Abstract
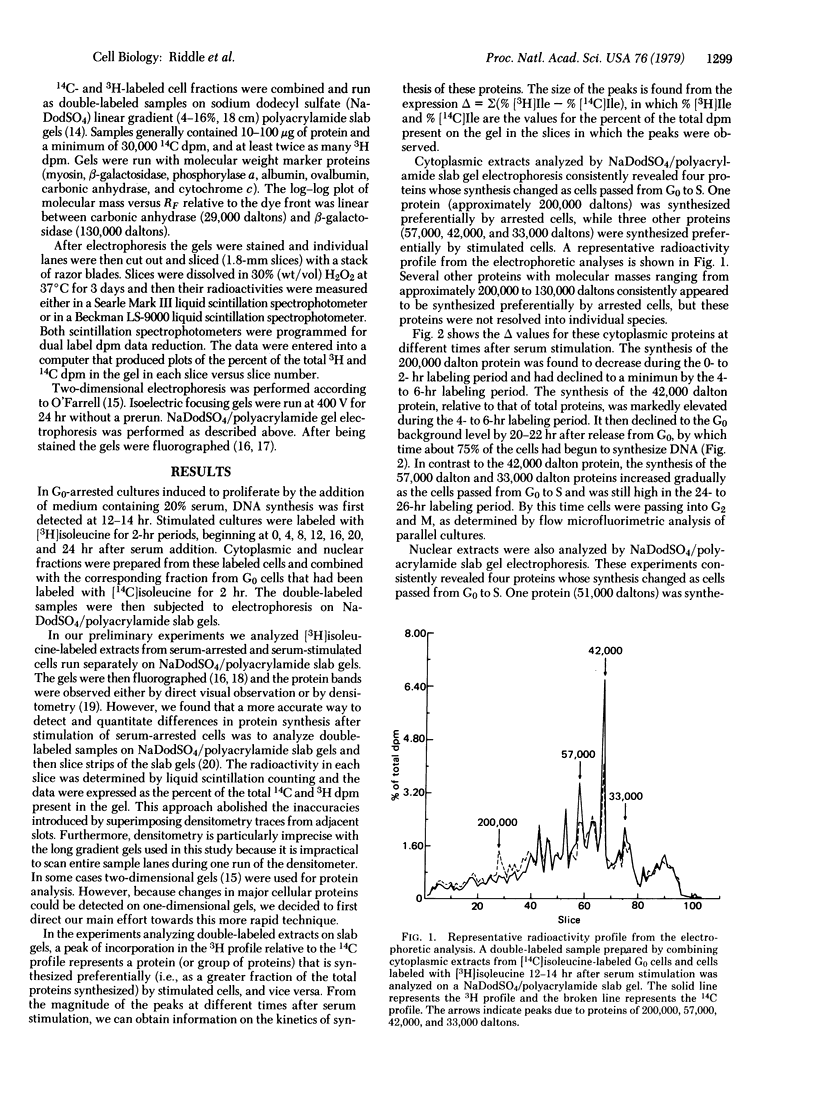
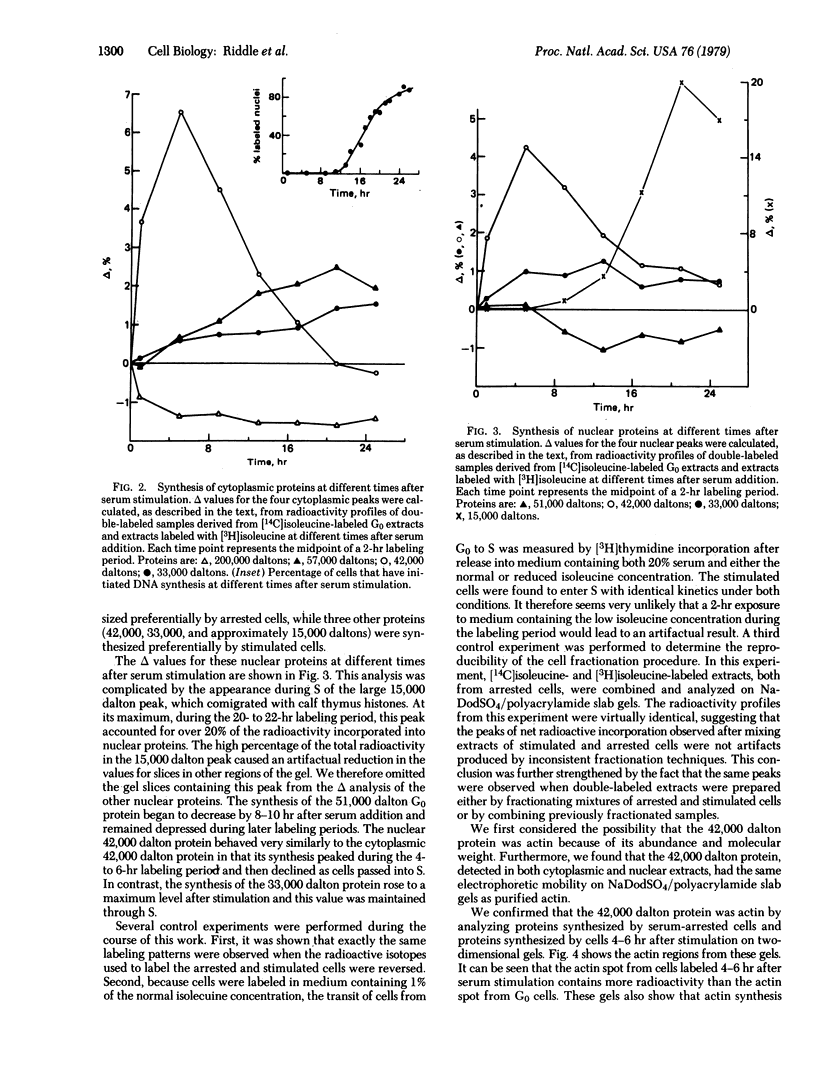
The synthesis of both cytoplasmic and nuclear proteins has been studied as quiescent, serum-deprived Swiss mouse 3T3 cells are stimulated to transit the cell cycle. In serum-arrested cells a 200,000 dalton cytoplasmic protein and a 51,000 dalton nuclear protein were found to be preferentially synthesized. In serum-stimulated cells the first major protein whose synthesis was seen to increase had a molecular mass of 42,000 daltons. This protein also showed the greatest change in synthesis during the transit from G0 to S phase. Its synthesis rose to a maximum 4--6 hr after stimulation and then declined as cells entered S phase. The protein was present in both nuclear and cytoplasmic extracts. It was identified as actin on the basis of its mobility on sodium dodecyl sulfate and isoelectric focusing polyacrylamide gels. Other proteins synthesized preferentially by stimulated cells had molecular masses of 57,000 daltons (cytoplasmic), 33,000 daltons (cytoplasmic and nuclear), and 15,000 daltons (nuclear). The synthesis of the 57,000 and 33,000 dalton proteins increased gradually after stimulation and remained high during S phase. The 15,000 dalton proteins began to be synthesized as cells entered S phase. The preferential synthesis of these proteins provides biochemical markers for the transition from quiescence to proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker H., Stanners C. P. Control of macromolecular synthesis in proliferating and resting Syrian hamster cells in monolayer culture. 3. Electrophoretic patters of newly synthesized proteins in synchronized proliferating cells and resting cells. J Cell Physiol. 1972 Aug;80(1):51–62. doi: 10.1002/jcp.1040800107. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Epifanova O. I., Abuladze M. K., Zosimovskaya A. I. Effects of low concentrations of actinomycin D on the initiation of DNA synthesis in rapidly proliferating and stimulated cell cultures. Exp Cell Res. 1975 Apr;92(1):23–30. doi: 10.1016/0014-4827(75)90632-1. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Pardee A. B. Proteins made in the mammalian cell cycle. J Biol Chem. 1971 Oct 25;246(20):6159–6165. [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gates B. J., Friedkin M. Mid-G1 marker protein(s) in 3T3 mouse fibroblast cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4959–4961. doi: 10.1073/pnas.75.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Collagen synthesis as a marker for cell type in mouse 3T3 lines. Cell. 1977 May;11(1):169–172. doi: 10.1016/0092-8674(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., James R., Paradee A. B. Evidence of the involvement of an outer membrane protein in DNA initiation. J Biol Chem. 1976 Jun 10;251(11):3470–3479. [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. I. Synthesis of histone fractions during the life cycle of mammalian cells. Arch Biochem Biophys. 1968 Nov;128(2):285–292. doi: 10.1016/0003-9861(68)90034-9. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. II. Conservation and turnover of histone fractions in mammalian cells. Arch Biochem Biophys. 1969 Mar;130(1):1–6. doi: 10.1016/0003-9861(69)90002-2. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Pardee A. B. S phase synchrony in monolayer CHO cultures. Exp Cell Res. 1976 Jul;100(2):265–275. doi: 10.1016/0014-4827(76)90147-6. [DOI] [PubMed] [Google Scholar]
- Hancock R. Conservation of histones in chromatin during growth and mitosis in vitro. J Mol Biol. 1969 Mar 28;40(3):457–466. doi: 10.1016/0022-2836(69)90165-x. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hnilica L. S. Methods for analysis of histones. Methods Enzymol. 1975;40:102–138. doi: 10.1016/s0076-6879(75)40011-8. [DOI] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Ley K. D. Detection of G1 proteins in Chinese hamster cells synchronized by isoleucine deprivation or mitotic selection. J Cell Biol. 1975 Jul;66(1):95–101. doi: 10.1083/jcb.66.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Zahn K. The synthesis of ninety proteins including actin throughout the HeLa cell cycle. J Cell Biol. 1978 Dec;79(3):833–838. doi: 10.1083/jcb.79.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S. Control of translation in cultured cells: continued synthesis and accumulation of messenger RNA in nondividing cultures. Proc Natl Acad Sci U S A. 1974 Mar;71(3):750–754. doi: 10.1073/pnas.71.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Coen D. M., Rich A. Tissue-specific forms of actin in the developing chick. Cell. 1976 Aug;8(4):521–527. doi: 10.1016/0092-8674(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Tsai R. L., Green H. Study of intracellular collagen precursors using DNA-cellulose chromatography. Nat New Biol. 1972 Jun 7;237(75):171–173. doi: 10.1038/newbio237171a0. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebel F., Baserga R. Early alterations in amino acid pools and protein synthesis of diploid fibroblasts stimulated to synthesize DNA by addition of serum. J Cell Physiol. 1969 Oct;74(2):191–202. doi: 10.1002/jcp.1040740211. [DOI] [PubMed] [Google Scholar]
- Yen A., Warrington R. C., Pardee A. B. Serum-stimulated 3T3 cells undertake a histidinol-sensitive process which G1 cells do not. Exp Cell Res. 1978 Jul;114(2):458–462. doi: 10.1016/0014-4827(78)90509-8. [DOI] [PubMed] [Google Scholar]



