Abstract
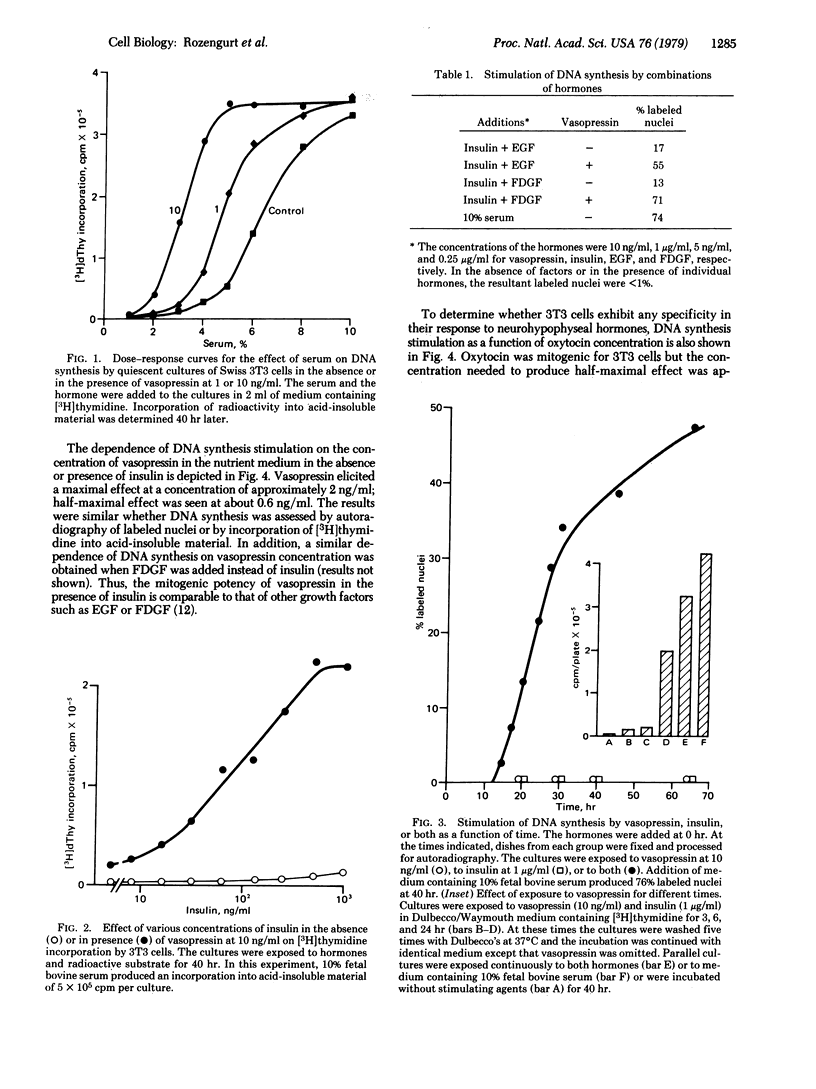
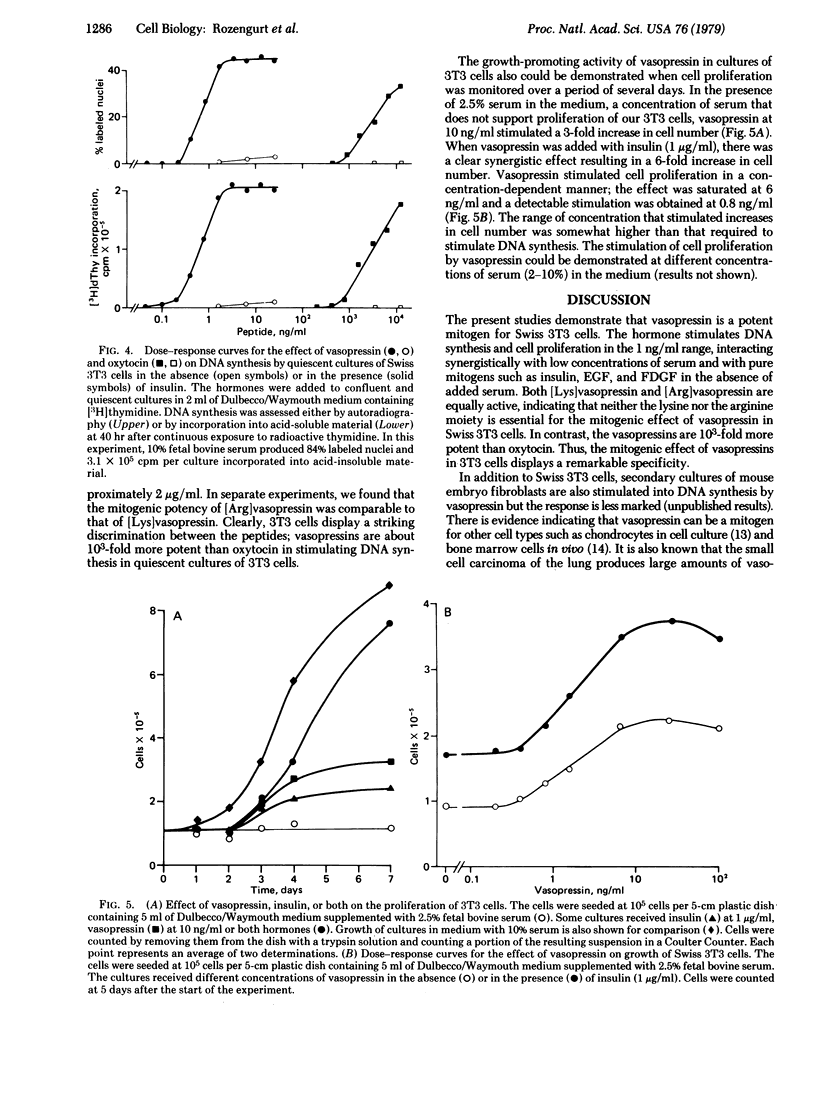
Vasopressin is shown to be a potent mitogen for Swiss 3T3 cells. The hormone (1--10 ng/ml) causes a striking shift of the dose--response curve for the effect of serum on thymidine incorporation by cultures of 3T3 cells arrested in the G1/G0 phase of the cell cycle. In the absence of added serum, the effect of vasopressin on DNA synthesis is greatly potentiated by insulin, epidermal growth factor, and a factor isolated from medium conditioned by simian virus 40-infected baby hamster kidney cells. The mitogenic effect of vasopressin is dependent on time and hormone concentration. In the presence of insulin, the half-maximal effect elicited by the peptide is obtained at 0.6 ng/ml. [Arg]Vasopressin and [Lys]vasopressin are equally potent. The vasopressins are 10(3)-fold more potent than oxytocin. In the presence of a low (2.5%) concentration of serum, vasopressins stimulate cell proliferation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P. Cyclic nucleotides in the cellular action of neurohypophyseal hormones. Fed Proc. 1977 May;36(6):1867–1871. [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Hechter O., Terada S., Spitsberg V., Nakahara T., Nakagawaga S. H., Flouret G. Neurohypophyseal hormone-responsive renal adenylate cyclase. III. Relationship between affinity and intrinsic activity in neurohypophyseal hormones and structural analogs. J Biol Chem. 1978 May 10;253(9):3230–3237. [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Hunt N. H., Perris A. D., Sandford P. A. Role of vasopressin in the mitotic response of rat bone marrow cells to haemorrhage. J Endocrinol. 1977 Jan;72(1):5–16. doi: 10.1677/joe.0.0720005. [DOI] [PubMed] [Google Scholar]
- Jard S., Roy C., Barth T., Rajerison R., Bockaert J. Antidiuretic hormone-sensitive kidney adenylate cyclase. Adv Cyclic Nucleotide Res. 1975;5:31–52. [PubMed] [Google Scholar]
- Mierzejewski K., Rozengurt E. Vitamin B12 enhances the stimulation of DNA synthesis by serum in resting cultures of 3T6 cells. Exp Cell Res. 1977 May;106(2):394–397. doi: 10.1016/0014-4827(77)90187-2. [DOI] [PubMed] [Google Scholar]
- Miller R. P., Husain F., Svensson M., Lohin S. Enhancement of [3H-methyl]thymidine incorporation and replication of rat chondrocytes grown in tissue culture by plasma, tissue extracts and vasopressin. Endocrinology. 1977 May;100(5):1365–1375. doi: 10.1210/endo-100-5-1365. [DOI] [PubMed] [Google Scholar]
- Moore G., Lutterodt A., Burford G., Lederis K. A highly specific antiserum for arginine vasopressin. Endocrinology. 1977 Nov;101(5):1421–1435. doi: 10.1210/endo-101-5-1421. [DOI] [PubMed] [Google Scholar]
- Pettengill O. S., Faulkner C. S., Wurster-Hill D. H., Maurer L. H., Sorenson G. D., Robinson A. G., Zimmerman E. A. Isolation and characterization of a hormone-producing cell line from human small cell anaplastic carcinoma of the lung. J Natl Cancer Inst. 1977 Mar;58(3):511–518. doi: 10.1093/jnci/58.3.511. [DOI] [PubMed] [Google Scholar]
- Primack A. The production of markers by bronchogenic carcinoma: a review. Semin Oncol. 1974 Sep;1(3):235–244. [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L. Increased ouabain-sensitive 86Rubidium uptake after mitogenic stimulation of quiescent chicken embryo fibroblasts with purified multiplication-stimulating activity. J Cell Biol. 1977 Jun;73(3):761–767. doi: 10.1083/jcb.73.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Lithium transport by fibroblastic mouse cells: characterization and stimulation by serum and growth factors in quiescent cultures. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):441–449. doi: 10.1002/jcp.1040970319. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Zorgniotti F., Mills B. Potassium transport and content during G1 and S phase following serum stimulation of 3T3 cells. J Cell Physiol. 1977 Jun;91(3):429–440. doi: 10.1002/jcp.1040910313. [DOI] [PubMed] [Google Scholar]


