Abstract
Background
Borderline personality disorder (BPD) and substance use disorders frequently cooccur; their dual presence predicts poor prognosis. The genetic underpinnings of BPD have not been well-characterized and could offer insight into comorbidity. The current report focuses on the association of Neurexin 3 (NRXN3) single nucleotide polymorphisms (SNPs) with BPD symptoms in heroin dependent cases and controls.
Methods
The sample of the Comorbidity and Trauma Study, a genetic association study of heroin dependence, consists of Australian heroin dependent cases ascertained from opioid replacement therapy clinics and controls ascertained in nearby economically-disadvantaged neighborhoods. The assessment included a screening instrument for BPD, used previously in Australian population surveys. Genotypic and BPD phenotypic data were available for 1439 cases and 507 controls. We examined the association of 1430 candidate gene SNPs with BPD phenotypes.
Results
One or more NRXN3 SNPs were nominally associated with all BPD phenotypes; however, none met the conservative significance threshold we employed to correct for multiple testing. The most strongly associated SNPs included rs10144398 with identity disturbance (p=4.9 × 10−5) and rs10151731 with affective instability (p=8.8 × 10−5). The strongest association with screening positive for BPD was found for the NRXN3 SNP, rs10083466 (p=.0013). Neither the correlation of BPD phenotypes nor the linkage disequilibrium relationships of the SNPs account for the number of observed associations involving NRXN3 SNPs.
Conclusions
Our findings provide intriguing preliminary evidence for the association of NRXN3 with BPD phenotypes. The strongest associations were found for traits (i.e., affective instability; identity disturbance) also observed with other disorders.
Keywords: borderline personality disorder, NRXN3, genetic association study, heroin dependence
1. Introduction
Borderline personality disorder (BPD) is a public health issue of great concern. Although BPD is a relatively uncommon disorder with a 1–2% general population prevalence (Jackson and Burgess, 2000; Torgersen, 2005), those afflicted have markedly increased morbidity and mortality that includes risk for suicide (Duberstein and Conwell, 1997; Skodol et al., 2002b;; Black et al., 2004).The long term outcome of BPD is characterized by severe and persistent impairment in social functioning (Skodol et al., 2002a; Gunderson et al., 2011) and utilization of substantial mental health resources (Skodol et al., 2002b). Despite its vast impact on the lives of patients, their families and on the health system, our understanding of factors contributing to BPD liability and the treatment options available to patients remain fairly limited (Feurino and Silk, 2011).
Additionally, epidemiological research supports that comorbid substance use disorders, including opioid dependence, are very prevalent in BPD with more than 40% of patients with BPD also having a diagnosis of a drug use disorder (Trull et al., 2000; Zanarini et al., 2011; Trull et al., in press). Borderline traits, and the disorder itself, are also overrepresented in patients with opioid dependence with rates of 44.1% for BPD in patients seeking buprenorphine treatment (Trull et al., 2000; Sansone et al., 2008) and this comorbidity is a predictor of poor outcome (Moos et al., 2001; Darke et al., 2005; Darke et al., 2007). The endogenous opioid system dysfunction hypothesis has been proposed as a potential explanation of the pathophysiology of BPD and the high rates of comorbidity with opioid dependence (Bandelow et al., 2010; New and Stanley, 2010). In support of this hypothesis, differences in μ-opioid receptors and in the function of the endogenous opioid system between patients with BPD and healthy controls have been reported (Prossin et al., 2010). Hence, characterizing the genetic underpinnings of BPD in a sample of cases with heroin dependence may prove to be of particular clinical importance.
Genetic and environmental contributions to the liability to BPD have been estimated (Leichsenring et al., 2011). In twin and family studies, the heritability of the disorder ranged from 35% to 69% (Torgersen et al., 2000; Distel et al., 2008; Kendler et al., 2008; Torgersen et al., 2008). Several candidate gene association studies have been conducted for BPD or for correlated personality traits; however, findings thus far remain unreplicated (Siever et al., 2002; Joyce et al., 2006; Ni et al., 2006; McCloskey et al., 2009; Wilson et al., 2009; Maurex et al., 2010; Nemoda et al., 2010; Perez-Rodriguez et al., 2010; Stoltenberg et al., 2011). The recent revision of the diagnostic classification of psychiatric illness to produce DSM-V included active discussion of a transition from a strictly categorical approach according to which a psychiatric illness is either present or not in any given individual to a more continuous, dimensional approach (Kupfer et al., 2002). This transition has been considered particularly pertinent for personality disorders, including BPD (Regier, 2007), and has been reinforced by a methodological shift in the study of the genetics of BPD where more emphasis has been applied on the identification of endophenotypes (Siever et al., 2002). In this context, most examinations of BPD's genetic underpinnings have traditionally utilized broad, categorical approaches. More recently, studies have examined the heritability of individual borderline personality traits and not the diagnosis per se, based on the rationale that this approach may improve understanding of component phenotypes (Siever et al., 2002; McCloskey et al., 2009).
The current investigation examines the association of single nucleotide polymorphisms (SNPs) with individual borderline personality traits in the Comorbidity and Trauma Study (CATS), a genetic association study of opioid dependence. This report focuses on neurexin 3 (NRXN3) SNPs for which evidence of nominal association was observed for all examined BPD phenotypes. NRXN3 polymorphisms have been previously reported to be associated with substance dependence (Liu et al., 2005; Hishimoto et al., 2007; Lachman et al., 2007; Docampo et al., 2012). A recent study observed an association of NRXN3 polymorphisms with attentional impulsivity and alcohol problems in men (Stoltenberg et al., 2011).
2. Methods
The methods for CATS have been described in detail elsewhere (Maloney et al., 2009; Shand et al., 2011). A brief summary is provided below.
2.1 Sample
Cases were recruited from opioid replacement therapy (ORT) clinics in the greater Sydney region and were required to be age 18 or older, understand English, and have participated in ORT for opioid dependence. Participants reporting recent suicidal intent or current psychosis were excluded. Individuals recruited from geographic areas in proximity to ORT clinics, termed “neighborhood controls,” were excluded for recreational opioid use more than five times lifetime (data were included from 23 controls who denied opioid use more than 5 times at screening, but reported greater use with no dependence symptoms at interview). All other inclusion and exclusion criteria were identical to cases. Data are reported here from heroin dependent cases (N=1439; 39.1% female) and neighborhood controls (N=507; 55.4% female) for whom both genotypic and BPD data were available. The mean age of cases [36.4 years (SD 8.6)] was significantly greater (p=.0006) than that of controls [34.6 years (SD 10.5)]. A significantly greater percentage of controls than cases reported have completed high school and received a diploma (54.4% versus 17.5%; p<.0001). Although both populations are primarily of European ancestry, both groups also contained individuals of Asian ancestry. Institutional review board (IRB) approval was obtained from University of New South Wales, Washington University School of Medicine, Queensland Institute of Medical Research, and all New South Wales area health service ethics committees governing participating clinics. Data were collected between 2004 and 2008. Participants provided written informed consent and were reimbursed AU$50.00.
2.2 Assessment
All interviews were conducted in person by experienced interviewers. Diagnostic sections on illicit drug and alcohol dependence were modified from the Semi-Structured Assessment for the Genetics of Alcoholism - Australia (SSAGA-OZ; Bucholz et al., 1994; Hesselbrock et al., 1999); the nicotine dependence section was modified from the Nicotine Addiction Genetics Study assessment (Saccone et al., 2007; Agrawal et al., 2008). Additionally, a screener for BPD, adopted from the International Personality Examination (IPDE; Loranger et al., 1994), was also administered. It utilizes the ICD-10 criteria to determine whether 10 borderline traits are characteristic of the participant's usual personality (World Health Organization, 1992). The individual BPD items and the two summary phenotypes derived from them are displayed in Table 1. This has also been used in the Australian National Survey of Mental Health and Well-Being and is considered to provide an adequate screening assessment for BPD (Lewin et al., 2005). Participants who endorsed having at least 3 out of the 10 assessed BPD symptoms, and reported that these symptoms persisted for most of their adult life and caused significant interference with their lives and activities, were classified as screening positive for BPD. The threshold of 3 symptoms is based on Loranger and colleagues' work (1994) and has been adapted elsewhere (Lewin et al., 2005; Maloney et al., 2009). An additional measure of self-reported impulsivity, the 30-item Barratt Impulsiveness Scale (BIS; Patton et al., 1995; Maloney et al., 2009; Stanford et al., 2009), was added to the study's assessment protocol after data collection had commenced; it is available on 1521 participants.
Table 1. Borderline Personality Items (True-false) and Prevalence of Endorsement (%).
| I can't decide what kind of person I want to be.(undecided) | 44.9 |
| I go to extremes to try and keep people from leaving me.(extreme) | 28.5 |
| I get into very intense relationships that don't last.(intense) | 34.7 |
| I argue or fight when people try to stop me from doing what I want.(argue) | 56.0 |
| I've never threatened suicide or injured myself on purpose.(threat) | 38.9 |
| I don't stick with a plan if I don't get results right away.(stick) | 45.3 |
| Sometimes I get so angry I break or smash things.(angry) | 38.9 |
| I often feel “empty” inside.(empty) | 62.4 |
| I'm very moody. (moody) | 50.7 |
| I take chances and do reckless things.(reckless) | 57.6 |
| Total symptoms endorsed (sxtotal) | mean 4.6 (SD 2.8) |
| Screened positive (screen)* | 52.3 |
Endorsed ≥3 symptoms, their persistence for most of adult life, and interference from them
2.3 Marker selection
The pair-wise option of Tagger (de Bakker et al., 2005), implemented in Haploview, (Barrett et al., 2005) with a threshold of r2>0.8 for most genes was used to select a custom set of 1536 SNPs. Since NRXN3 is a large gene, we primarily selected SNPs for genotyping that are in relatively low linkage disequilibrium (LD) to provide coverage in an efficient manner with some redundancy allowed for areas where prior reports had observed associations (see Figure 1). The set provided coverage of 72 candidate genes selected on the basis of relevance for heroin dependence, 47 additional SNPs previously reported to be associated with heroin dependence, and 30 ancestry-informative markers (AIMs).
Figure 1.
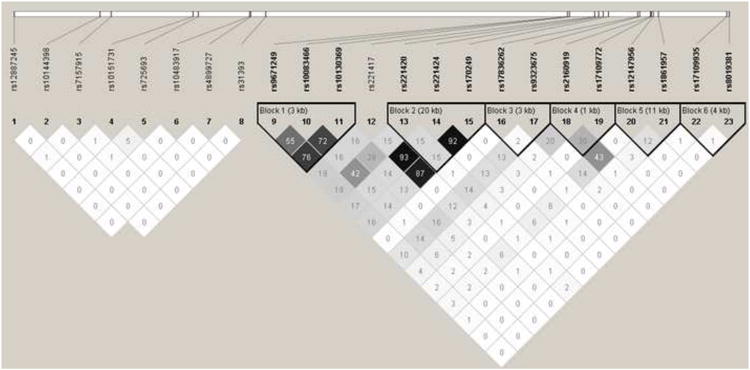
Linkage disequilibrium analysis of select NRXN3 SNPs (r2 values are shown).
2.4 Genotyping
Genotyping was performed on an Illumina BeadStation using GoldenGate technology (Peters et al., 2008). DNA samples from CEPH trio 1334 obtained from the Coriell Cell Repository served as internal quality controls for clustering and reproducibility. Primary genotypic data analyses with Illumina BeadStudio software were followed by visual inspection and assessment of data quality and clustering.
2.5 Statistical Analyses
2.5.1 Data Cleaning
Details of data cleaning have been reported previously (Nelson et al., 2012). The mean call rate for SNPs remaining after data cleaning exceeded 99.9%.The 1430 SNPs that remained after data cleaning included 23 NRXN3 polymorphisms. All 1430 SNPs were examined for association with BPD and related phenotypes (Table 2).
Table 2. NRXN3 SNPs Genotyped.
| SNP | Location | Classification | Minor Allele |
|---|---|---|---|
| rs12887245 | 77901980 | intronic | A |
| rs10144398 | 78081097 | intronic | A |
| rs7157915 | 78103930 | intronic | C |
| rs10151731 | 78275372 | intronic | C |
| rs725693 | 78285563 | intronic | C |
| rs10483917 | 78392399 | intronic | A |
| rs4899727 | 78394606 | intronic | G |
| rs31393 | 78426368 | intronic | G |
| rs9671249 | 79053658 | intronic | G |
| rs10083466 | 79054471 | intronic | A |
| rs10130369 | 79057542 | intronic | A |
| rs221417 | 79111379 | intronic | A |
| rs221420 | 79119291 | intronic | G |
| rs221424 | 79128774 | intronic | G |
| rs170249 | 79139680 | intronic | A |
| rs17836262 | 79200816 | intronic | C |
| rs9323675 | 79204384 | coding | A |
| rs2160919 | 79227549 | intronic | A |
| rs17109772 | 79229104 | intronic | G |
| rs12147956 | 79232192 | intronic | G |
| rs1861957 | 79243988 | intronic | A |
| rs17109935 | 79386064 | intronic | G |
| rs8019381 | 79390336 | intronic | A |
2.5.2 Admixture
Principal components analysis (PCA) was conducted using the smartpca program in the Eigensoft 3.0 package (Patterson et al., 2006) to determine whether correction for admixture was necessary. The kill r2 setting of 0.8 was used to remove SNPs in high LD with others in the panel. Data from 1123 of the 1430 SNPs were retained. PCA found that comparisons of cases to neighborhood controls did not require inclusion of principal components as covariates.
2.5.3 Association
Logistic regression analyses were performed in PLINK (Purcell et al., 2007) to examine the association between the log-additive effects of minor allele dosage and binary outcome measures (i.e., individual BPD items, screening positive for BPD). Linear regression was used to examine association with BPD symptom count. Heroin dependence status was included as a covariate in all analyses, and sex served as an additional covariate for analyses involving × chromosome SNPs. After applying the most conservative correction for multiple testing, a strict Bonferroni correction that assumes all SNPs and BPD variables are independent measures, the significance threshold was revised to 2.91 × 10−6 (i.e., .05/1430 SNPs/12 phenotypic comparisons).
3. Results
The prevalence of BPD symptoms and of screening positive for BPD is shown in Table 1. All symptoms were endorsed at rates far exceeding those of general population samples, as expected. Overall, 58.6% of opioid dependent cases and 34.3% of neighborhood controls screened positively for BPD. The increased prevalence of BPD in these samples has been discussed in more detail previously (Maloney et al., 2009). All symptoms other than “intense relationships” were also more commonly reported by opioid dependent cases than controls. The strongest associations with opioid dependence were found for “emptiness” (OR 2.84; 95%CI 2.31-3.50) and screening positively for BPD (OR 2.71; 95%CI 2.19-3.36). Opioid dependent individuals also endorsed a significantly higher number of BPD symptoms than controls (4.98 versus 3.41; t=11.39; p<0.001). All genetic association analyses thus include a control for opioid dependence status. Compared with anticipated rates in the general population, the neighborhood controls, thus not surprisingly showed an overrepresentation of individuals screening for BPD, as they were drawn from neighborhoods with higher exposure opportunity to and rates of substance use (excluding opioid dependence, but including subjects with dependence on licit and other illicit drugs).
The genetic association analyses found that two NRXN3 SNPs were the strongest observed associations: rs10144398 with “undecided” (i.e., identity disturbance; 4.9 × 10−5) and rs10151731 with “moody” (i.e., affective instability; 8.8x 10−5). Interestingly, one or more NRXN3 SNPs were found to be nominally associated with each examined BPD phenotype (Table 3). The LD relationships (Figure 1) explained some (e.g., involving rs10083466, rs10130369, and rs9671249), but not all (e.g., not rs10144398 and rs17109935) instances in which we found multiple NRXN3 SNPs associated with the same BPD phenotype. Supplementary Tables 1-51 display the 10 SNPs most significantly associated with each dependent variable. Interestingly, NRXN3 SNPs were included among the top hits for 7 of the dependent variables (“screen”, “sxtotal”, “undecided”, “empty”, “moody”, “threat”, “stick”). Several NRXN3 SNPs were associated with multiple BPD phenotypes. For each such SNP, the direction of risk remained the same across phenotypes (Table 4). Although all individual BPD items were significantly correlated (Table 5), the correlations are modest (ranging from 0.15-0.41) and thus did not definitively account for the multiple associations involving individual SNPs. Higher correlations were observed between the individual and summary items (“sxtotal” and “screen”) as well as between the summary items.
Table 3. Association of NRXN3 SNPs with individual and total borderline personality disorder symptoms (p values are shown).
| SNP | angry | argue | empty | extreme | intense | moody | reckless | stick | threat | undecided | sxtotal | screen |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs12887245 | .79 | .13 | .36 | .83 | .07 | .70 | .23 | .005 | .92 | .07 | .06 | .16 |
| rs10144398 | .79 | .99 | .83 | .16 | .15 | .76 | .32 | .016 | .61 | .000049 | .047 | .68 |
| rs7157915 | .74 | .62 | .46 | .88 | .56 | .96 | .66 | .72 | .95 | .31 | .97 | .36 |
| rs10151731 | .70 | .44 | .09 | .48 | .21 | .000088 | .37 | .85 | .59 | .15 | .029 | .22 |
| rs725693 | .95 | .49 | .11 | .10 | .78 | .07 | .037 | .14 | .40 | .78 | .43 | .59 |
| rs10483917 | .17 | .78 | .012 | .95 | .26 | .16 | .15 | .09 | .003 | .82 | .023 | .96 |
| rs4899727 | .007 | .58 | .45 | .74 | .50 | .18 | .08 | .23 | .65 | .27 | .09 | .39 |
| rs31393 | .41 | .26 | .53 | .15 | .13 | .89 | .58 | .70 | .34 | .51 | .15 | .35 |
| rs9671249 | .57 | .51 | .35 | .51 | .51 | .65 | .83 | .21 | .93 | .85 | .74 | .03 |
| rs10083466 | .52 | .42 | .09 | .27 | .35 | .09 | .12 | .25 | .35 | .59 | .046 | .0013 |
| rs10130369 | .86 | .27 | .49 | .59 | .92 | .96 | .93 | .46 | .60 | .80 | .83 | .04 |
| rs221417 | .52 | .13 | .56 | .82 | .99 | .77 | .86 | .76 | .76 | .97 | .81 | .86 |
| rs221420 | .98 | .06 | .82 | .40 | .49 | .52 | .65 | .86 | .29 | .83 | .28 | .24 |
| rs221424 | .34 | .07 | .77 | .82 | .91 | .64 | .76 | .92 | .82 | .86 | .88 | .62 |
| rs170249 | .32 | .09 | .87 | .99 | .69 | .62 | .96 | .82 | .73 | .60 | .87 | .57 |
| rs17836262 | .04 | .48 | .06 | .39 | .12 | .025 | .045 | .004 | .97 | .23 | .006 | .12 |
| rs9323675 | .88 | .03 | .40 | .16 | .26 | .32 | .93 | .07 | .78 | .78 | .61 | .40 |
| rs2160919 | .67 | .67 | .88 | .36 | .62 | .48 | .052 | .35 | .72 | .36 | .68 | .39 |
| rs17109772 | .84 | .38 | .92 | .87 | .08 | .73 | .07 | .71 | .028 | .37 | .23 | .43 |
| rs12147956 | .58 | .11 | .89 | .34 | .46 | .35 | .21 | .50 | .45 | .83 | .55 | .08 |
| rs1861957 | .24 | .19 | .50 | .34 | .67 | .96 | .96 | .09 | .27 | .96 | .99 | .99 |
| rs17109935 | .74 | .53 | .018 | .036 | .08 | .48 | .09 | .008 | .55 | .027 | .035 | .15 |
| rs8019381 | .55 | .82 | .14 | .17 | .04 | .044 | .045 | .44 | .89 | .88 | .08 | .12 |
Table 4. Odds ratios for nominally associated NRXN3 SNPs (beta values provided for sxtotal).
| Phenotype | SNP | OR | SNP | OR | SNP | OR | SNP | OR |
|---|---|---|---|---|---|---|---|---|
| angry | rs4899727 | 0.64 | rs17836262 | 0.81 | ||||
| argue | rs9323675 | 0.81 | ||||||
| empty | rs10483917 | 0.55 | rs17109935 | 1.25 | ||||
| extreme | rs17109935 | 1.22 | ||||||
| intense | rs8019381 | 1.26 | ||||||
| moody | rs10151731 | 1.42 | rs17836262 | 0.80 | rs8019381 | 1.26 | ||
| reckless | rs725693 | 1.15 | rs17836262 | 0.82 | rs8019381 | 1.27 | ||
| stick | rs17836262 | 0.75 | rs12887245 | 1.43 | rs17109935 | 1.26 | rs10144398 | 0.84 |
| threat | rs10483917 | 0.43 | rs17109772 | 1.32 | ||||
| undecided | rs10144398 | 0.75 | rs17109935 | 1.21 | ||||
| screen | rs10083466 | 0.78 | rs10130369 | 0.85 | ||||
| sxtotal | rs17836262 -0.36; rs10483917 -0.71; rs10151731 0.25; rs17109935 0.24; rs10083466 -0.20; rs10144398 -0.19 | |||||||
Table 5. Pearson correlations of individual borderline personality disorder items, screen, and total symptoms*.
| angry | argue | empty | extreme | intense | moody | reckless | stick | threat | undecided | screen | sxtotal | BIS total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| angry | --- | 0.28 | 0.24 | 0.22 | 0.18 | 0.35 | 0.28 | 0.20 | 0.19 | 0.21 | 0.31 | 0.56 | 0.28 |
| argue | --- | 0.24 | 0.25 | 0.25 | 0.30 | 0.31 | 0.23 | 0.15 | 0.19 | 0.35 | 0.57 | 0.28 | |
| empty | --- | 0.29 | 0.28 | 0.41 | 0.27 | 0.29 | 0.28 | 0.33 | 0.53 | 0.64 | 0.33 | ||
| extreme | --- | 0.35 | 0.26 | 0.21 | 0.22 | 0.20 | 0.28 | 0.35 | 0.57 | 0.26 | |||
| intense | --- | 0.25 | 0.25 | 0.21 | 0.17 | 0.24 | 0.35 | 0.56 | 0.27 | ||||
| moody | --- | 0.33 | 0.21 | 0.22 | 0.27 | 0.45 | 0.64 | 0.32 | |||||
| reckless | --- | 0.22 | 0.16 | 0.19 | 0.39 | 0.57 | 0.36 | ||||||
| stick | --- | 0.13 | 0.29 | 0.39 | 0.53 | 0.34 | |||||||
| threat | --- | 0.15 | 0.31 | 0.47 | 0.21 | ||||||||
| undecided | --- | 0.39 | 0.56 | 0.31 | |||||||||
| screen | --- | 0.67 | 0.40 | ||||||||||
| sxtotal | --- | 0.52 | |||||||||||
| BIS total | --- |
All values are significant at p <0.0001
Other genes with SNPs associated with multiple items include GRIN2A, which was represented among the top 10 SNPs for 6 BPD phenotypes. The strongest associations observed for GRIN2A SNPs included rs4782080 with “intense” (p=1.4 × 10−4) and rs7191784 with “angry” (p=8.1 × 10−4). Although only a single strong association involving an NPY SNP was noted, rs3025120 was strongly associated with “threat” (p=1.2 × 10−4; OR 1.91).
4. Discussion
We report evidence of association involving multiple SNPs within the NRXN3 gene and BPD phenotypes. The strongest associations we observed, while not meeting the stringent Bonferroni correction for multiple testing that we conservatively employed, have p values that, in a candidate gene association study, are worthy of further investigation.
Neurexins are presynaptic, transmembrane, cell adhesion proteins that are believed to play an important role in functional synapse formation and maturation (Dean and Dresbach, 2006; Südhof, 2008; Knight et al., 2011). The pre-synaptic neurexins interact widely with post-synaptic neuroligins to stabilize transient points of contact between axons and dendrites (Krueger et al., 2012). Numerous isoforms and splice variants that exist for each neurexin and neuroligan are believed to play important roles in determining key elements of synaptic differentiation, a process that is now recognized to be much more complex than previously appreciated and also involve several other families of proteins (Krueger et al., 2012).
A prior report (Stoltenberg et al., 2011) had observed gender-specific associations of NRXN3 polymorphisms with impulsivity, a cardinal feature of BPD (American Psychiatric Association, 2000) and a well-known risk factor for addiction (Verdejo-García et al., 2008). We performed several post-hoc analyses to determine whether our findings could be explained by the hypothesis that NRXN3 polymorphisms serve as vulnerability factors for impulsivity and thus ultimately for a variety of externalizing disorders, including BPD and substance use disorders. We first determined that BIS total score is significantly correlated with all BPD phenotypes observing the strongest correlations with the two composite measures, sxtotal and screening positively (see Table 5). We next conducted an examination of the association of BIS total score with NRXN3 polymorphisms, and found that two SNPs were nominally associated: rs17836262 (p=0.021) and rs10083466 (p=0.030). Although these associations are modest in magnitude, it is noteworthy that these same SNPs were the most highly associated with the two variables representing broader BPD constructs (the former with sxtotal and the latter with screen). A recent report (Docampo et al., 2012) also observed an association of rs1424850, a NRXN3 SNP in high LD with rs10083466, with smoking behavior. Consistent with our results, the effects of the minor allele were also protective in their report. Since Stoltenberg and colleagues (Stoltenberg et al., 2011) had observed an association involving a NRXN3 SNP, rs11624704, and attentional impulsivity in men, we examined whether any NRXN3 SNPs was associated with BIS attentional impulsivity in our sample. We observed modest nominal association involving rs17109772 (p=0.04) in men and rs10083466 in women (p=0.03). Because rs11624704 is not in strong LD with any SNPs we genotyped, our data cannot address replication of the prior report (Stoltenberg et al., 2011). Three SNPs that we genotyped (rs10151731, rs10144398, and rs725693) are in low-level LD with rs11624704.The strongest associations that we observed involved the former two SNPs; in addition, the latter SNP has the strongest (nominal) association with recklessness in our sample. Overall, the results of these post-hoc analyses suggest that impulsivity and the composite BPD phenotypes are modestly associated with a similar set of NRXN3 SNPs. The strongest associations that we observed involve other single-item phenotypes, moody and undecided, for which impulsivity does not appear to explain our findings. Thus, additional research will be necessary to characterize these phenotypes more clearly.
Prior reports have found evidence of association of NRXN3 SNPs with alcohol, nicotine, and illicit drug use phenotypes (Liu et al., 2005; Hishimoto et al., 2007; Stoltenberg et al., 2011; Docampo et al., 2012). BPD has been observed in epidemiologic and clinical samples to be associated with significant risk for licit and illicit drug dependence. Investigators have posited a role for dysfunction in opioid receptor neurotransmission in BPD (Stanley and Siever, 2010). Despite extensive coverage of opioid receptor and related genes in our study, our findings of scattered nominal associations offer at best minimal support for this hypothesis.
4.1 Limitations
A number of methodological limitations should be considered when interpreting our findings. Foremost, we acknowledge that this investigation was not designed to comprehensively examine BPD liability. The assessment was not meant to diagnose BPD but rather as a screening instrument for the disorder. However, it has been deemed to be an adequate assessment for BPD despite its omission of assessment for DSM-IV criterion 9, which refers to transient, stress-related paranoid ideation or severe dissociative symptoms, and has been used in other studies (Lewin et al., 2005). Additionally, the threshold used for a positive screen (i.e., 3 endorsed symptoms out of a total of 10) is low in comparison to the 5 out of 9 symptoms required by DSM-IV. This low threshold may also be contributing to the somewhat higher than expected prevalence of positive screening for BPD of our samples in comparison to related samples. We conducted a post-hoc factor analysis of BPD symptoms to extract an estimate of variance common to these items and found that a one factor solution fit the data well. When we performed association analyses for the BPD symptom factor score, the results were nearly identical to those for our sxtotal variable (e.g., lowest p value =0.0058 for rs17836262) suggesting that the latter variable is a reasonable composite measure of BPD symptoms. The psychometric properties of the individual true-false BPD items that we used as binary dependent variables have not been carefully examined. Because of these limitations, we adopted what many might consider as overly conservative threshold for statistical significance, reporting even our strongest findings as suggestive of nominal association. We acknowledge that a less conservative approach (e.g., false discovery rate) would have provided a less stringent threshold for statistical significance.
Another important potential limitation is the ability to generalize from our sample of opioid dependent cases and neighborhood controls to clinical or general population samples ascertained explicitly for BPD. Although we acknowledge that our results will need to be replicated in other samples, we also believe that the associations with BPD symptom constructs in a sample not ascertained for BPD may actually be more broadly useful in improving understanding of risk across a spectrum of externalizing disorders with overlapping symptom profiles. It is possible that we may be underestimating the association with correlated phenotypes by including heroin dependence as a covariate in all analyses. Finally, NRXN3 is a large gene for which we genotyped tagged SNPs that were selected to provide reasonable coverage. The relative lack of LD observed across the vast majority of these SNPs precludes performing haplotype-based analyses that could help pinpoint causal variants. It is very likely that additional variants (i.e., not genotyped in the current report) that are more strongly associated with BPD phenotypes exist; however, further investigation will be necessary to identify them.
4.2 Conclusion
In conclusion, our findings provide intriguing preliminary evidence for association of NRXN3 SNPs with multiple BPD phenotypes. Further research and replication of these findings are warranted to determine their true clinical significance.
Supplementary Material
Acknowledgments
The authors would like to thank Anthony Caracella for his work in sample receipt and preparation, Megan Campbell for project coordination, Lisa Bowdler and Sara Smith for their efforts in sample genotyping and Fiona Shand, Michelle Torok, Elizabeth Conroy, Caitlin McCue and Cherie Kam for assistance with data collection.
Role of the Funding Source: This work was supported by the National Institute on Drug Abuse (R01 DA017305 to ECN), the National Drug and Alcohol Research Centre and the Australian National Health and Medical Research Council (to LD). The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Author Disclosures: Conflict of Interest: The authors have no conflicts of interest.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors: ECN, MTL, ACH, LD, NGM, and GWM were responsible for the study concept and design. ECN performed the data analyses. AA assisted with data analysis and interpretation of findings. VNP and ECN drafted the manuscript. TJT, ALG, MTL, ACH, AA, AKH, LW, AAT, PAFM, EM, LD, NGM, and GWM provided critical revision of the manuscript and contributed to its intellectual content. All authors critically reviewed content and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Pergadia ML, Saccone SF, Lynskey MT, Wang JC, Martin NG, Statham D, Henders A, Campbell M, Garcia R, Broms U, Todd RD, Goate AM, Rice J, Kaprio J, Heath AC, Montgomery GW, Madden PAF. An autosomal linkage scan for cannabis use disorders in the nicotine addiction genetics project. Arch Gen Psychiatry. 2008;65:713–721. doi: 10.1001/archpsyc.65.6.713. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSMIV-TR. Fourth-Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychol Rev. 2010;117:623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Black DW, Blum N, Pfohl B, Hale N. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18:226–239. doi: 10.1521/pedi.18.3.226.35445. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Williamson A, Mills KL, Havard A, Teesson M. Borderline personality disorder and persistently elevated levels of risk in 36-month outcomes for the treatment of heroin dependence. Addiction. 2007;102:1140–1146. doi: 10.1111/j.1360-0443.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Williamson A, Teesson M. The impact of borderline personality disorder on 12-month outcomes for the treatment of heroin dependence. Addiction. 2005;100:1121–1130. doi: 10.1111/j.1360-0443.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, Willemsen G, Boomsma DI. Heritability of borderline personality disorder features is similar across three countries. Psychol Med. 2008;38:1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Docampo E, Ribasés M, Gratacòs M, Bruguera E, Cabezas C, Sánchez-Mora C, Nieva G, Puente D, Argimon-Pallàs JM, Casas M, Rabionet R, Estivill X. Association of Neurexin 3 polymorphisms with smoking behavior. Genes Brain Behav. 2012;11:704–711. doi: 10.1111/j.1601-183X.2012.00815.x. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Conwell Y. Personality disorders and completed suicide: a methodological and conceptual review. Clin Psychol(NY) 1997;4:359–376. [Google Scholar]
- Feurino L, 3rd, Silk KR. State of the art in the pharmacologic treatment of borderline personality disorder. Curr Psychiatry Rep. 2011;13:69–75. doi: 10.1007/s11920-010-0168-9. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Stout RL, McGlashan TH, Shea MT, Morey LC, Grilo CM, Zanarini MC, Yen S, Markowitz JC, Sanislow C, Ansell E, Pinto A, Skodol AE. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68:827–837. doi: 10.1001/archgenpsychiatry.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hishimoto A, Liu QR, Drgon T, Pletnikova O, Walther D, Zhu XG, Troncoso JC, Uhl GR. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- Jackson HJ, Burgess PM. Personality disorders in the community: a report from the Australian National Survey of Mental Health and Wellbeing. Soc Psychiatry Psychiatr Epidemiol. 2000;35:531–538. doi: 10.1007/s001270050276. [DOI] [PubMed] [Google Scholar]
- Joyce PR, McHugh PC, McKenzie JM, Sullivan PF, Mulder RT, Luty SE, Carter JD, Frampton CM, Cloninger CR, Miller AM, Kennedy MA. A dopamine transporter polymorphism is a risk factor for borderline personality disorder in depressed patients. Psychol Med. 2006;36:807–813. doi: 10.1017/S0033291706007288. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Røysamb E, Tambs K, Torgersen S, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Arch Gen Psychiatry. 2008;65:1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D, Xie W, Boulianne G. Neurexins and neuroligins: recent insights from invertebrates. Mol Neurobiol. 2011;44:426–440. doi: 10.1007/s12035-011-8213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol. 2012;22:412–422. doi: 10.1016/j.conb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, First MB, Regier DA, editors. A research agenda for DSM-V. American Psychiatric Association; Washington, D.C: 2002. [Google Scholar]
- Lachman HM, Fann CS, Bartzis M, Evgrafov OV, Rosenthal RN, Nunes EV, Miner C, Santana M, Gaffney J, Riddick A, Hsu CL, Knowles JA. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Hum Mol Genet. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. Lancet. 2011;377:74–84. doi: 10.1016/S0140-6736(10)61422-5. [DOI] [PubMed] [Google Scholar]
- Lewin TJ, Slade T, Andrews G, Carr VJ, Hornabrook CW. Assessing personality disorders in a national mental health survey. Soc Psychiatry Psychiatr Epidemiol. 2005;40:87–98. doi: 10.1007/s00127-005-0878-1. [DOI] [PubMed] [Google Scholar]
- Liu QR, Drgon T, Walther D, Johnson C, Poleskaya O, Hess J, Uhl GR. Pooled association genome scanning: validation and use to identify addiction vulnerability loci in two samples. Proc Natl Acad Sci USA. 2005;102:11864–11869. doi: 10.1073/pnas.0500329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger AW, Sartorius N, Andreoli A, Berger P, Buchheim P, Channabasavanna SM, Coid B, Dahl A, Diekstra R, Ferguson B, Jacobsberg L, Mombour W, Pull C, Ono Y, Regier D. The International Personality Disorder Examination: The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215–224. doi: 10.1001/archpsyc.1994.03950030051005. [DOI] [PubMed] [Google Scholar]
- Maloney E, Degenhardt L, Darke S, Nelson EC. Impulsivity and borderline personality as risk factors for suicide attempts among opioid-dependent individuals. Psychiatry Res. 2009;169:16–21. doi: 10.1016/j.psychres.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurex L, Zaboli G, Ohman A, Asberg M, Leopardi R. The serotonin transporter gene polymorphism (5-HTTLPR) and affective symptoms among women diagnosed with borderline personality disorder. Eur Psychiatry. 2010;25:19–25. doi: 10.1016/j.eurpsy.2009.05.001. [DOI] [PubMed] [Google Scholar]
- McCloskey MS, New AS, Siever LJ, Goodman M, Koenigsberg HW, Flory JD, Coccaro EF. Evaluation of behavioral impulsivity and aggression tasks as endophenotypes for borderline personality disorder. J Psychiatr Res. 2009;43:1036–1048. doi: 10.1016/j.jpsychires.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS, Finney JW. Predictors of deterioration among patients with substance-use disorders. J Clin Psychol. 2001;57:1403–1419. doi: 10.1002/jclp.1105. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand FL, Henders AK, Wallace L, Todorov AA, Schrage AJ, Madden PAF, Degenhardt L, Martin NG, Montgomery GW. Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00445.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoda Z, Lyons-Ruth K, Szekely A, Bertha E, Faludi G, Sasvari-Szekely M. Association between dopaminergic polymorphisms and borderline personality traits among at-risk young adults and psychiatric inpatients. Behav Brain Funct. 2010;6:1–11. doi: 10.1186/1744-9081-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Stanley B. An opioid deficit in borderline personality disorder: self-cutting, substance abuse, and social dysfunction. Am J Psychiatry. 2010;167:882–885. doi: 10.1176/appi.ajp.2010.10040634. [DOI] [PubMed] [Google Scholar]
- Ni X, Chan K, Bulgin N, Sicard T, Bismil R, McMain S, Kennedy JL. Association between serotonin transporter gene and borderline personality disorder. J Psychiatr Res. 2006;40:448–453. doi: 10.1016/j.jpsychires.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez MM, Weinstein S, New AS, Bevilacqua L, Yuan Q, Zhou Z, Hodgkinson C, Goodman M, Koenigsberg HW, Goldman D, Siever LJ. Tryptophan-hydroxylase 2 haplotype association with borderline personality disorder and aggression in a sample of patients with personality disorders and healthy controls. J Psychiatr Res. 2010;44:1075–1081. doi: 10.1016/j.jpsychires.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K, Wiltshire S, Henders AK, Dragovic M, Badcock JC, Chandler D, Howell S, Ellis C, Bouwer S, Montgomery GW, Palmer LJ, Kalaydjieva L, Jablensky A. Comprehensive analysis of tagging sequence variants in DTNBP1 shows no association with schizophrenia or with its composite neurocognitive endophenotypes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1159–1166. doi: 10.1002/ajmg.b.30741. [DOI] [PubMed] [Google Scholar]
- Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167:925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA. Dimensional approaches to psychiatric classification: refining the research agenda for DSM-V: an introduction. Int J Methods Psychiatr Res. 2007;16(1):S1–S5. doi: 10.1002/mpr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, Agrawal A, Dick DM, Heath AC, Todorov AA, Maunu H, Heikkila K, Morley KI, Rice JP, Todd RD, Kaprio J, Peltonen L, Martin NG, Goate AM, Madden PAF. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. Am J Hum Genet. 2007;80:856–866. doi: 10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone RA, Whitecar P, Wiederman MW. The prevalence of borderline personality among buprenorphine patients. Int J Psychiatry Med. 2008;38:217–226. doi: 10.2190/PM.38.2.h. [DOI] [PubMed] [Google Scholar]
- Shand FL, Degenhardt L, Slade T, Nelson EC. Sex differences amongst dependent heroin users: histories, clinical characteristics and predictors of other substance dependence. Addict Behav. 2011;36:27–36. doi: 10.1016/j.addbeh.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Torgersen S, Gunderson JG, Livesley WJ, Kendler KS. The borderline diagnosis III: identifying endophenotypes for genetic studies. Biol Psychiatry. 2002;51:964–968. doi: 10.1016/s0006-3223(02)01326-4. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS, Grilo CM, Shea MT, Zanarini MC, Morey LC, Sanislow CA, Oldham JM. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002a;159:276–283. doi: 10.1176/appi.ajp.159.2.276. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personaltit structure. Biol Psychiatry. 2002b;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, J HP. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Individ Dif. 2009;47:385–395. [Google Scholar]
- Stanley B, Siever LJ. The interpersonal dimension of borderline personality disorder: toward a neuropeptide model. Am J Psychiatry. 2010;167:24–39. doi: 10.1176/appi.ajp.2009.09050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Lehmann MK, Christ CC, Hersrud SL, Davies GE. Associations among types of impulsivity, substance use problems and neurexin-3 polymorphisms. Drug Alcohol Depend. 2011;119:e31–e38. doi: 10.1016/j.drugalcdep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S. Epidemiology. In: Oldham JM, Skodol AE, Bender DS, editors. The American Psychiatric Publishing Textbook of Personality Disorders. American Psychiatric Publishing; Washington, D.C: 2005. pp. 129–141. [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Røysamb E, Neale MC, Kendler KS. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Lygren S, Øien PA, Skre I, Onstad S, Edvardsen J, Tambs K, Kringlen E. A twin study of personality disorders. Compr Psychiatry. 2000;41:416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher J, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Brown WC, Tomko RL, Schaefer L, Jahng S. Substance use disorders and personality disorders. In: Sherk K, editor. Oxford Handbook of Substance Use Disorders. Oxford University Press; New York: In Press. [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wilson ST, Stanley B, Brent DA, Oquendo MA, Huang YY, Mann JJ. The tryptophan hydroxylase-1 A218C polymorphism is associated with diagnosis, but not suicidal behavior, in borderline personality disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:202–208. doi: 10.1002/ajmg.b.30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (WHO) ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. World Health Organisation; Geneva: 1992. [Google Scholar]
- Zanarini MC, Frankenbur FR, Weingeroff JL, Reich DB, Fitzmaurice GM, Weiss RD. The course of substance use disorders in patients with borderline personality disorder and Axis II comparison subjects: a 10-year follow-up study. Addiction. 2011;106:342–348. doi: 10.1111/j.1360-0443.2010.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


