Abstract
Planarians possess a rudimentary brain with many features in common with vertebrate brains. They also display a remarkable capacity for tissue regeneration including the complete regeneration of the nervous system. Using the induction of planarian seizure-like movements (pSLMs) as a behavioral endpoint, we demonstrate that an intact nervous system is necessary for this organism to react to cocaine exposure, but not necessary to react to nicotine administration. Decapitated planarians (Girardia tigrina) display pSLMs indistinguishable from intact worms when exposed to nicotine, but cocaine-induced pSLMs are reduced by about 95% upon decapitation. Decapitated worms recover their normal sensitivity to cocaine within five days after head amputation. In worms where half of the brain was removed or partially dissected, the expression of cocaine-induced pSLMs was reduced by approximately 75 %. Similar amputations at the level of the tail did not show a significant decrease to cocaine exposure. To the best of our knowledge, our work is the first report that explores how regenerating planarians react to the exposure of cocaine.
Keywords: planaria, nicotine, cocaine, regeneration, seizure-like behavior
Planarians are proving to be useful and relevant models to study pharmacological effects in biological systems; they represent the earliest extant example of organisms displaying cephalization, including a brain which possesses many features in common with more advanced nervous systems. These commonalities include, among others, the general morphology and physiology of their neurons, the presence of dendritic spines and the use of several major neurotransmitter systems described in mammals, including humans (Cebrià, 2007; Sarnat and Netsky, 1985). The planarian brain is sometimes referred to as cerebral ganglia or cephalic ganglia (Newmark and Sanchez Alvarado, 2001; Sarnat and Netsky, 1985).
Many species of planaria also display a unique characteristic, a remarkable capacity for regeneration. This includes complete morphological and functional regeneration of their nervous system, including their brain. There is plenty of information about the molecular basis of the development and morphogenesis of the regenerating brain in planarians (Cebrià, 2007; Gentile et al., 2011; Umesono et al., 2011) and about the recovery of behavioral functions during this regenerative process (Inoue et al., 2004; Kato et al., 2004; Nishimura et al., 2007). However, we are not aware of any work relating planarian regeneration with the behavioral effects induced by abused drugs, including nicotine and cocaine.
Given the power of modern molecular techniques, it is easy to forget that the ultimate objective of physiological discoveries is to find their possible significance within the context of the whole organism, particularly its behavior. Recent work has demonstrated the usefulness of two distinct planarian behavioral endpoints in pharmacological experiments, namely the observation of changes in the normal motility of the worms (Raffa et al., 2001) and the induction of planaria seizure-like movements (pSLMs; Rawls et al., 2009, 2011). pSLMs have also been referred as C-like hyperkinesias (Pagán et al., 2008; Palladini et al., 1996) and are characterized by sudden, fast writhing and twitching (hence seizure-like_ instead of the passive gliding that these animals normally display (Figure 1A,B). Both motile behavior and pSLMs are easily quantified and have been used to document the behavioral effects of a wide variety of psychoactive agents and abused drugs, including cocaine, amphetamines, nicotine and opiates among others. Furthermore, the expression of pSLMs can also be prevented in a concentration-dependent manner by a variety of established and novel antagonists of the abused substances listed above. It is unlikely that they represent a nonspecific behavioral response (Pagán et al., 2008; 2012; Ramakrishnan and Desaer, 2011; Ramoz et al., 2012; Rawls et al., 2009, 2010, 2011). Even though cocaine and the cholinergic compound nicotine affect planarian behavior, there are no studies that relate regeneration with these drugs or that explore the recovery of behavioral sensitivity to these drugs in regenerating planarians. In this work, we studied the induction of pSLMs by acute exposure to cocaine or nicotine in intact planarians and in worms where the cephalic ganglia were partially or completely separated. We also measured the regain of behavioral sensitivity to these compounds in regenerating planarians over time. The main purpose of this work is to obtain a first approximation of the relative distribution of the binding sites for cocaine or nicotine that control these seizure-like behaviors in our experimental organism and to determine whether an intact brain is required for these substance to induce such behavioral responses.
Figure 1.
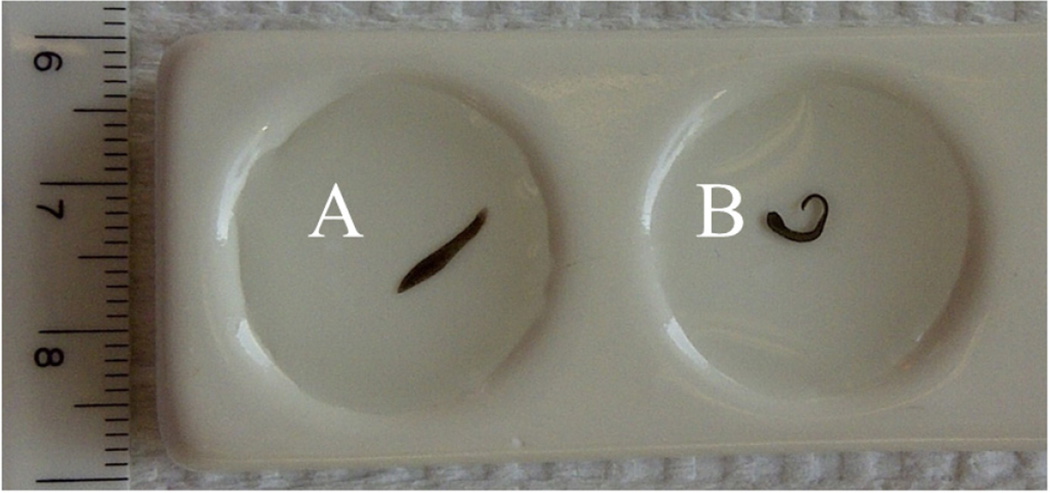
Examples of a control worm (A) and a planarian showing a seizure-like movement (pSLM) in response to cocaine (B). The scale at left is in cm.
EXPERIMENTAL PROCEDURES
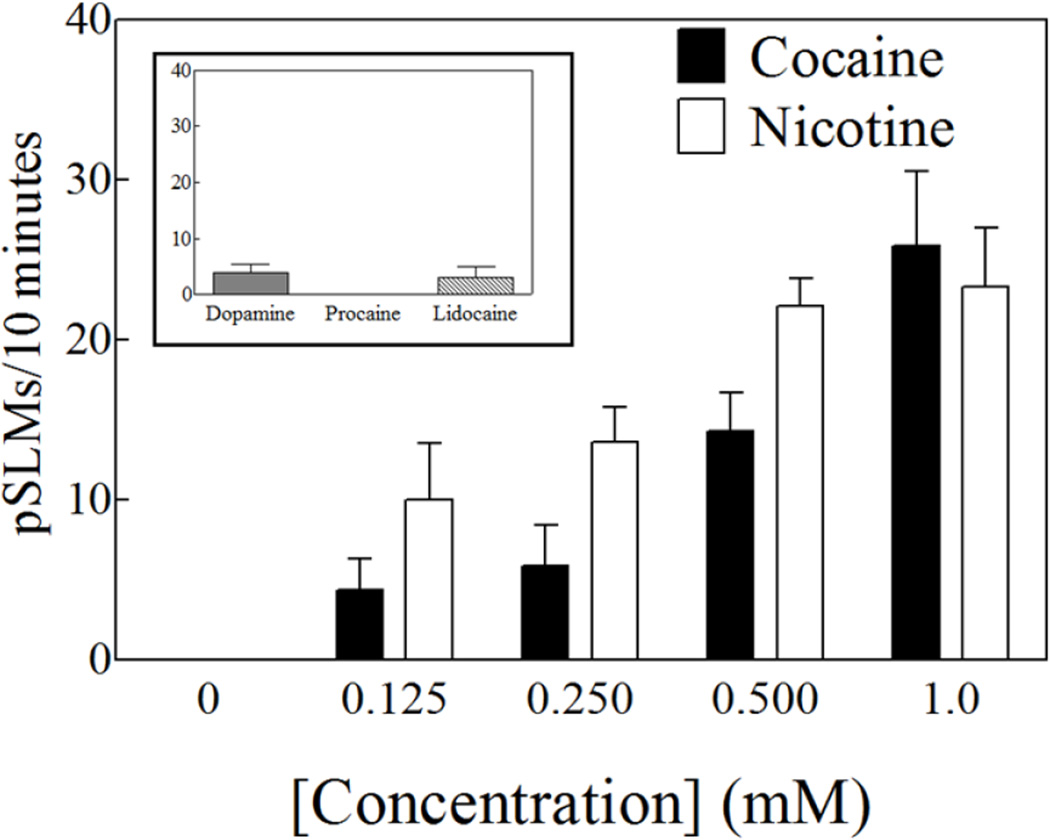
The worms (Girardia tigrina, also known as Dugesia tigrina) were purchased from Ward's (Rochester, NY). The experimental compounds were obtained through Sigma-Aldrich (St. Louis, MO). General laboratory materials were purchased from Fisher Scientific (Suwanee, GA). All graphs and statistical procedures were done using the Prism software package (GraphPad Inc., La Jolla, CA). The experiments were conducted in artificial pond water (APW: NaCl, 6 mM; NaHCO3, 0.1 mM; CaCl2, 0.6 mM) at room temperature. Cocaine and nicotine were tested at a concentration of 1 mM, based on defined plateaus in concentration-response curves of the induced pSLMs as a function of the concentration of either cocaine or nicotine in intact planarians (Pagán et al., 2012). These curves are reproduced in Figure 2. For the decapitation experiments, an equal number of worms (1–1.5 cm long) were placed in separate plastic containers and kept next to each other in the dark. Using a new scalpel, the planarians in one of the containers were decapitated or semi-decapitated depending on the experiment. The planarians in the other container were used intact.
Figure 2.
Concentration-dependent induction of pSLMs by cocaine or nicotine. Each bar is the average of 5–8 worms. The error bars represent the standard error of the mean. This was redrawn from: Pagán et al., 2012, Int J Dev Biol. 56(1–3):193-6. Courtesy of the International Journal of Developmental Biology. Used with permission. Inset: Induction of pSLMs by 1 mM dopamine, procaine or lidocaine. Procaine did not induce any pSLMs at all and lidocaine or dopamine did not significantly induce them either (p = 0.214 and 0.056 respectively, One sample t-test). Each bar is the average of 4–7 worms. The error bars represent the standard error of the mean.
In experiments in which partially decapitated planarians were tested, the worm was gently placed on a piece of filter paper soaked with ice-cold APW and the heads were completely or partially excised under a dissecting microscope. To measure any seizure-like activity induced by cocaine or nicotine, a worm was placed into a well of a ceramic plate (Figure 1A,B) using a soft paintbrush and a 1 mM solution of the experimental compound or APW (control) was added to the well. Any pSLMs (Figure 1B) were visually counted over a period of ten minutes.
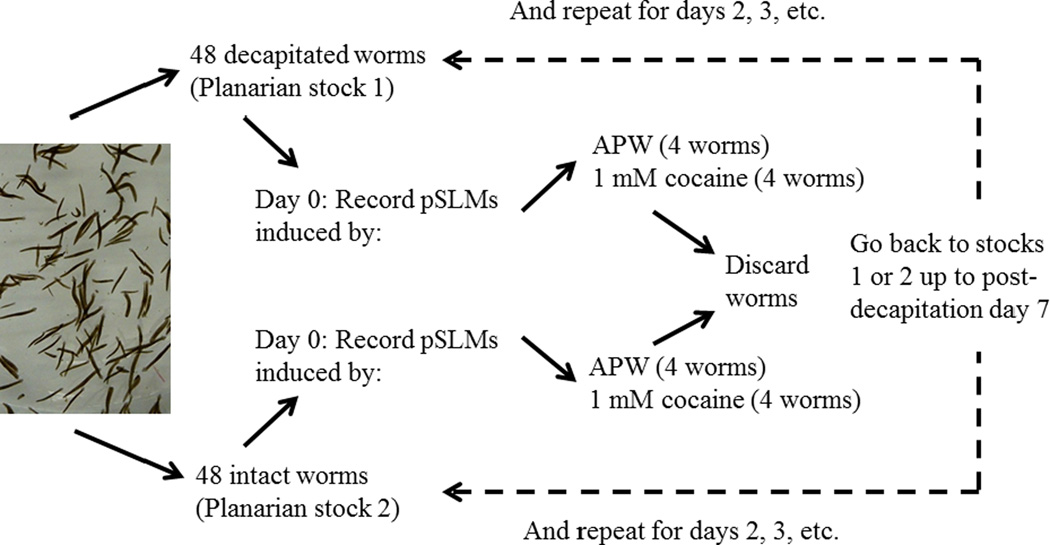
Figure 3 shows the experimental setup to test the recovery of the cocaine-induced pSLMs in regenerating planarians. Briefly, 48 worms were decapitated as described above and set aside (Planarian stock 1). An equal number of intact worms were also set aside for the experiments (Planarian stock 2). The pSLM responses of intact or decapitated planarians exposed to plain APW (control) or exposed to 1 mM cocaine were recorded on the day of decapitation (day 0); these planarians were discarded and the next day this procedure was repeated with planarians taken from stocks 1 (decapitated) or 2 (intact). This was repeated until post-decapitation day 7. Thus, each planarian was acutely exposed to 1 mM cocaine and then discarded. There was no repeated administration. All pSLM measurements were taken between 8–11am.
Figure 3.
Outline of the experimental protocol to assess the recovery of sensitivity to the experimental compounds in regenerating planarians (see text).
RESULTS
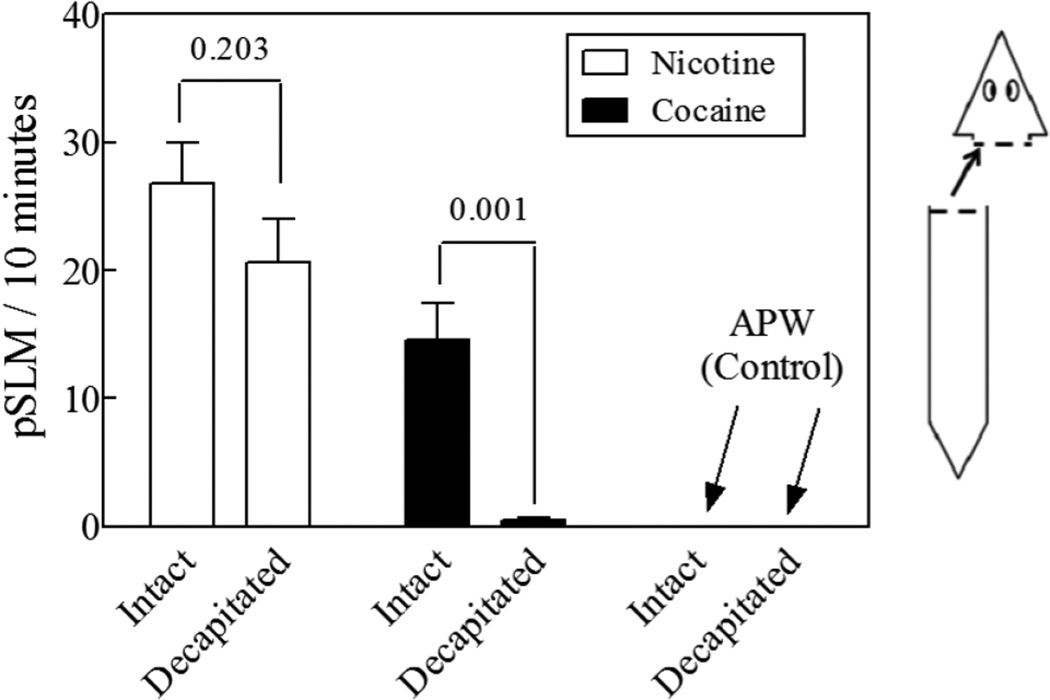
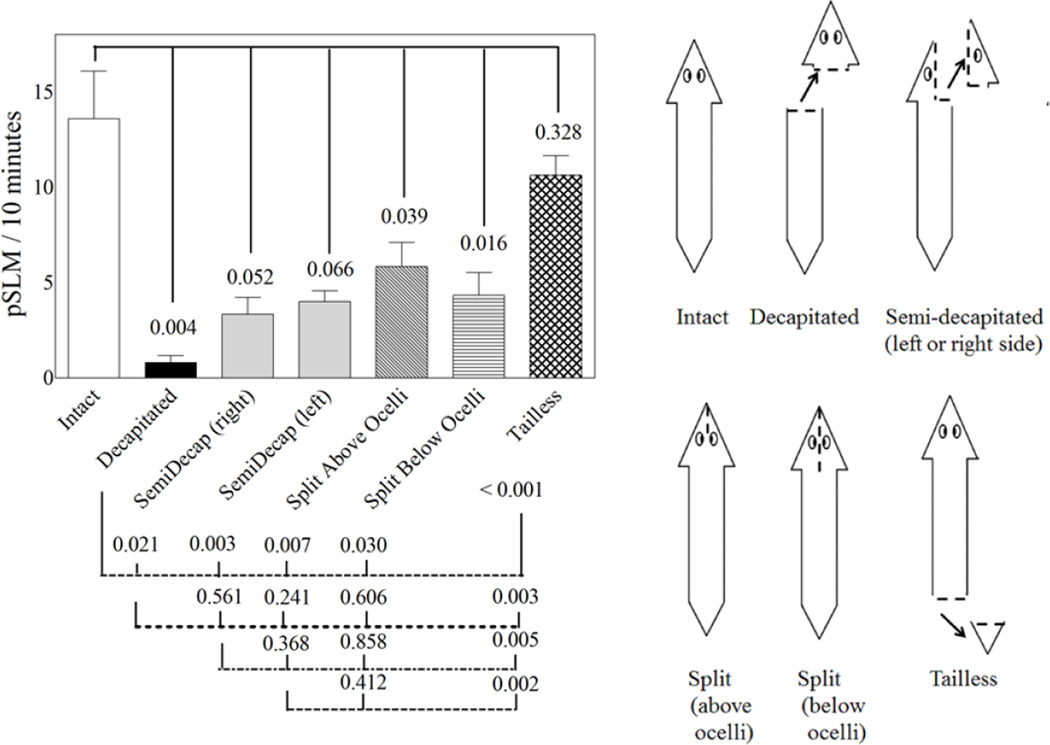
Upon decapitation, planarians became hypokinetic, this is, they moved at a much slower rate than intact animals; in fact, they tended to stay in the same place unless disturbed. Figure 4 shows the induction of pSLMs in response to the exposure of cocaine, nicotine or plain APW (control) in intact and decapitated planarians, as indicated. Cocaine and nicotine induced pSLMs at levels consistent with previous reports (Pagán et al., 2008, 2012; Rawls et al., 2009, 2011, Figure 2). Our results show that the absence of the cephalic ganglia reduced the ability of the planarians to exhibit pSLMs in response to cocaine by about 95%. In contrast, the cephalic ganglia are not necessary for the worms to respond to nicotine. The local anesthetics lidocaine and procaine did not display significant pSLMs at a concentration of 1mM (Figure 2). Interestingly, the neurotransmitter dopamine did not induce significant pSLMs either. Also, partial decapitation results in partial responses to cocaine exposure, as indicated in Figure 5. The excision of the right or left sides of the cerebral ganglia or just the surgical separating the brain hemispheres (split above or below the ocelli) results in similar partial responses to cocaine exposure (Figure 5). The excision of the tail tip did not significantly affect the response to cocaine (Figure 5).
Figure 4.
pSLMs induced by 1 mM of the experimental compounds in intact and decapitated planarians (shown at right). N = 6–14 worms. The numbers in the figure indicate the p-value of the comparison of intact vs. decapitated planarians for each compound through an unpaired, two-tailed t-test. The error bars represent the standard error of the mean.
Figure 5.
pSLMs induced by 1 mM cocaine in intact, decapitated, semi-decapitated, head-split or tailless planarians as indicated in the drawing below the bar graph. The groups were compared by two-tailed unpaired t-tests. The numbers shown represent the p-values obtained from the comparison of the experimental groups. The error bars represent the standard error of the mean.
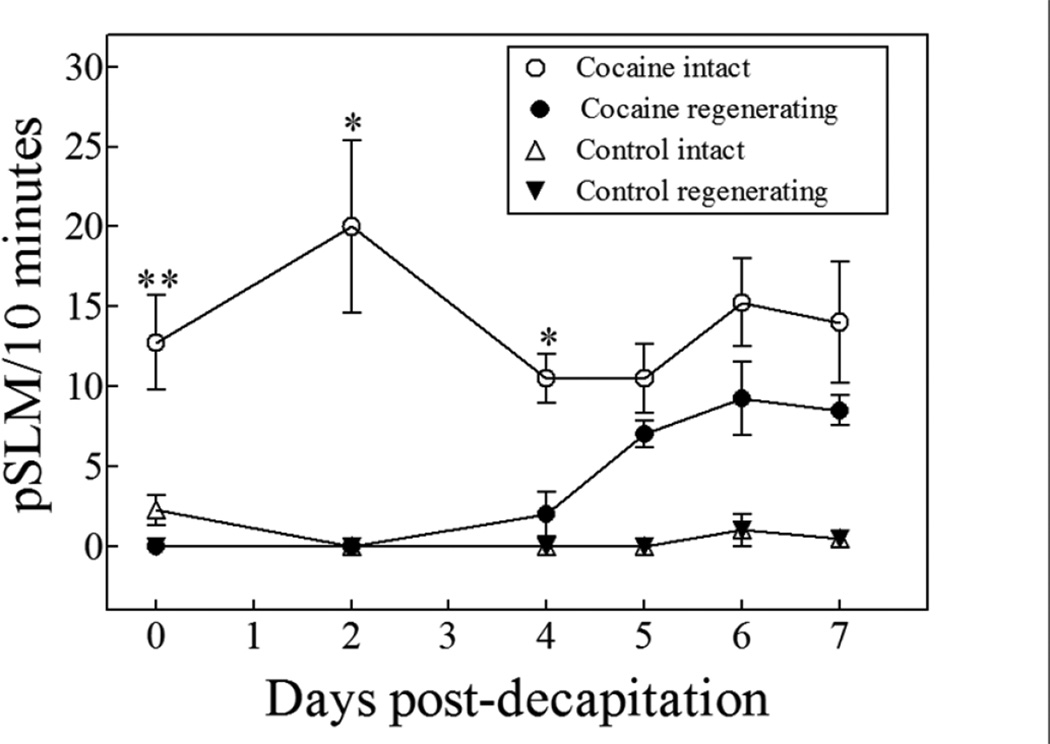
Since planarians are able to regenerate their brain, and a brain is necessary for them to react to cocaine, do they recover their normal sensitivity to cocaine upon regeneration? Further, if they do recover their cocaine sensitivity, at which point in time does this happen? To answer these questions, we measured the response of decapitated worms exposed to cocaine as the worms were regenerating their heads over an 8-day period as indicated in Figure 3.
Figure 6 shows that the induction of pSLMs in intact planarians by cocaine was constant over the 8-day observation period; there was no significant difference in the pSLMs induced by 1 mM cocaine from days 0 to 7 (two-tailed unpaired t-test). The regenerating planarians responded differently than the intact planarians to the exposure of cocaine (p < 0.0001; Two-way ANOVA). The regenerating planarians were insensitive to cocaine until day 4, when they began to exhibit pSLMs and displayed pSLM activity statistically indistinguishable from the intact worms by day 5. Our results are consistent with other reports indicating that regenerating planarians recover their brain functionality by days four to five postdecapitation (Cebrià, 2007; Inoue et al., 2004; Kato et al., 2004).
Figure 6.
Acute cocaine (1 mM) exposure to intact and regenerating planarians over a period of eight days; each symbol represent the average of ≥ 3 planarians. In brief, intact or regenerating planarians were exposed to cocaine on the day of decapitation (day 0) and discarded after the experiment. Each subsequent day, another set of planarians was experimented on and discarded, all the way to postdecapitation day 7 (See text and Figure 3). The regenerating planarians responded differently than the intact planarians to the exposure of cocaine (p < 0.0001, Two-way ANOVA). The comparison of intact vs. decapitated planarians for each day was done through a paired, two-tailed t-test (“*” ≤ 0.05; “**” ≤ 0.01; “***” ≤ 0.001). The error bars represent the standard error of the mean.
DISCUSSION
To the best of our knowledge, our work is the first report on how planarians undergoing regeneration react to the exposure of the abused drugs cocaine and nicotine. The simplest explanation for our results is that cocaine elicits the pSLM response through targets located in the cephalic ganglia of the worms, while nicotine can induce this behavior in the absence of the planarian brain, implying extracephalic binding sites (Figure 7). The fact that the presence of the cephalic ganglia is not required for the worms to react to nicotine does not imply that nicotine acts exclusively at peripheral sites. It is possible that binding sites for nicotine are also present in the planarian head, but we cannot examine the activity of isolated heads using the pSLM model. However, we can certainly conclude that most of the pSLM-inducing cocaine binding sites in planaria are located at the head region, specifically at the level of their cephalic ganglia. Even though we did not characterize the specific nature of the pharmacological targets for cocaine or nicotine in our experimental organism our results are consistent with the operational definition of receptor targets. This means that a biochemical target does not need to be identified to infer the presence of a receptor (Kenakin et al., 1992). We demonstrate in this work that cocaine seems to induce its effects mostly at the level of the planarians CNS while this does not seem to be the case for nicotine, which in our view, strongly argues for an initial characterization of the relative localization of the cocaine and nicotine receptor sites in these organisms. It is important to point out that in this sense the term “receptor” also implies targets such as transporters, enzymes, etc., in addition to the usual proteins characterized as true receptors.
Figure 7.
Interpretation of the relative localization of the putative binding sites for cocaine or nicotine in planarians (see text).
Interestingly, the deletion of half a brain results in a decrease in the cocaine-induced pSLMs of about 75 %, rather than the expected 50 % reduction due to the excision of half of the cephalic ganglia (Figure 5). This was our initial hypothesis, since it is the simplest case scenario. However, our results suggest that not only an intact brain is required for the worms to respond to cocaine, but that the connections between the two brain hemispheres is necessary to fully display cocaine-induced pSLMs. There was no significant difference between the semi-decapitated planarians and worms where the two brain hemispheres were separated, but not excised (Figure 5). The planarian brain hemispheres are connected by anterior commissures rich in interneurons; these structures have been proposed as a signal processing centers in this organism (Cebrià, 2007; 2008; Cebrià et al., 2002; Okamoto et al., 2005; Umesono et al., 1999). Based on our results on the split- yet not-severed head experiments (Figure 5) it seems that by severing the hemispheric connections, the planaria response to cocaine is disrupted, but not eliminated. The experiments where planarians recovered their ability to respond to cocaine are consistent with work by others in which the cephalic ganglia regeneration of a related planarian species (Schmidtea mediterranea) was studied using antibodies against several neurotransmitter substances, including serotonin, allostatin, neuropeptide F, GYRFamide and FMRFamide (Fraguas et al., 2012). Based on this study, a model of brain regeneration was proposed. In this model, the process of planarian anterior CNS regeneration is divided into four distinct stages. Stages one, two, three and four roughly correspond to post-decapitation days 1–2, 2–3, 4 and 7 respectively in our experiments. In Fraguas’ model, the anterior commisure begins to form at stage 2, followed by the further, but not complete, regeneration of the cephalic ganglia by stage 3 (corresponding to our post-decapitation day 4). Their results are in close agreement with similar studies in D. tigrina using antibodies against serotonin (Reuter et al., 1996).
The results and model described above are consistent with our observations on the recovery of cocaine sensitivity upon head regeneration. Our data shows that sub-maximal responses to cocaine begin to be observed precisely at day 4, which corresponds to post-decapitation stage 3 in the model proposed (Fraguas et al., 2012.).
In vertebrates, nicotine mainly interacts with ligand-gated ion channels known as nicotinic acetylcholine receptors (nAChR). These receptors are divided into two main classes; muscle-type (peripheral) and neuronal type (Millar and Gotti, 2009). Nicotine, by definition activates all types of nAChRs, of which there are dozens of different subtypes in vertebrate nervous systems (Albuquerque et al., 2009). Moreover, it has been reported that in vertebrates, nicotine can modulate the ubiquitin-proteasome system in a partially nAChR-independent manner (Kane et al., 2004; Rezvani et al., 2007).
The behavioral effects of cocaine in mammals are due to its interaction with presynaptic proteins in central neurons, including the serotonin, norepinephrine and dopamine transporters, especially this latter one (Torres et al., 2003). Additionally, cocaine inhibits the function of neuronal sodium channels, which account for the local anesthetic properties of cocaine and related compounds (Catalayud and González, 2003). To try to differentiate between the transporter- vs. the sodium channel-effects in our experimental system, we tested the local anesthetics lidocaine and procaine at a concentration of 1mM. In contrast to cocaine, neither local anesthetic significantly induced pSLMs at 1mM. This suggests that the observed cocaine effects were not likely due to cocaine’s interaction with sodium channels. Also, 1mM dopamine did not significantly induced pSLMs in our experiments (Figure 2). This is inconsistent with a possible link between an increase in synaptic dopamine and cocaine’s block of dopamine transporters, but further experiments are needed to explore this possibility. As in other models, the cholinergic and dopaminergic systems are related in planarians (Butarelli et al., 2000)
It is very likely that homologues of proteins targeted by nicotine and cocaine exist in D. tigrina. A representative genome of a related planarian species (Schmidtea mediterranea) has been sequenced (Robb et al., 2008). By using the S. mediterranea database (online at http://smedgd.neuro.utah.edu/index.html), we have identified approximately 100 homologous sequences to nAChRs, close to 20 homologous sequences to monoamine transporters, more than 100 homologous sequences to sodium channels and about 20 homologous sequences to candidate sequences for proteasome subunits which as we saw before, seem to be a nicotine target. The systematic exploration of the nicotine and cocaine molecular targets in planaria is possible using a variety of molecular techniques (Cebrià, 2007; Gentile et al., 2011; Nishimura et al., 2010; Umesono et al., 2011; Zamanian et al., 2011) which can help elucidate the nature and specific localization of proteins responsible for the behavioral effects of these psychoactive substances in planarians undergoing regeneration.
Our work provides further support for planarians as an animal model in pharmacology, with possible insights into mammalian pharmacology (Buttarelli et al., 2008). There is already an example of a compound, parthenolide, which was reported to prevent the behavioral effects induced by cocaine, but not the behavioral effects of other compounds, in planarians (Pagán et al., 2008, 2012; Rowlands and Pagán, 2008). Parthenolide was further proven effective against the cocaine effect on constitutive dopaminergic firing within the ventral tegmental area in the rat brain (Schwarz et al., 2011). The parthenolide example illustrates how pharmacological research using planarians can be applied to vertebrates. Further, our work integrates the fields of regeneration / developmental biology with the field of neuropharmacology; this interdisciplinary research may lead to fundamental discoveries in these combined research areas.
Decapitated planarians lose most of their behavioral responses to cocaine exposure
Decapitated planarians fully respond to nicotine exposure
Regenerating planarians recover their cocaine sensitivity
ACKNOWLEDGMENTS
We thank Dr. Kiyokazu Agata (Kyoto University, Japan) for suggesting the use of decapitated planarians to study psychoactive substances and Drs. Emili Saló (University of Barcelona, Spain), Robert B. Raffa (Temple University, Philadelphia, PA, USA) and Gregg Phares (West Chester University, PA, USA) for their critical reading of the manuscript and useful suggestions. We are very grateful for the financial and institutional support from the Department of Biology, the College of Arts & Sciences and the Office of Sponsored Research, West Chester University. We especially acknowledge the funds provided by the National Institutes of Health (NIH; R03-DA026518) to O.R.P. The NIH did not have any role in this report's study design, in the collection, analysis and interpretation of data, in the writing of the report or in any process related to the submission of this the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
D.B. and S.D. performed experiments, contributed to the design of the experiments and to the data analyses/interpretation. E. M., G.W., M.T. and J.S. performed experiments and contributed to the data analyses/interpretation. O.R. Pagán designed and performed experiments, compiled, analyzed and interpreted the data, designed and prepared the figures, wrote the original manuscript as well as the reworked revision. All authors proofread the paper.
None of the authors of this report have any known conflict of interest.
REFERENCES
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89(1):73–120. doi: 10.1152/physrev.00015.2008. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttarelli F, Pellicano C, Pontieri FE. Neuropharmacology and behavior in planarians: translations to mammals. Comp. Biochem Physiol C Toxicol Pharmacol. 2008;147:399–408. doi: 10.1016/j.cbpc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125(2):225–231. doi: 10.1016/s0742-8413(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Calatayud J, González A. History of the development and evolution of local anesthesia since the coca leaf. Anesthesiology. 2003;98(6):1503–1508. doi: 10.1097/00000542-200306000-00031. [DOI] [PubMed] [Google Scholar]
- Cebrià F. Organization of the nervous system in the model planarian Schmidtea mediterranea: an immunocytochemical study. Neurosci Res. 2008;61(4):375–384. doi: 10.1016/j.neures.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Cebrià F. Regenerating the central nervous system: how easy for planarians! Dev Genes Evol. 2007;217:733–748. doi: 10.1007/s00427-007-0188-6. [DOI] [PubMed] [Google Scholar]
- Cebrià F, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. Dissecting planarian central nervous system regeneration by the expression of neural-specific genes. Dev Growth Differ. 2002;44(2):135–146. doi: 10.1046/j.1440-169x.2002.00629.x. [DOI] [PubMed] [Google Scholar]
- Fraguas S, Barberán S, Ibarra B, Stöger L, Cebrià F. Regeneration of neuronal cell types in Schmidtea mediterranea: an immunohistochemical and expression study. Int J Dev Biol. 2012;56(1–2–3):143–153. doi: 10.1387/ijdb.113428sf. [DOI] [PubMed] [Google Scholar]
- Gentile L, Cebrià F, Bartscherer K. The planarian flatworm: an in vivo model for stem cell biology and nervous system regeneration. Dis Model Mech. 2011;4:12–19. doi: 10.1242/dmm.006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kumamoto H, Okamoto K, Umesono Y, Sakai M, Sánchez Alvarado A, Agata K. Morphological and functional recovery of the planarian photosensing system during head regeneration. Zoolog Sci. 2004;21:275–283. doi: 10.2108/zsj.21.275. [DOI] [PubMed] [Google Scholar]
- Kane JK, Konu O, Ma JZ, Li MD. Nicotine coregulates multiple pathways involved in protein modification/degradation in rat brain. Brain Res Mol Brain Res. 2004;132(2):181–191. doi: 10.1016/j.molbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Kato C, Mihashi K, Ishida S. Motility recovery during the process of regeneration in freshwater planarians. Behav Brain Res. 2004;150:9–14. doi: 10.1016/j.bbr.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kenakin TP, Bond RA, Bonner TI. Definition of pharmacological receptors. Pharmacol Rev. 1992;44(3):351–362. [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Newmark PA, Sanchez Alvarado A. Regeneration in planaria. Encyclopedia of life sciences. 2001 [Google Scholar]
- Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Girardia japonica. Neuroscience. 2010;168(1):18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Inoue T, Umesono Y, Sano S, Yoshimoto K, Inden M, Takata K, Taniguchi T, Shimohama S, Agata K. Reconstruction of dopaminergic neural network and locomotion function in planarian regenerates. Dev Neurobiol. 2007;67:1059–1078. doi: 10.1002/dneu.20377. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zoolog Sci. 2005;22(5):535–546. doi: 10.2108/zsj.22.535. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Baker D, Deats S, Montgomery E, Tenaglia M, Randolph C, Kotturu D, Tallarida C, Bach D, Wilk G, Rawls S, Raffa Planarians in pharmacology: Parthenolide is a specific behavioral antagonist of cocaine in the planarian Girardia tigrina. Int. J Dev Biol. 2012;56:193–196. doi: 10.1387/ijdb.113486op. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Holland LJ, Schulingkamp RJ. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. J Pharmacol Toxicol Methods. 2001;45:223–226. doi: 10.1016/s1056-8719(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Desaer C. Carbamazepine inhibits distinct chemoconvulsant-induced seizure-like activity in Girardia tigrina. Pharmacol Biochem Behav. 2011;99:665–670. doi: 10.1016/j.pbb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Ramoz L, Lodi S, Bhatt P, Reitz AB, Tallarida C, Tallarida RJ, Raffa RB, Rawls SM. Mephedrone ("bath salt") pharmacology: insights from invertebrates. Neuroscience. 2012;208C:79–84. doi: 10.1016/j.neuroscience.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Thomas T, Adeola M, Patil T, Raymondi N, Poles A, Loo M, Raffa RB. Topiramate antagonizes NMDA- and AMPA-induced seizure-like activity in planarians. Pharmacol Biochem Behav. 2009;93:363–367. doi: 10.1016/j.pbb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: Clues from planarians. Drug Alcohol Depend. 2011;118:274–279. doi: 10.1016/j.drugalcdep.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Yuvasheva E, Raffa RB. First evidence that drugs of abuse produce behavioral sensitization and cross sensitization in planarians. Neuroscience. 2010;169:1800–1804. doi: 10.1097/FBP.0b013e32833b0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27(39):10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb SM, Ross E, Sánchez Alvarado A. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:D599–D606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur J Pharmacol. 2008;583(1):170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Netsky MG. The brain of the planarian as the ancestor of the human brain. Can J Neurol Sci. 1985;12:296–302. doi: 10.1017/s031716710003537x. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Bloom D, Castro R, Pagán OR, Jiménez-Rivera CA. Parthenolide blocks cocaine's effect on spontaneous firing activity of dopaminergic neurons in the ventral tegmental area. Curr Neuropharmacol. 2010;9:17–20. doi: 10.2174/157015911795017010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Tasaki J, Nishimura K, Inoue T, Agata K. Regeneration in an evolutionarily primitive brain-the planarian Girardia japonica model. Eur J Neurosci. 2011;34:863–869. doi: 10.1111/j.1460-9568.2011.07819.x. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Watanabe K, Agata K. Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Dev Genes Evol. 1999;209:31–39. doi: 10.1007/s004270050224. [DOI] [PubMed] [Google Scholar]
- Zamanian M, Kimber MJ, McVeigh P, Carlson SA, Maule AG, Day TA. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genomics. 2011;12:596. doi: 10.1186/1471-2164-12-596. [DOI] [PMC free article] [PubMed] [Google Scholar]