Abstract
Purpose
The osteoprotogerin/receptor activator of NF-kappa β/receptor activator of NF-kappa β ligand (OPG/RANK/RANKL) pathway plays a critical role in bone remodeling. This study investigated associations between serum levels of OPG, soluble RANKL (sRANKL), and the ratio of OPG/sRANKL to risk of incident hip fracture.
Methods
A nested case–control study was conducted among postmenopausal, Caucasian women aged 50–79 at baseline (1993–1998), followed for hip fracture through March 2005 in the Women's Health Initiative Observational Study. 400 incident hip fracture cases were selected and individually matched to 400 controls with noprior fracture or incident hip fracture. Matching factors were baseline age, enrollment date and hormone therapy (HT) exposure. Baseline serum OPG and sRANKL levels were measured using high sensitivity ELISA. Odds ratios were computed for quartiles of each biomarker adjusting for matching factors and hip fracture risk factors.
Results
Serum OPG was significantly associated with older age, low physical activity and poorer physical function in control women. sRANKL was inversely associated with total calcium intake in control women, but not associated with age or other fracture risk factors. The odds ratio for hip fracture comparing the highest to lowest quartiles of OPG was 2.28 (95% confidence interval (CI), 1.45–3.61) after adjusting for the matching variables (p-value for linear trend <0.001), and 1.87 (95% CI, 1.15–3.04; p for linear trend = 0.02) after adjusting for self-rated health status, physical activity and physical functioning. No significant associations between sRANKL or the ratio of OPG/sRANKL and hip fracture risk were observed.
Conclusion
Serum OPG levels were independently associated with a nearly twofold increased risk of hip fracture in postmenopausal women.
Keywords: RANKL, Osteoprotogerin, Hip fracture, Osteoporosis, Postmenopausal women
Introduction
The discovery of osteoprotegerin (OPG) in 1997 led quickly to the understanding that the OPG/RANKL/RANK System is central to the coupling of osteoclasts and osteoblasts in bone biology [1]. At the level of the bone remodeling unit, receptor activator of NF-kappa β ligand (RANKL) is expressed on the cell surface of both osteoblasts and stromal cells. RANKL binds to RANK and leads to differentiation and maturation of osteoclasts, leading to bone resorption. OPG is a protein made by osteoblasts that binds to RANKL as a decoy receptor and blocks its interaction with RANK, thus blocking osteoclast formation and bone resorption. Other tissues besides osteoblasts produce RANKL and OPG and thus, measurements of these biomarkers in the serum may reflect both skeletal and non-skeletal sources, as well as age-related differences in clearance [2]. The balance between RANKL and OPG may be regulated by other cytokines or hormones that promote bone resorption.
Associations between OPG, bone turnover markers, and BMD have been inconsistent [2–4]. There are limited data examining the relationship between OPG and/or RANKL and fracture risk. OPG levels were not associated with fracture in one study [5], but high levels were associated with increased risk in a small case–control study of hip fracture [6] and height loss in postmenopausal women [7]. sRANKL has been associated with fracture in one study with 31 fractures [8], but no association was seen between sRANKL and height loss in a larger cohort of postmenopausal women [7]. The early studies are limited by small sample sizes, low numbers of fracture events, and lack of sensitivity of early generation assays.
The sole epidemiologic study to examine a high sensitivity marker of OPG in relation to incident hip fracture found that the highest levels of OPG were associated with hip fracture risk [9]. We conducted a nested case–control study within the Women's Health Initiative Observational Study (WHI-OS) to examine serum OPG and soluble RANKL (sRANKL) levels and the ratio of OPG to sRANKL to risk of incident hip fracture risk. A second objective was to determine if associations between these biomarker levels and incident hip fracture differed for women who were users vs. nonusers of HT, those with high and low body mass index, those with high vs. low total calcium intake (diet plus supplement intake) and those with high and low FRAX scores(ameasure of 10-year fracture risk).
Materials and methods
Study group
The WHI-OS is a prospective cohort study that enrolled 93,676 women ages 50–79 years from 1994–1998 at 40 clinical centers throughout the United States [10]. The women were eligible if they were post-menopausal, unlikely to move or die within three years, not enrolled in the WHI Clinical Trials and not currently participating in any other clinical trial. At baseline, the women completed screening and enrollment questionnaires by interview and self-report, a physical examination and blood specimen collection. The study was reviewed and approved by the Human Subjects Review Committees at each participating institution.
Follow-up and outcome ascertainment
The women were sent questionnaires annually to report the occurrence of any hospitalization and a wide variety of outcomes including clinical fractures of any type. The median (interquartile range) of follow-up time was 8.0 (7.0–9.0) years per participant as of September, 2005. At that time, 4.8% of WHI-OS participants had withdrawn or were lost to follow-up and 6.7% had died. Hip fractures were verified by review of radiological, magnetic resonance imaging, or operative reports by trained physicians at each clinical center and then confirmed by blinded central adjudicators [11]. Hip fractures with a possible or confirmed pathological cause (from malignancy, infection or focal bone lesion) were excluded. There was no attempt to distinguish fractures caused by excessive trauma from other hip fractures.
Nested case–control study design
The study population was restricted to Caucasian women who had been part of a previous whole genome association study. From this group, 400 randomly selected incident cases of hip fracture and their matched controls were identified. Controls were selected using random sampling in a 1:1 ratio with cases from the subpopulation of women who reported no postmenopausal fractures (self-reported fracture at age ≥55 years) at baseline and no incident hip fracture through the planned study closeout (March 31, 2005) with individual matching by age (+/−1 year), enrollment date (+/1 year) and current hormone therapy use at baseline (exact). Matching by enrollment date serves the dual purpose of matching on length of follow-up and length of frozen storage for the serum specimens. Bio-marker levels were obtained in 396 cases and 397 controls. This study design provided 80% power to detect a difference of 0.20 standard deviations in mean biomarker levels between cases and controls at the 0.05 level of statistical significance. This corresponds to 80% power to detect a statistically significant odds ratio of 1.22 for hip fracture per 1 standard deviation difference in biomarker levels.
Baseline clinical variables
Baseline questionnaires ascertained information on race/ethnicity, treated diabetes, history of myocardial infarction, coronary revascularization or stroke, current and past smoking, parental history of hip fracture, and self-rated health status. Detailed information on current and past HT use was ascertained by questionnaire. Alcohol consumption was estimated using questionnaire items as servings per week. Physical activity was classified on the basis of frequency and duration of four speeds of walking and mild, moderate and strenuous activities in the prior week. Kilocalories of energy expended in a week on leisure time activity was calculated (MET score = kcal h/week/kg) [12]. Physical function was measured using the 10-item Rand-36 physical function scale which includes items measuring whether health now limits physical function in moderate/vigorous activities, strength to lift, carry, stoop, or bend, stair climb, ability to walk various distances without difficulty, and self-care [13]. Weight was measured to the nearest 0.1 kg on a balance beam scale with the participant dressed in indoor clothing without shoes. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Body mass index was calculated as weight (kg)/height (m2).
Current use of prescription medications including corticosteroids was recorded by clinic interviewers at the first screening visit by direct inspection of medicine containers. Prescription names were entered into the WHI database which assigned drug codes using Medispan software. Dietary supplements, including calcium preparations, taken at least twice weekly for the prior two weeks were entered directly from medicine containers as described above. Dietary intake of calcium was measured using a semi-quantitative food frequency questionnaire [14]. Total calcium intake was defined as the sum of calcium from diet, supplements, and medications. FRAX scores were provided by the WHO Collaborative Group based on women's individual clinical risk factors without bone mineral density [15].
Cases and controls did not have previous measurements of bone mineral density, serum 25-hydroxyvitamin D (Vitamin D) levels or bone metabolism markers, except as detailed below.
Laboratory procedures
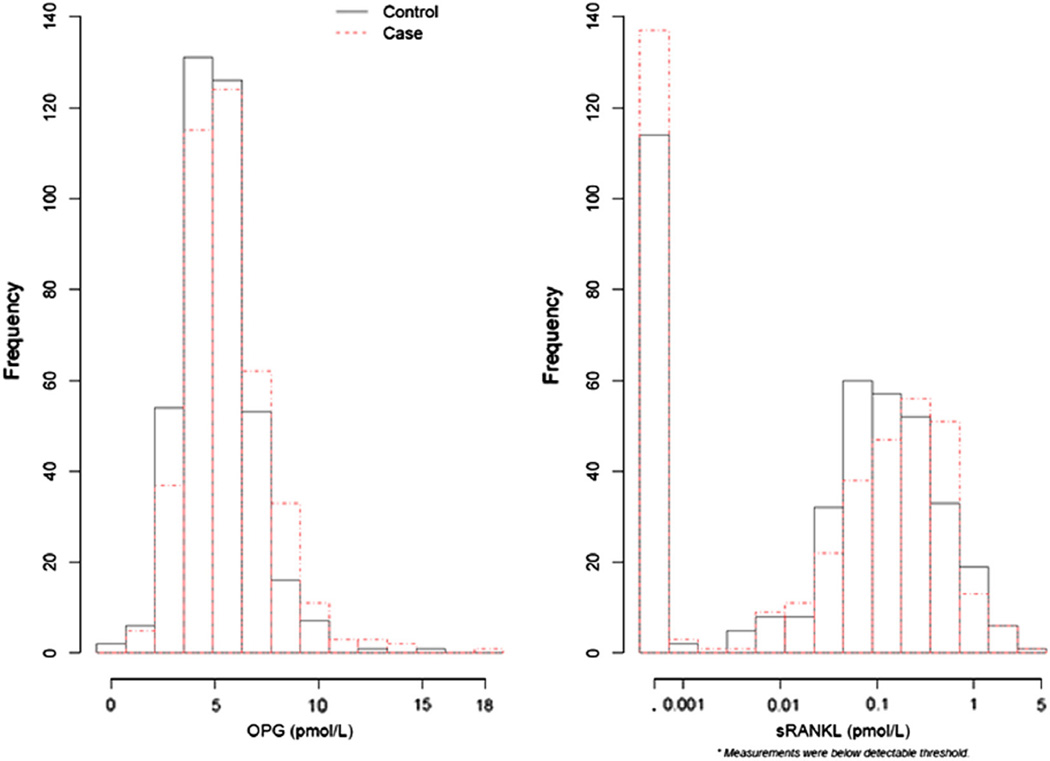
Laboratory personnel were blinded to case–control status for all measurements. OPG and sRANKL were measured by ELISA (Osteoprotegerin EIA, and ampli-sRANKL EIA, Biomedica kits distributed by American Laboratory Products Company, LTD; Salem, NH) at Ohio State University in baseline serum that had been stored at − 70° Celsius within two hours of collection. The OPG assay measures both free and complexed OPG-RANKL and detects both the monomeric and dimeric forms of OPG. The minimum detection limit is 0.14 pmol/L (2.8 pg/ml). The range of OPG levels in serum sample from normal healthy individuals is 0.65–4.2 pmol/L (13.0–84 pg/ml). The intra-assay and inter-assay coefficients of variation are 6% and 8%, respectively. The sRANKL ELISA measures free RANK-L and uses an enzyme catalyzed amplification cycle to enhance the detection limit. The median value for normal healthy women is 0.37 pmol/L The detection limit of the assay is 0.001 pmol/L The intra-assay and inter-assay coefficients of variation are 8% and 6%, respectively. Serum sRANKL levels were undetectable in one-third of cases and controls overall (35% of cases, 28% of controls).
Plasma “whole” parathyroid hormone (PTH) levels were measured by immunoradiometric assay utilizing a polyclonal 1–84 PTH antibody (Whole PTH™) at Ohio State University. This third generation assay specifically measures the entire biologically active 1–84 PTH molecule. The minimum detection limit is 1 pg/ml and functional sensitivity is assessed at 3 pg/ml. The range of PTH levels in samples from normal healthy individuals is 5–39 pg/ml. The intra-assay and inter-assay coefficients of variation are 4% and 7%, respectively. A subgroup of 125 participants (all but two were hip fracture cases) were part of a separate case–control study that measured 25-hydroxyvitamin D, cross-linked C-telopeptide (CTX) and amino-terminal propeptide of type I procollagen (PINP) in baseline serum using methods described previously [16]. These measurements were made available to the present study in order to examine correlations with serum OPG and sRANKL.
Statistical methods
Baseline characteristics were compared between hip fracture cases and matched controls, with corresponding p-values calculated from chi-square tests for categorical variables and t-tests for continuous variables. To further assess the potential for confounding, baseline characteristics were compared across quartiles of OPG and sRANKL levels in control participants. Spearman correlations were calculated to assess the strength of association between each biomarker and select continuous covariates.
Distributions of OPG and sRANKL were compared for hip fracture cases and controls. Histograms of the biomarker distributions were plotted for cases and controls; measurements of sRANKL varied by three orders of magnitude so a log10 scale was used to better display the distribution of sRANKL. Associations between OPG and sRANKL levels and the ratio of OPG/SRANKL levels and incident hip fracture were assessed in logistic regression models adjusting for the matching baseline factors of age, blood draw date, and current HT use, as well as plate. Odds ratios were computed for quartiles of each biomarker compared to the lowest quartile, and per standard deviation of the continuous form of the log transformed biomarker unless the association with hip fracture was found to be non-linear. Quartiles cutpoints were defined based on the distribution in controls. Therefore, for sRANKL, undetectable levels defined the lowest quartile which was coded at 0.0005, the midpoint between 0 and 0.001 (the lower detection limit) for purposes of analysis. Associations were first examined without any additional adjustment and then with adjustment for body mass index (continuous), and finally in a fully adjusted model. Covariates were selected for inclusion in the full multivariate model based on their association with incident hip fracture in the initial univariate analysis and their correlation with each biomarker level in controls. Correlations between biomarker levels and covariates were assessed using Spearman correlation coefficients.
Stratified analyses were conducted for HT, body mass index and FRAX score, estimating odds ratios within binary categorizations of each of these variables. The statistical significance of differences in the odds ratios was tested using cross-product terms added to the fully-adjusted multivariate models for each biomarker.
Statistical analyses were performed using SAS statistical software (version 9.2; SAS Institute, Cary, North Carolina), generalized additive models [17] and corresponding figure were computed with R (version 2.11; R Development Core Team (2010) – http://www.R-project.org) and the R library mgcv.
Theory
Because OPG acts as a decoy receptor for RANKL to block bone re-sorption within the bone remodeling unit, it would be biologically plausible for higher serum levels of OPG to be associated with reduced bone resorption, higher bone density and a decreased risk of hip fracture. Alternatively, higher serum levels of OPG could occur to counteract high RANKL bioactivity resulting in increased bone resorption and therefore higher serum levels could reflect a compensatory mechanism that is related to increased hip fracture risk. Thus, the results of this study could inform which of these mechanisms is more supported by the data.
Results
Cases of hip fracture were identical to controls on the matching factors of age (69.8 years) and current HT use (37.8%; Table 1). Cases were significantly more likely to be current smokers, to have a parent who broke their hip, to use corticosteroids, to have lower physical function and higher 10-year probabilities of hip fracture as estimated by FRAX compared to controls. Cases were significantly less likely than controls to drink alcohol, and had lower levels of physical activity, self-rated health status, and body mass index (Table 1).
Table 1.
Baseline characteristics of hip fracture cases and matched controls from the Women's Health Initiative Observational Study.
| Case (N= 400) |
Control (N = 400) |
P-valuea | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Current HT | 151 | 37.8 | 151 | 37.8 | Matching variable |
| Smoking | 0.004 | ||||
| Never | 183 | 46.4 | 215 | 54.4 | |
| Past | 176 | 44.7 | 165 | 41.8 | |
| Current | 35 | 8.9 | 15 | 3.8 | |
| Alcohol consumption | 0.06 | ||||
| Non/past drinker | 136 | 34.3 | 103 | 25.8 | |
| <1 drink/week | 115 | 29.0 | 136 | 34.0 | |
| 1–14 drinks/week | 128 | 32.3 | 144 | 36.0 | |
| >14 drinks/week | 17 | 4.3 | 17 | 4.3 | |
| Episodes/week mod-stren phys activity ≥20 min (categorical) | 0.05 | ||||
| No activity | 65 | 16.4 | 47 | 12.0 | |
| Some activity | 170 | 42.8 | 151 | 38.4 | |
| 2 – <4 episodes per week | 72 | 18.1 | 78 | 19.8 | |
| 4+ episodes per week | 90 | 22.7 | 117 | 29.8 | |
| Parent broke hip | 83 | 21.3 | 56 | 14.3 | 0.01 |
| Treated diabetes (pills or injections) | 24 | 6.0 | 19 | 4.8 | 0.43 |
| Hx of angina, MI or stroke | 55 | 13.8 | 49 | 12.3 | 0.54 |
| Self-reported health | <0.001 | ||||
| Excellent | 41 | 10.3 | 66 | 16.5 | |
| Very good | 137 | 34.3 | 190 | 47.6 | |
| Good | 156 | 39.1 | 119 | 29.8 | |
| Fair/poor | 65 | 16.3 | 24 | 6.0 | |
| Corticosteroid use | 14 | 3.5 | 5 | 1.3 | 0.04 |
| Mean | SD | Mean | SD | P-value | |
| Age at screening, years | 69.8 | (6.4) | 69.8 | (6.4) | Matching variable |
| Body-mass index (BMI), kg/m2 | 25.6 | (5.0) | 27.0 | (5.8) | <0.001 |
| Total energy expenditure/week from phys act, MET-hours | 10.9 | (10.9) | 14.6 | (14.6) | <0.001 |
| Rand-36 physical function score | 71.6 | (22.7) | 80.8 | (18.6) | <0.001 |
| Total calcium intake (mg) | 1220.4 | (717.8) | 1281.5 | (721.8) | 0.24 |
| Total vitamin D intake (IU) | 395.6 | (293.0) | 394.9 | (257.1) | 0.97 |
| Serum parathyroid hormone (PTH; PG/mL) | 22.4 | (13.3) | 21.9 | (7.9) | 0.52 |
| Frax 10 year probability (%) of hip fracture without BMD | 6.6 | (7.8) | 4.3 | (4.3) | <0.001 |
Test of association based on Chi-squared tests (categorical variables) or t-tests (continuous variables).
Controls with higher OPG levels were older, and after adjusting for age were significantly more likely to have treated diabetes, poorer self-rated health status, and lower physical function scores compared to controls with lower OPG levels (Table 2). The mean age of controls in the lowest quartile (≤4 pmol/L) was 67.7 years compared to 72.6 years in the highest quartile (>6 pmol/L; p < 0.001; r = 0.33). Serum OPG levels were modestly correlated with PTH levels (r = 0.11). Serum sRANKL levels were not associated with age in controls (Table 3). The only covariate that showed significant associations with sRANKL was total calcium intake which appeared lower in the lowest quartile of sRANKL (0.0005 pmol/L). Current HT use did not significantly differ across quartiles of either biomarker (Tables 2 and 3). sRANKL levels were not correlated with serum PTH (r = 0.02).
Table 2.
Baseline characteristics of controls (n = 397) by quartilesa of OPG.
| ≤4 pmol/L (N = 100) |
4 < –5 pmol/L (N = 100) |
5 < –6 pmol/L (N = 99) |
>6 pmol/L (N = 98) |
P-valueb | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Current HT | 43 | 43.0 | 36 | 36.0 | 33 | 33.3 | 38 | 38.8 | 0.30 |
| Smoking | 0.93 | ||||||||
| Never | 54 | 54.5 | 48 | 48.5 | 63 | 64.9 | 48 | 49.5 | |
| Past | 42 | 42.4 | 48 | 48.5 | 29 | 29.9 | 45 | 46.4 | |
| Current | 3 | 3.0 | 3 | 3.0 | 5 | 5.2 | 4 | 4.1 | |
| Alcohol consumption | 0.90 | ||||||||
| Non/past drinker | 24 | 24.0 | 22 | 22.0 | 31 | 31.3 | 26 | 26.5 | |
| <1 drink/week | 33 | 33.0 | 38 | 38.0 | 30 | 30.3 | 32 | 32.7 | |
| 1–14 drinks/week | 38 | 38.0 | 36 | 36.0 | 35 | 35.4 | 35 | 35.7 | |
| >14 drinks/week | 5 | 5.0 | 4 | 4.0 | 3 | 3.0 | 5 | 5.1 | |
| Episodes/week mod-stren phys activity >= 20 min | 0.18 | ||||||||
| No activity | 13 | 13.1 | 10 | 10.3 | 12 | 12.2 | 12 | 12.5 | |
| Some activity | 36 | 36.4 | 33 | 34.0 | 43 | 43.9 | 37 | 38.5 | |
| 2–<4 episodes per week | 17 | 17.2 | 16 | 16.5 | 19 | 19.4 | 25 | 26.0 | |
| 4+ episodes per week | 33 | 33.3 | 38 | 39.2 | 24 | 24.5 | 22 | 22.9 | |
| Parent broke hip | 15 | 15.2 | 9 | 9.0 | 17 | 17.9 | 14 | 14.7 | 0.81 |
| Treated diabetes (pills or shots) | 3 | 3.0 | 1 | 1.0 | 4 | 4.1 | 11 | 11.2 | 0.007 |
| Hx of angina, MI or stroke | 12 | 12.1 | 6 | 6.1 | 14 | 14.1 | 16 | 16.5 | 0.30 |
| Self-reported health | 0.003 | ||||||||
| Excellent | 22 | 22.0 | 24 | 24.0 | 9 | 9.2 | 10 | 10.2 | |
| Very good | 55 | 55.0 | 40 | 40.0 | 53 | 54.1 | 42 | 42.9 | |
| Good | 18 | 18.0 | 28 | 28.0 | 34 | 34.7 | 38 | 38.8 | |
| Fair/poor | 5 | 5.0 | 8 | 8.0 | 2 | 2.0 | 8 | 8.2 | |
| Use of corticosteroids | 0 | 0.0 | 0 | 0.0 | 3 | 3.0 | 2 | 2.0 | 0.22 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | |
| Age at screening, years | 67.6 | 6.6 | 68.5 | 6.2 | 70.5 | 5.7 | 72.6 | 6.0 | <0.001 |
| Body-mass index (BMI), kg/m2 | 26.9 | 4.7 | 26.9 | 5.5 | 27.4 | 6.8 | 26.8 | 6.0 | 0.10 |
| Total energy expenditure/week from phys act, MET-hours | 14.9 | 14.5 | 16.0 | 14.8 | 14.0 | 13.8 | 14.0 | 15.6 | 0.44 |
| Rand-36 physical function score | 85.9 | 14.8 | 83.5 | 16.2 | 76.4 | 20.8 | 77.5 | 19.5 | 0.002 |
| Total calcium intake (mg) | 1239.7 | 730.8 | 1311.5 | 749.5 | 1350.2 | 703.0 | 1238.0 | 714.9 | 0.80 |
| Total vitamin D intake (IU) | 369.7 | 255.1 | 407.5 | 266.7 | 415.1 | 240.2 | 396.4 | 265.6 | 0.88 |
| Serum parathyroid hormone (PTH; PG/mL) | 21.7 | 7.9 | 21.8 | 7.6 | 22.2 | 8.1 | 21.9 | 8.2 | 0.98 |
| 10 year probability (%) of hip fracture without BMD | 3.1 | 3.4 | 3.3 | 3.4 | 4.8 | 5.0 | 5.8 | 4.8 | 0.74 |
Quartiles of OPG based on controls.
Test of association adjusted for age.
Table 3.
Baseline characteristics of controls (n = 397) by quartilesa of sRANKL.
| Below detectable threshold (N = 114) |
0.001 <–0.1 pmol/L (N = 93) |
0.1 <–0.2 pmol/L (N = 96) |
>0.2 pmol/L (N = 94) |
P-valueb | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Current HT | 37 | 32.5 | 33 | 35.5 | 41 | 42.7 | 39 | 41.5 | 0.14 |
| Smoking | 0.49 | ||||||||
| Never | 60 | 53.6 | 43 | 46.2 | 55 | 59.1 | 55 | 58.5 | |
| Past | 46 | 41.1 | 48 | 51.6 | 34 | 36.6 | 36 | 38.3 | |
| Current | 6 | 5.4 | 2 | 2.2 | 4 | 4.3 | 3 | 3.2 | |
| Alcohol consumption | 0.71 | ||||||||
| Non/past drinker | 30 | 26.3 | 27 | 29.0 | 26 | 27.1 | 20 | 21.3 | |
| <1 drink/week | 40 | 35.1 | 31 | 33.3 | 28 | 29.2 | 34 | 36.2 | |
| 1–14 drinks/week | 39 | 34.2 | 29 | 31.2 | 40 | 41.7 | 36 | 38.3 | |
| >14 drinks/week | 5 | 4.4 | 6 | 6.5 | 2 | 2.1 | 4 | 4.3 | |
| Episodes/week mod-stren phys activity ≥20 min (categorical) | 0.13 | ||||||||
| No activity | 20 | 17.7 | 8 | 8.7 | 11 | 11.8 | 8 | 8.7 | |
| Some activity | 41 | 36.3 | 45 | 48.9 | 32 | 34.4 | 31 | 33.7 | |
| 2–<4 episodes per week | 22 | 19.5 | 13 | 14.1 | 15 | 16.1 | 27 | 29.3 | |
| 4+ episodes per week | 30 | 26.5 | 26 | 28.3 | 35 | 37.6 | 26 | 28.3 | |
| Parent broke hip | 20 | 18.0 | 11 | 12.1 | 10 | 10.4 | 14 | 15.4 | 0.46 |
| Treated diabetes (pills or shots) | 4 | 3.5 | 7 | 7.5 | 5 | 5.2 | 3 | 3.2 | 0.87 |
| Hx of angina, MI or stroke | 18 | 16.1 | 10 | 10.8 | 8 | 8.3 | 12 | 12.9 | 0.36 |
| Self-reported health | 0.15 | ||||||||
| Excellent | 16 | 14.2 | 16 | 17.2 | 16 | 16.7 | 17 | 18.1 | |
| Very good | 49 | 43.4 | 42 | 45.2 | 48 | 50.0 | 51 | 54.3 | |
| Good | 39 | 34.5 | 30 | 32.3 | 27 | 28.1 | 22 | 23.4 | |
| Fair/poor | 9 | 8.0 | 5 | 5.4 | 5 | 5.2 | 4 | 4.3 | |
| Use of corticosteroids | 1 | 0.9 | 2 | 2.2 | 1 | 1.0 | 1 | 1.1 | 0.99 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | |
| Age at screening, years | 70.1 | 6.9 | 69.7 | 5.6 | 69.9 | 6.5 | 69.3 | 6.3 | 0.47 |
| Body-mass index (BMI), kg/m2 | 27.4 | 6.6 | 26.8 | 5.4 | 26.9 | 5.8 | 26.8 | 4.9 | 0.37 |
| Total energy expenditure/week from phys act, MET-hours | 15.3 | 16.8 | 13.6 | 14.8 | 15.4 | 15.4 | 14.5 | 10.1 | 0.89 |
| Rand-36 physical function score | 79.6 | 21.1 | 81.9 | 18.0 | 79.1 | 18.4 | 83.2 | 14.5 | 0.38 |
| Total calcium intake (mg) | 1098.2 | 632.5 | 1364.0 | 696.0 | 1383.2 | 827.7 | 1330.9 | 711.0 | 0.01 |
| Total vitamin D intake (IU) | 349.6 | 239.4 | 450.7 | 263.1 | 408.4 | 264.2 | 391.1 | 257.4 | 0.30 |
| Serum parathyroid hormone (PTH; PG/mL) | 22.0 | 7.3 | 20.8 | 7.9 | 22.9 | 8.7 | 21.8 | 7.8 | 0.37 |
| 10 year probability (%) of hip fracture without BMD | 5.0 | 5.4 | 3.8 | 3.6 | 3.9 | 3.4 | 4.1 | 4.4 | 0.69 |
Quartiles of sRANKL based on controls.
Test of association adjusted for age.
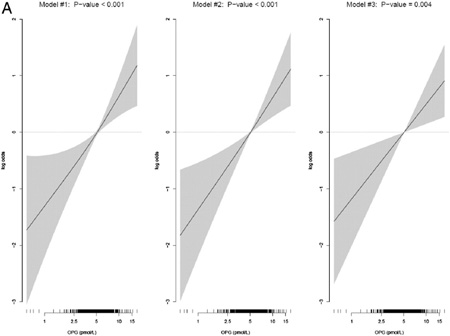
The odds ratio for hip fracture comparing the highest quartile of OPG to the lowest was 2.28 (95% confidence interval (CI), 1.45–3.61) after adjusting for the matching variables (p-value for linear trend <0.001) and there was no attenuation after additional adjustment for BMI (Models 1 and 2; Table 4). After additional adjustments for self-rated health status, physical activity and physical functioning in model 3, the odds ratio for the highest compared to lowest quartile was 1.87 (95% CI, 1.15–3.04; p for linear trend = 0.02). This association persisted after additional adjustment for serum PTH (model 4). When OPG was examined as a continuous variable, the odds ratio for a 1 standard deviation difference was 1.31 (95% CI, 1.09–1.57) and the p-values for linear trend were highly significant in all four models (p = 0.01); Table 3. Examination of the GAM plot (Appendix I) for OPG shows a linear trend in the odds ratios across the levels of OPG.
Table 4.
Multivariable adjusted odds ratios for the associations of serum OPG and sRANKL levels with risk of hip fracture.
| Quartiles of OPG | Case (Ne) | Control (N) | Model #1a |
P-trend | Model #2b |
P-trend | Model #3c |
P-trend | Model #4d |
P-trend | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |||||||
| >6 pmol/L | 136 | 93 | 2.28 | (1.45, 3.61) | <0.001 | 2.33 | (1.47, 3.70) | <0.001 | 1.87 | (1.15, 3.04) | 0.02 | 1.78 | (1.09, 2.91) | 0.03 |
| 5<–6 pmol/L | 85 | 93 | 1.32 | (0.85, 2.07) | 1.36 | (0.87, 2.13) | 1.32 | (0.82, 2.12) | 1.28 | (0.79, 2.07) | ||||
| 4<–5 pmol/L | 89 | 93 | 1.39 | (0.89, 2.16) | 1.45 | (0.93, 2.26) | 1.36 | (0.85, 2.18) | 1.35 | (0.84, 2.17) | ||||
| <4 pmol/L | 70 | 94 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| OPG linearf (1 SD increase) | 380 | 373 | 1.39 | (1.17, 1.65) | <0.001 | 1.40 | (1.17, 1.66) | <0.001 | 1.31 | (1.09, 1.57) | 0.004 | 1.28 | (1.07, 1.54) | 0.009 |
| Quartiles of sRANKL | ||||||||||||||
| >0.2 pmol/L | 104 | 89 | 0.95 | (0.64, 1.40) | 0.67 | 0.91 | (0.62, 1.35) | 0.55 | 0.99 | (0.65, 1.50) | 0.90 | 1.00 | (0.66, 1.53) | 0.99 |
| 0.001<–0.1 pmol/L | 73 | 90 | 0.67 | (0.44, 1.01) | 0.64 | (0.43, 0.97) | 0.70 | (0.45, 1.07) | 0.72 | (0.46, 1.12) | ||||
| 0.001<–0.1 pmol/L | 69 | 89 | 0.65 | (0.43, 0.99) | 0.63 | (0.41, 0.95) | 0.63 | (0.40, 0.98) | 0.63 | (0.40, 0.98) | ||||
| Below detectable threshold | 134 | 106 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| sRANKL Linear (1 SD increase) | 380 | 374 | NAg | |||||||||||
Note: For sRANKL, 3-df test of association (i.e., not assuming trend) yielded p-values of 0.08, 0.06, 0.09, and 0.10 for models #1 to 4, respectively
Model #1 adjustments include age, current HT use, year of enrollment and plate.
Model #2 includes model #1 adjustments plus BMI.
Model #3 includes model #2 adjustments plus self-reported health, physical activity, and physical functioning.
Model #4 includes model #3 adjustments plus PTH (continuous variable; log-scale).
Total number of cases and controls after excluding participants with missing/invalid biomarker data, missing plate information, or incomplete covariate information for model #3.
Estimated OR (95%CI) where OPG was fit as a linear variable used model #3 adjustments.
Estimates that assumed a linear relationship between sRANKL and the log-odds of hip fracture were not computed. The association was decidedly non-linear (see Appendix I).
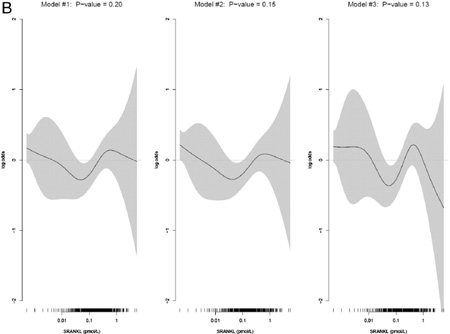
No significant associations between serum sRANKL levels and hip fracture were observed (Fig. 1, Table 4). One-third of the study population had undetectable sRANKL levels (134 (35%) of cases, 106 (28%) of controls; Fig. 1). The odds ratios comparing the highest to lowest (undetectable) quartiles were all close to 1.0 and the p-values for linear trend were non-significant in all models. Odds ratios in the middle two quartiles were in the direction of decreased risk of hip fracture suggesting a possible u-shaped relationship between sRANKL and hip fracture.
Fig.1.
Distribution of serum OPG and sRANKL levels by case–control status.
Examination of the GAM plot for sRANKL illustrates of the non-linearity of the observed association (Appendix I).
No associations were observed between the ratio of OPG/sRANKL levels and hip fracture in similar analyses to those described above. In sensitivity analyses, exclusion of non-detectable sRANKL cases and controls did not change the results, nor was there a disproportionate number of cases among non-detectable values of sRANKL (p = 0.08). The association of OPG with hip fracture did not vary by quartile of sRANKL. The OPG association with hip fracture was also not significantly modified by current HT use (p-interaction = 0.62), BMI (p = 0.07), total calcium intake (p = 0.55),or FRAX score (p = 0.97). Although not statistically significant, the odds ratios across categories of BMI suggested a stronger association of OPG levels in normal weight women, than in obese women. Odds ratios were 1.11 (95% CI 1.04–1.18) for women in the normal weight category (BMI ≤ 25); 1.07 (95% CI 1.02–1.13) for overweight women (BMI 25–<30); 1.05 (95% CI 0.98–1.12) for obese women (BMI 30–<35); and, 1.00 (95% CI 0.90–1.12) for extremely obese women (BMI ≥ 35).
In the 125 participants with available measurements, serum OPG was modestly correlated with baseline CTX (r = 0.11) and PINP (r = 0.13), whereas no correlation with baseline serum 25(OH)D (r = 0.02) was observed. sRANKL levels were uncorrelated with these biomarkers (coefficients ≤0.05 for all three comparisons).
Discussion
In this case–control study nested within the WHI Observational Study, OPG levels had an independent, linear association (log transformed scale) with increased risk of hip fracture consistent with a nearly twofold increased risk in the highest compared to lowest quartiles. No associations were observed between sRANKL or the ratio of OPG/sRANKL and incident hip fracture. Neither biomarker was associated with the current HT use. Serum OPG levels were significantly related to age, and after adjusting for age to markers of frailty [18] including low physical activity and poor physical function.
Evidence from large prospective studies regarding associations of serum OPG and sRANKL and incident fractures, particularly hip fracture, is extremely limited. Two smaller, previous studies have examined serum OPG levels in relation to incident hip fracture. In a case–control study of 490 women aged 65 and older (154 with fracture, number of hip fractures not reported) nested within the Study of Osteoporotic Fracture (SOF), the age-adjusted odds ratio for hip fracture by a 1 standard deviation difference in serum OPG was 1.3 (95% CI, 1.0–1.7) using an early generation assay [5], virtually identical to the odds ratio observed in this study. In that study, OPG levels were not associated with increased risks of total fractures or wrist fractures A case–control study of Danish women with hip fracture (n = 41) or lower forearm fracture (n = 66) compared to age-matched community controls found an odds ratio for the highest quartile of serum OPG compared to the lowest of 2.5 (95% CI, 1.3–4.7) [6]. Neither of these studies adjusted for possible confounders other than age. In the Tromso Study, serum OPG levels, but not sRANKL levels, were found to be associated with height loss in postmenopausal women, particularly those not using HT [7]. Most recently in the Tromso Study, high serum OPG levels were found to be associated with 2.8 times the risk of hip fracture in men, and 1.6 times the risk of hip fracture in women not using HT, but no association was seen in women taking HT [9]. In the present study, no interaction was seen between HT use and serum OPG levels, indicating a similar association in both users and non-users of HT. Discontinuation of HT use after baseline, which was common after publication of the WHI HT Trial results, could have contributed to this null interaction, or it may simply be absent in this population. Among the baseline HT users in the WHI Observational Study, nearly two-thirds had discontinued HT use by the eighth year of follow-up. The only study to report an association of sRANKL with any fracture was an Italian cohort of 906 (half women) who experienced 31 fractures classified as “non-traumatic” and 115 fractures classified as “traumatic” [8]. The multivariate-adjusted relative risk of nontraumatic fracture in the highest tertile (22 fractures) compared to the lowest (2 fractures) was 9.4 (95% CI, 2.1–40.1) whereas no association was found for traumatic fracture.
Associations between OPG and sRANKL with markers of bone resorption and turnover are inconsistent as are studies relating OPG and sRANKL to levels of bone mineral density [4]. For example, OPG was positively associated with BMD in women using estrogen in the Rancho Bernardo study, but no association was seen in women not using HT [3] whereas RANKL was not associated with BMD. The numerous conflicting reports may reflect either differences in study populations many of which were small and select, the assays used to measure the biomarkers, or approaches to controlling for confounding [4]. In general, more studies have found no association [2,5,19–23] between serum OPG levels and BMD, than have found either inverse or positive associations [4,7]. Most studies have found no association of sRANKL levels with bone density, even the one study reporting an association with nontraumatic fracture [4,8].
Prospective studies that have examined baseline serum OPG levels and future hip fracture suggest a hypothesis about possible mechanisms. OPG levels were inversely correlated with serum osteocalcin in the SOF case–control study [5]. Our findings support modest, positive associations between serum OPG and bone resorption markers in a subset of 125 women. Higher OPG levels were also associated with greater bone loss in women in the Tromso Study [24]. Taken together, these findings suggest the possibility that higher levels of serum OPG are indicative of higher bone resorption, lower bone formation and increased rates of bone loss leading to higher fracture risk. Measurements of bone metabolism markers and bone density were not available in all cases and controls in the present study, and therefore, we could not directly evaluate this mediation hypothesis. However, a recent study comparing cytokine production by bone marrow and bone cells in 37 women who had hip replacement due to femoral neck fractures and 15 women who had hip replacement due to osteoarthritis supports the hypothesis that hip fracture is associated with increased OPG expression, produced equally by the bone marrow and bone cells, compared to the osteoarthritis controls [25]. RANKL expression by bone marrow cells was also elevated in the fractured women, and the authors postulate that the increase in OPG was possibly a response to block RANKL in the presence of increased bone turnover.
Serum OPG, but not sRANKL levels, were directly correlated with age (r = 0.33; p < 0.01) in the present study, in agreement with many previous studies [2,4]. However, data on longitudinal changes in biomarker levels are lacking in postmenopausal women. In this study, no significant association between the current HT use and OPG levels was observed in the control women. Estrogen has been shown to increase the production of OPG by osteoblasts in vitro [26]. However, serum levels of OPG have shown no association with serum hormone levels in vivo in postmenopausal women [2]. Moreover, experimental studies examining changes in serum OPG levels after treatment with estrogen therapy in postmenopausal women show conflicting results with one study demonstrating decreased OPG levels after treatment [27] and another showing no significant change in OPG levels but a trend toward higher levels [28]. The evidence to date therefore suggests no consistent association between serum OPG and HT use in postmenopausal women.
Strengths of this study include the nested case–control design within a prospective study, the rigorous adjudication of hip fracture cases, the availability of information on a broad range of covariates, and the use of highly sensitive biomarker assays. Markers of bone resorption and measurements of BMD were not available in all cases and controls for testing hypotheses about mechanisms, but our purpose was to examine associations with hip fracture to fill this gap in the literature. As discussed above, the large volume of literature relating these biomarkers to BMD and bone metabolism markers is quite inconsistent rendering studies of fracture necessary to understand the ultimate impacts of these biomarkers. About one-third of the women had undetectable sRANKL levels which is less than the other published studies [7], but still limited the distribution on this variable.
Conclusions
We conclude that OPG levels were independently associated with a nearly twofold increased risk of hip fracture in postmenopausal women in the WHI-OS. sRANKL levels and the ratio of OPG/sRANKL were not associated with incident hip fracture. These results are consistent with the hypothesis that higher serum OPG levels are a response to elevated osteoclastogenesis and bone resorption in skeletal tissues which might lead to increased risk for hip fractures.
Acknowledgments
Funding
Funding for this project was provided by NHLBI contract # HHSN268200900002C.
The project described was supported by the National Center for Research Resources, grant UL1RR025755, KL2RR025754, and TL1RR025753, and is now at the National Center for Advancing Translational Sciences, grant 8KL2TR000112-05, 8UL1TR000090-05, 8TL1TR000091-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
WHI Investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
For a list of all the investigators who have contributed to WHI science, please visit: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Abbreviations
- OPG
osteoprotogerin
- RANK
receptor activator of NF-kappa β
- RANKL
receptor activator of NF-kappa β ligand
- sRANKL
soluble receptor activator of NF-kappa β ligand
- WHI
Women's Health Initiative
Appendix I
Fig A. Multivariable adjusted generalized additive models of the effect of SRANKL on hip fracture.
Fig B. Multivariable adjusted generalized additive models of the effect of OPG on hip fracture.
Footnotes
Conflict of Interest Disclosures
Dr. LaCroix reported serving on scientific advisory boards for Pfizer, sanofi-aventis and Amgen. No other authors reported having a financial conflict of interest.
Contributor Information
Andrea Z. LaCroix, Email: alacroix@whi.org.
Rebecca D. Jackson, Email: Rebecca.jackson@osumc.edu.
Aaron Aragaki, Email: aaragaki@whi.org.
Charles Kooperberg, Email: clk@fhcrc.org.
Jane A. Cauley, Email: jcauley@edc.pitt.edu.
Zhao Chen, Email: zchen@email.arizona.edu.
Meryl S. LeBoff, Email: mleboff@partners.org.
David Duggan, Email: dduggan@tgen.org.
Jean Wactawski-Wende, Email: jww@buffalo.edu.
References
- 1.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S, Arrighi HM, Melton LJ, III, Atkinson EJ, O'Fallon WM, Dunstan C, et al. Correlates of osteoprotegerin levels in women and men. Osteoporos Int. 2002;13:394–399. doi: 10.1007/s001980200045. [DOI] [PubMed] [Google Scholar]
- 3.Stern A, Laughlin GA, Bergstrom J, Barrett-Connor E. The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor kappaB legend with bone mineral density in older adults: the Rancho Bernardo study. Eur J Endocrinol. 2007;156:555–562. doi: 10.1530/EJE-06-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner D, Fahrleitner-Pammer A. Levels of osteoprotegerin (OPG) and receptor activator for nuclear factor kappa B ligand (RANKL) in serum: are they of any help? Wien Med Wochenschr. 2010;160:452–457. doi: 10.1007/s10354-010-0818-x. [DOI] [PubMed] [Google Scholar]
- 5.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen HL, Kusk P, Madsen B, Fenger M, Lauritzen JB. Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratios. J Bone Miner Metab. 2004;22:132–138. doi: 10.1007/s00774-003-0461-3. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen L, Hansen JB, Brox J, Mathiesen E, Vik A, Jacobsen BK. Serum osteoprotegerin levels are related to height loss: the Tromso Study. Eur J Epidemiol. 2011;26:305–312. doi: 10.1007/s10654-011-9555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schett G, Kiechl S, Redlich K, Oberhollenzer F, Weger S, Egger G, et al. Soluble RANKL and risk of nontraumatic fracture. JAMA. 2004;291:1108–1113. doi: 10.1001/jama.291.9.1108. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen L, Hansen JB, Ahmen L, Bjornerem A, Emaus N, Joakimsen R, et al. Osteoprotegerin is associated with hip fracture incidence: the Tromso Study. Int J Epidemiol. 2012;41:1033–1039. doi: 10.1093/ije/dys063. [DOI] [PubMed] [Google Scholar]
- 10.Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2002;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 11.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. WHI Morbidity and Mortality Committee, outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 13.Hays RD, Sherbourne CD, Mazel RN. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 14.Patterson RE, Kristal AR, Tinker LF, Carter TA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 16.Barbour KE, Boudreau R, Danielson ME, Youk AO, Wactawski-Wende J, Greep NC, et al. Inflammatory markers and the risk of hip fracture: the Women's Health Initiative. J Bone Miner Res. 2012;27:1167–1176. doi: 10.1002/jbmr.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–686. [Google Scholar]
- 18.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsen B, Hjelmborg JV, Kostenuik P, Stilgren LS, Kyvik K, Adamu S, et al. Circulating amounts of osteoprotegerin and RANK ligand: genetic influence and relationship with BMD assessed in female twins. Bone. 2005;36:727–735. doi: 10.1016/j.bone.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Indridason OS, Franzson L, Sigurdsson G. Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporos Int. 2005;16:417–423. doi: 10.1007/s00198-004-1699-x. [DOI] [PubMed] [Google Scholar]
- 21.Liu JM, Zhao HY, Ning G, Zhao YJ, Chen Y, Zhang Z, et al. Relationships between the changes of serum levels of OPG and RANKL with age, menopause, bone biochemical markers and bone mineral density in Chinese women aged 20–75. Calcif Tissue Int. 2005;76:1–6. doi: 10.1007/s00223-004-0007-2. [DOI] [PubMed] [Google Scholar]
- 22.Oh KW, Rhee EJ, Lee WY, Kim SW, Oh ES, Baek KH, et al. The relationship between circulating osteoprotegerin levels and bone mineral metabolism in healthy women. Clin Endocrinol (Oxf) 2004;61:244–249. doi: 10.1111/j.1365-2265.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- 23.Uemura H, Yasui T, Miyatani Y, Yamada M, Hiyoshi M, Arisawa K, et al. Circulating profiles of osteoprotegerin and soluble receptor activator of nuclear factor kappaB ligand in post-menopausal women. J Endocrinol Invest. 2008;31:163–168. doi: 10.1007/BF03345584. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen L, Vik A, Emaus N, Brox J, Hansen JB, Mathiesen E, et al. Bone loss in relation to serum levels of osteoprotegerin and nuclear factor-kappaB ligand: the Tromso Study. Osteoporos Int. 2010;21:931–938. doi: 10.1007/s00198-009-1035-6. [DOI] [PubMed] [Google Scholar]
- 25.D'Amelio P, Roato I, D'Amico L, Veneziano L, Suman E, Sassi F, et al. Bone and bone marrow pro-osteoclastogenic cytokines are up-regulated in osteoporosis fragility fractures. Osteoporos Int. 2011;22:2869–2877. doi: 10.1007/s00198-010-1496-7. [DOI] [PubMed] [Google Scholar]
- 26.Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur J Endocrinol. 2002;147:269–273. doi: 10.1530/eje.0.1470269. [DOI] [PubMed] [Google Scholar]
- 27.Han KO, Choi JT, Choi HA, Moon IG, Yim CH, Park WK, et al. The changes in circulating osteoprotegerin after hormone therapy in postmenopausal women and their relationship with oestrogen responsiveness on bone. Clin Endocrinol (Oxf) 2005;62:349–353. doi: 10.1111/j.1365-2265.2005.02221.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim JG, Kim JH, Lee DO, Kim H, Kim JY, Suh CS, et al. Changes in the serum levels of osteoprotegerin and soluble receptor activator for nuclear factor kappaB ligand after estrogen–progestogen therapy and their relationships with changes in bone mass in postmenopausal women. Menopause. 2008;15:357–362. doi: 10.1097/gme.0b013e318133a153. [DOI] [PubMed] [Google Scholar]