table 1.
Figure 1.
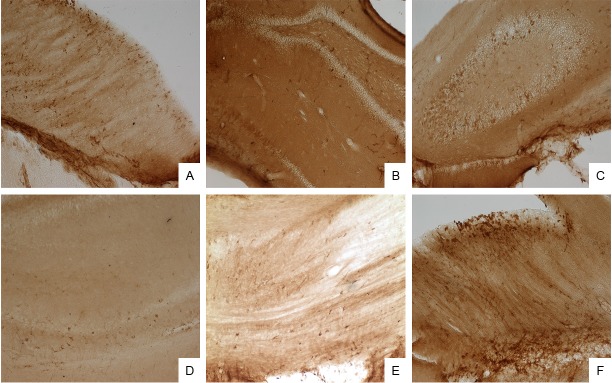
A: Immunohistochemistry for Nestin at 7 days after treatment in the control group (CA1 region; visualization with DAB, 100×); B: Immunohistochemistry for Nestin at 7 days after treatment in the HIBD group (CA1 region; visualization with DAB, 100×); C: Immunohistochemistry for Nestin at 7 days after treatment in the mNGF group (CA3 and DG regions; visualization with DAB, 100×); D: Immunohistochemistry for Nestin at 14 days after treatment in the control group (hippocampus; visualization with DAB, 100×); E: Immunohistochemistry for Nestin at 14 days after treatment in the HIBI group (hippocampus; visualization with DAB, 100×); F: Immunohistochemistry for Nestin at 14 days after treatment in the mNGF group (hippocampus; visualization with DAB, 100×).
Table 1.
Number of Nestin positive cells in the affected hippocampus of different groups (χ̅±s; n=10)
| Group | Number of Nestin positive cells | |||
|---|---|---|---|---|
|
| ||||
| 7 d | 14 d | 21 d | 28 d | |
| Control | 24.65±2.21 | 18.86±2.38 | 16.50±3.21 | 13.60±2.03 |
| HIBD | 22.03±1.59a | 18.56±2.23a | 16.80±1.77a | 14.90±2.35a |
| mNGF | 27.25±2.15a,b | 23.90±2.95a,b | 20.08±2.19a,b | 17.05±1.75a,b |
| F | 13.512c | 8.915d | 3.216e | 6.312d |
| P | <0.01 | <0.05 | >0.05 | <0.01 |
P<0.01 vs control group.
P<0.01 vs HIBD group.
P<0.01;
P<0.05;
P>0.05 vs HIBD group.
Number of apoptotic neurons in affected hippocampus
Seven days after treatment, the number of apoptotic cells in the mNGF group was significantly higher than that in the control group, but markedly lower than that in the HIBD group. Moreover, the number of apoptotic cells in the HIBD group was dramatically increased when compared with that in the control group 7 days after treatment. The number of apoptotic cells in three groups 14 days after treatment was significantly higher than those 7 days after treatment. However, the number of apoptotic cells became to reduce 21 and 28 days after treatment (Table 2).
Table 2.
Number of apoptotic cells of the affected hippocampus at different time points in different groups (χ̅±s; n=10)
| Group | Number of apoptotic cells | |||
|---|---|---|---|---|
|
| ||||
| 7 d | 14 d | 21 d | 28 d | |
| Control | 8.65±0.01 | 12.16±0.28 | 10.41±0.21 | 3.60±0.03 |
| HIBD | 29.12±0.31a | 37.46±0.23a | 35.36±0.77a | 25.70±0.21a |
| mNGF | 21.20±0.15a,b | 30.60±0.95a,b | 15.08±0.18a,b | 9.25±0.43a,b |
| F | 9.315c | 7.213d | 8.712c | 12.428c |
| P | P<0.01 | P<0.05 | P<0.01 | P<0.01 |
P<0.01 vs control group.
P<0.01;
P<0.01;
P<0.05 vs HIBD group.
Discussion
NGF is widely expressed in different tissues, can exert neurotrophic effect on the injured nerves and promote the neurogenesis, and is closely related to the development, functional maintenance and repair of nervous system [5,6]. NGF not only exerts neurotrophic effect in the central nervous system, but can promote the regeneration of injured neurons, improve the pathology of neuron and protect the nerves against hypoxia/ischemia. NGF can promote the generation of dendrites, and the extension and survival of neurons, increase the density of nerve fibers in the targeted regions, especially the cholinergic neurons in the hippocampus, basal forebrain and striatum. In addition, NGF can facilitate the budding of protrusion of sympathetic neurons and sensory neurons, maintain the survival of sympathetic neurons and sensory neurons, promote the differentiation of neurons, facilitate the mitosis, increase the number of neurons and determine the direction of axonal growth. Moreover, for mature neurons, NGF not only exerts neurotrophic effect on normal neurons but protects and repairs the injured neurons [7,8]. Nakatomi et al [9] found that intraventricular injection of NGF following cerebral ischemia could significantly promote the activation and proliferation of endogenous neural stem cells (NSCs), and the migration of NSCs into the hippocampus to integrate into the brain loop leading to the improvement of neurofunction. mNGF (Jin Lu Jie) is one of NGFs. In China, Xue et al [3] applied mNGF in the treatment of 86 children with HIBD achieving favorable outcome, but the specific mechanism of therapeutic effect of NGF is still unclear.
NSCs are a group of cells with potentials of differentiation into multiple cells. Following brain injury, NSCs are activated and then differentiated into nerve cells which migrate into the injured sites for repair and reconstruction. Thus, NSCs play an important role in the post-injury repair of the brain. Nestin expression is mainly found in the neurula. With the development and differentiation of neurons, Nestin expression becomes to be absent. Thus, Nestin can be used as a marker of NSCs [10,11]. In respect of the source of Nestin following brain injury, there are two opinions currently: Some investigators propose that the astrocytes around the injured sites replay the embryonic development [12] but others suggest that the Nestin positive cells are derived from the NSCs in the subependymal zone [13]. Under the physiological state, the NSCs in the brain are in a quiescent stage. In the presence of stimulation, some cytokines may activate these NSCs leading to their in situ or ectopic proliferation. In the aid of chemokines, these NSCs migrate into the injured sites and differentiate into functional cells [9]. Following the central nervous system injury, we can induce the differentiation of endogenous NSCs to repair the brain injury [14].
Neonatal HIE is a perinatal disease as a result of hypoxia/ischemia of any cause and reduction or discontinuation in cerebral blood flow in neonates or fetus. HIE is a common disease at the perinatal stage and usually results in sequela. To date, effective treatment has not developed for the HIE. To investigate the mechanism underlying the pathogenesis of HIE may guide the clinical treatment of HIE. Our results showed mNGF could significantly increase the number of nestin positive cells. This may be explained that the astrocytes may secret NGF following HIBD, but this secretion is transient. Supplement with exogenous mNGF may change the microenvironment in which the NSCs are activated to proliferate, the inhibitors are inactivated. In the presence of chemokines, these NSCs migrate into the injured sites and differentiate into specific cell types. After HIBD, treatment with exogenous mNGF may stimulate the embryonic transformation of cytoskeleton in mature cells, leading to the presence of characteristics of embryonic neuroepithelial cells which might be the precursors of astrocytes. At the same time, the NSCs in a quiescent stage are activated and present with in situ or ectopic proliferation. In the presence of chemokines, these NSCs migrate into the injured sites and differentiate into functional cells. Following the central nervous system injury, we may induce the differentiation and proliferation of endogenous NSCs to repair the nervous system injury. Our findings were consistent with previously reported [12,14]. In addition, our results also revealed that mNGF treatment could reduced the apoptosis of neurons, which was in accordance with reported previously [15].
Clinical treatment of HIBI with mNGF achieves favorable outcome, but the specific mechanism is still unclear. Our results demonstrate that the therapeutic effect of mNGF is related to the induction of proliferation of endogenous NSCs and the inhibition of apoptosis of neurons following mNGF treatment.
Acknowledgements
This work was supported by grants from the China Postdoctoral Science Foundation (No. 20070410505).
Disclosure of conflict of interest
None.
References
- 1.Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol. 2011;2011:609813. doi: 10.1155/2011/609813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou CL, Chen HJ, Yu RJ. Ultrasound diagnostics of neonatal brain. Peking University Medical Press; 2007. pp. 85–8. [Google Scholar]
- 3.Xue Y, Yin XJ, Yan L. Clinical observation of effects of nerve growth factor on management of hypoxic-ischemic encephalopathy. Chongqing Med. 2006;35:2030–1. [Google Scholar]
- 4.Gong M, Bi Y, Jiang W, Zhang Y, Chen L, Hou N, Liu Y, Wei X, Chen J, Li T. Immortalized mesenchymal stem cells: an alternative to primary mesenchymal stem cells in neuronal differentiation and neuroregeneration associated studies. J Biomed Sci. 2011;18:87–106. doi: 10.1186/1423-0127-18-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vera C, Tapia V, Kohan K, Gabler F, Ferreira A, Selman A, Vega M, Romero C. Nerve growth factor induces the expression of chaperone protein calreticulin in human epithelial ovarian cells. Horm Metab Res. 2012;44:639–43. doi: 10.1055/s-0032-1311633. [DOI] [PubMed] [Google Scholar]
- 6.Guo L, Yeh ML, Cuzon Carlson VC, Johnson-Venkatesh EM, Yeh HH. Nerve growth factor in the hippocamposeptal system: evidence for activity-dependent anterograde delivery and modulation of synaptic activity. J Neurosci. 2012;32:7701–10. doi: 10.1523/JNEUROSCI.0028-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salama-Cohen P, Arévalo MA, Meier J, Grantyn R, Rodríguez-Tébar A. NGF controls dendrite development in hippocampal neurons by binding to p75NTR and modulating the cellular targets of Notch. Mol Biol Cell. 2005;16:339–47. doi: 10.1091/mbc.E04-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HY, Zhu XF, Wang LM, Luo ZH, Yang ZJ, Liu DY, Yuan DX, Nie L, Wu YJ, Wang SX. [Brain-derived neurotrophic factor and neural stem cells transplantation in treatment of hypoxic-ischemic brain injury in rats] . Zhonghua Er Ke Za Zhi. 2008;46:544–9. [PubMed] [Google Scholar]
- 9.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 10.Uchida K, Okano H, Hayashi T, Mine Y, Tanioka Y, Nomura T, Kawase T. Grafted swine neuroepithelial stem cells can form myelinated axons and both efferent and afferent synapses with xenogeneic rat neurons. J Neurosci Res. 2003;72:661–9. doi: 10.1002/jnr.10628. [DOI] [PubMed] [Google Scholar]
- 11.An YH, Wan H, Zhang ZS, Wang HY, Gao ZX, Sun MZ, Wang ZC. Effect of rat Schwann cell secretion on proliferation and differentiation of human neural stem cells. Biomed Environ Sci. 2003;16:90–4. [PubMed] [Google Scholar]
- 12.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–33. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9:65–75. doi: 10.1111/j.1460-9568.1997.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 14.Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, Neugebauer E, Snyder EY, McIntosh TK. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51:1043–52. doi: 10.1097/00006123-200210000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett. 2004;361:168–71. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]


