Abstract
Background:
For patients with localized esophageal cancer (EC) who can withstand surgery, the preferred therapy is chemoradiation followed by surgery (trimodality). However, after achieving a clinical complete response [clinCR; defined as both post-chemoradiation endoscopic biopsy showing no cancer and physiologic uptake by positron emission tomography (PET)], some patients decline surgery. The literature on the outcome of such patients is sparse.
Method:
Between 2002 and 2011, we identified 622 trimodality-eligible EC patients in our prospectively maintained databases. All patients had to be trimodality eligible and must have completed preoperative staging after chemoradiation that included repeat endoscopic biopsy and PET among other routine tests.
Results:
Out of 622 trimodality-eligible patients identified, 61 patients (9.8%) declined surgery. All 61 patients had a clinCR. The median age was 69 years (range 47–85). Males (85.2%) and Caucasians (88.5%) were dominant. Baseline stage was II (44.2%) or III (52.5%), and histology was adenocarcinoma (65.6%) or squamous cell carcinoma (29.5%). Forty-two patients are alive at a median follow-up of 50.9 months (95% CI 39.5–62.3). The 5-year overall and relapse-free survival rates were 58.1 ± 8.4 and 35.3 ± 7.6%, respectively. Of 13 patients with local recurrence during surveil-lance, 12 had successful salvage resection.
Conclusion:
Although the outcome of 61 EC patients with clinCR who declined surgery appears reasonable, in the absence of a validated prediction/prognosis model, surgery must be encouraged for all trimodality-eligible patients.
Keywords: Esophageal cancer, Trimodality, Surgery, Chemoradiation, Outcomes
Introduction
The estimated number of new cases of upper gastrointestinal cancers in the United States in 2011 is 38,500, with a high fatality rate (estimated 25,050 deaths) [1]. It is also a global health problem with approximately 482,000 new esophageal cancer (EC) cases with high mortality (84%) around the world in 2008 [2].
In North America, trimodality (chemoradiation followed by surgery) is often recommended for localized (clinical stage II or III) EC patients if they can withstand surgery [3, 4]. However, surgical resection is not always possible following recovery from chemoradiation therapy due to the deterioration of the general condition in some patients, the recommendation of the surgical team to delay surgery, or the patients’ refusal to undergo surgery. The literature lacks information about the fate of patients who decline surgery. There are no optimally powered randomized trials reported to suggest that surgery can be safely avoided in certain patients after chemo-radiation. Finally, there are no models based on clinical variables or biomarkers to segregate patients for specific therapies. In patients with squamous cell carcinoma, two randomized trials provide some guidance on the utility of different components of multimodality therapies but not without shortcomings [3, 5]. In this report, we review the outcome of one of the largest cohorts of trimodality-eligible patients who declined surgery.
Patients and Methods
Objective
The primary objective of this study was to characterize the outcome of trimodality-eligible patients who completed chemo-radiation but declined to proceed with surgery.
Patient Selection
We searched prospectively maintained EC databases of the Departments of Gastrointestinal Medical Oncology and Thoracic and Cardiovascular Surgery at the University of Texas MD Anderson Cancer Center (UTMDACC) between 2002 and 2011. Patients with localized, histologically confirmed gastroesophageal junction or EC were eligible. All patients had extensive baseline staging included a computed tomography, positron emission tomography (PET), esophagogastroduodenoscopy, and endoscopic ultrasonography. Trimodality-eligible patients were defined as having (1) technically resectable cancer and (2) physiologic ability to withstand surgery. All patients were evaluated by a multidisciplinary team and discussed in the conference that consisted of medical oncologists, thoracic surgical oncologists, radiation oncologists, gastroenterologists, pathologists, and many supporting team personnel. The UTMDACC Institutional Review Board approved this analysis.
Chemoradiotherapy
All patients had chemoradiation. Concurrent chemotherapy consisted of fluoropyrimidine (intravenous or oral) and a second drug which was either a platinum compound or taxane. The median total radiation dose was 50.4 Gy (range 39–66), in daily fractions of 1.8 Gy.
Assessments after Chemoradiotherapy
Approximately 5–6 weeks after the completion of chemoradiation, patients had preoperative staging workup that included an endoscopic biopsy and a PET. Patients were then assigned to one of two categories: clinical complete response (clinCR) group or less than clinical complete response (<clinCR) group. We defined clinCR as having a negative endoscopic biopsy for cancer and a physiologic range of the glucose uptake by PET. The details of this definition were referred to those published from our institution [6, 7].
Follow-Up and Survival
Patients were followed periodically until 5 years or death. Follow-up data were obtained from the UTMDACC tumor registry and the hospital records or social security database.
Statistical Analysis
The Kaplan-Meier method was used to estimate the probability of survival analyses. Survival time or relapse-free time was defined as the time from the date of initial treatment to the event. If an event date was not available, the date of the last follow-up was taken. All statistical calculations were performed using IBM SPSS statistics 19.0.
Results
Patient Characteristics
We identified 622 trimodality-eligible EC patients in our database. A total of 425 (68.3%) of 622 patients achieved a clinCR after preoperative chemoradiation. However, of the entire population of 622 patients, 61 (9.8%) declined surgery despite our recommendations. All 61 patients who declined surgery had a clinCR. Table 1 shows the characteristics of these 61 patients. Males and Caucasians were dominant. Most patients had adenocarcinoma (n = 40; 65.6%) or squamous cell carcinoma (n = 18; 29.5%). At baseline endoscopic ultrasonography staging, the frequency of stage II and III cancers was 44.2 and 55.2%, respectively. Eighteen patients (29.5%) received two cycles of induction chemotherapy prior to preoperative chemoradiation.
Table 1.
Patients characteristics (n = 61)
| Characteristics | Patients | % |
|---|---|---|
| Age, years | ||
| Median | 69 | |
| Range | 47–85 | |
| Gender | ||
| Male | 52 | 85.2 |
| Female | 9 | 14.8 |
| Race | ||
| Caucasian | 54 | 88.5 |
| Asian | 4 | 6.6 |
| Black | 2 | 3.3 |
| Arabic | 1 | 1.6 |
| Histology | ||
| Adenocarcinoma | 40 | 65.6 |
| Squamous cell carcinoma | 18 | 29.5 |
| Adenosquamous carcinoma | 1 | 1.6 |
| Neuroendocrine carcinoma | 2 | 3.3 |
| Primary site | ||
| AEG1 | 26 | 42.6 |
| AEG2 | 19 | 31.1 |
| Esophagus | 16 | 26.2 |
| Tumor grade | ||
| Moderate | 31 | 50.8 |
| Poor | 30 | 49.2 |
| Baseline T stage | ||
| T1 | 4 | 6.6 |
| T2 | 7 | 11.5 |
| T3 | 49 | 80.3 |
| T4a | 1 | 1.6 |
| Baseline N stage | ||
| N0 | 26 | 42.6 |
| N1 | 34 | 55.8 |
| N2 | 1 | 1.6 |
| Baseline stage | ||
| IB | 2 | 3.3 |
| IIA | 5 | 8.1 |
| IIB | 22 | 36.1 |
| IIIA | 27 | 44.3 |
| IIIB | 4 | 6.6 |
| IIIC | 1 | 1.6 |
| AEG = Adenocarcinoma of the esophagogastric junction. |
Overall Survival
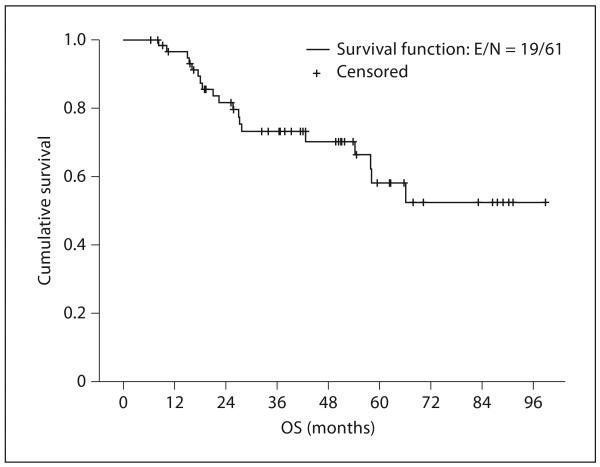
After a median follow-up time of 50.9 months (range 6.5–98.8), 19 patients (31.1%) have died. Thus, the median overall survival (OS) for all 61 patients has not been reached. Two of 19 patients had non-EC-related deaths. The estimated 5-year OS rate was 58.1% (95% CI 41.3–74.9; fig. 1).
Fig. 1.
Kaplan-Meier overall survival plot for all 61 patients. OS denotes overall survival.
Relapse-Free Survival
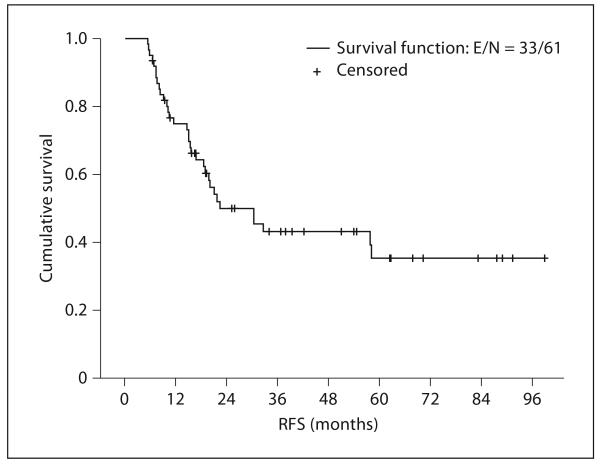
Thirty-three patients (54.1%) have had documented recurrences. Among these, 13 (39.4%) had local recurrence and 20 (60.6%) had evidence of metastatic cancer. The median relapse-free survival (RFS) was 22.4 months (95% CI 9.4–35.3), and the estimated 5-year RFS rate was 35.3% (95% CI 20.1–50.5; fig. 2). In total, 23 of 40 adenocarcinoma patients and 7 of 18 squamous cell carcinoma patients relapsed. The median RFS of the adenocarcinoma cohort was 22 months and that of the squamous cell carcinoma group was 58 months. The median dose of radiation in relapsing and non-relapsing patients was the same.
Fig. 2.
Kaplan-Meier relapse-free survival plot for all 61 patients. RFS denotes relapse-free survival.
Salvage Surgery
Among the 13 patients with local-regional recurrence, 12 patients (92.3%) underwent salvage surgery. Salvage surgery was defined as the delayed esophagectomy performed upon documentation of local-regional-only recurrence that occurred more than 3 months following the completion of chemoradiation. In this salvaged population, the median time from the end date of chemoradiation to surgery was 9.3 months (range 4.9–31.4). Salvage surgery in all 12 patients led to an R0 resection. Three patients had EC and 9 had type I or II cancer. Three patients had squamous cell carcinoma. The most common surgery was Ivor-Lewis (n = 10). One patient had an anastomotic leak, 1 had sepsis, 2 had pneumonia, and 1 died as a result of surgery. At this writing, 9 of 12 patients with salvage surgery were still alive.
Discussion
For patients with potentially resectable cancer of the esophagus, three strategies are in use: primary surgery, preoperative chemotherapy, and preoperative chemoradiation. Preoperative chemoradiation provides the strongest evidence for OS [8]. However, a small fraction of patients who are initially recommended a trimodality strategy does not undergo surgery. There are three reasons for patients assigned to trimodality therapy not do undergo surgery: (1) a fraction of patients becomes medically unfit to undergo surgery. Such patients are frail to begin with and chemoradiation is used as the ‘stress test’. (2) Development of metastatic cancer before surgery. These patients have cancers that are already metastatic but need time to manifest. (3) Patients decline to undergo surgery.
Two reports describing randomized trials that compared chemoradiation alone to trimodality therapy shed some light on this issue [3, 5]. Both trials reported that the local control rate was significantly better in the surgery arm than it was for patients who received only chemoradiation (2-year local recurrence-free rate: 64.3 vs. 40.7%; p = 0.003 for Stahl et al. [3] and 66.4 vs. 57.0%; p = 0.0014 for Bedenne et al. [5]), but the OS were not different (3-year OS rate: 31.3 vs. 24.4%; p = 0.02 and 33.6 vs. 39.8%; p = 0.04, respectively). However, both of these trials were underpowered and did not specifically ask a direct question. Furthermore, these trials were conducted in a predominantly squamous cell carcinoma patient population.
The majority of patients in our study are not disease free, although the survival is respectable. The rate of metastatic EC in relapsing patients is similar to that observed in trimodality patients. The better-than-expected OS is likely due to the selection bias. All patients achieved a clinCR and ended up declining surgery. Better OS is also contributed by successful salvage surgery performed in 12 of 13 patients.
There are obvious strengths and weaknesses in our report. The weaknesses include: (i) retrospective analysis, (ii) small series, and (iii) the absence of a validated or structured approach to clinical decisions. The strengths include: (i) first report of EC patients who declined surgery and (ii) salvage surgery data in patients who declined surgery.
In conclusion, achievement of clinCR after preoperative chemoradiation provides an enticement for some patients to decline surgery; however, RFS data are not satisfactory and local recurrence rates are too high. Although we can expect a low rate cure from definitive chemoradiation [9], the results of the CROSS trial also support the role of surgery in this population [8]. Most importantly, we lack any model based on clinical variables and/or bio-markers to make smart clinical decisions. Until we have such tools, we strongly recommend surgery for all trimodality-eligible patients.
Funding
This work was supported in part by the Dallas, Park, Smith, and Cantu family funds, the Kevin Fund, the Sultan Fund, the River Creek Foundation, and the Aaron and Martha Schecter Private Foundation. This work was also supported by the Multidisciplinary Research Program at MD Anderson and by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 5.Bedenne L, Michel P, Bouche O, et al. Chemo-radiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A, Xiao L, Hayashi Y, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer. 2011;117:4823–4833. doi: 10.1002/cncr.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javeri H, Xiao L, Rohren E, et al. Influence of the baseline 18F-fluoro-2-deoxy-D-glucose positron emission tomography results on survival and pathologic response in patients with gastroesophageal cancer undergoing chemoradiation. Cancer. 2009;115:624–630. doi: 10.1002/cncr.24056. [DOI] [PubMed] [Google Scholar]
- 8.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01) Radiation Therapy Oncology Group. Jama. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]