Abstract
Background
The ExtaviJect® 30G autoinjector was developed to facilitate parenteral self-administration of interferon beta-1b (Extavia®), a first-line disease-modifying therapy in patients with multiple sclerosis. Our aim was to assess patient compliance with treatment when using the autoinjector, patients’ and nurses’ experiences of using the device, its tolerability, and patient satisfaction.
Methods
This was a 12-week, real-world, prospective, observational, noninterventional study conducted in nine European countries. Questionnaires were used to measure patient compliance and to assess patients’ and nurses’ experiences. All adverse events were recorded by severity, including injection site reactions or pain. Patient satisfaction and health-related quality of life were assessed using the Treatment Satisfaction Questionnaire for Medication-9 (TSQM-9) and EuroQol-5 Dimension (EQ-5D) instruments, respectively.
Results
Of 582 patients enrolled, 568 (98%) received at least one injection and attended the first follow-up visit at 6 weeks, and 542 (93%) attended the second follow-up visit at 12 weeks. For the whole study, 548 of 568 (97%) patients were compliant with treatment. Among the various questions assessing whether the device was easy and quick to use accurately, without fear of the needle, 56%–98% of patients and 59%–98% of nurses were in agreement. There were nine serious adverse events (four disease-related) reported among the 227 (39%) patients reporting adverse events. Scores increased in the TSQM-9 convenience domain between weeks 6 and 12 (P=0.0009), and in the EQ-5D visual analog scale between baseline and week 12 (P<0.0001), indicating improvement in health-related quality of life.
Conclusion
ExtaviJect 30G was convenient to use and was associated with high levels of compliance.
Keywords: autoinjector, injections, subcutaneous, interferon beta, multiple sclerosis, relapsing-remitting, patient preference, self-administration
Introduction
Subcutaneous interferon beta-1b is widely used for the treatment of multiple sclerosis (MS). Its clinical benefits have been demonstrated in numerous randomized, controlled Phase III studies in patients with clinically isolated syndrome, relapsing-remitting MS, and relapsing secondary progressive MS.1–5 Recently, a long-term follow-up study found a clinically important survival benefit associated with early interferon beta-1b treatment.6 Comparing survival rates among patients randomized to interferon beta-1b therapy with those simultaneously randomized to receive placebo during their first 2 years of therapy, a 47% reduction in all-cause mortality was found in the former group at 21 years.6
There is also a wealth of evidence supporting the long-term safety and tolerability of interferon beta-1b,7 which has accumulated since its first clinical use over 25 years ago and also from 1.3 million patient-years of exposure since it first received marketing authorization (data on file; Novartis Pharma AG, Basel, Switzerland; Bayer Schering Pharma AG, Berlin, Germany). Among the most common side effects of injectable disease-modifying therapies experienced by patients with MS are injection site pain (ISP) and injection site reactions (ISRs).8–10 Rates of ISP and ISRs associated with subcutaneous administration of interferons are generally higher than those associated with intramuscular administration, and are also higher for subcutaneous administration of interferon beta-1a than for high-dose, high-frequency administration of interferon beta-1b.8,11 Furthermore, studies investigating the role of needle diameter in ISP/ISRs have shown that administration of interferon formulations using thinner needles is clinically advantageous.11,12 A study of 120 healthy volunteers found that less ISP was associated with needles of a smaller compared with a larger outer diameter.12
Improvements in injection-related tolerance and satisfaction among patients who self-administer interferons can be achieved by using autoinjectors.9,12–18 Use of autoinjectors has also been shown to be a strong predictor of adherence17 and, in a crossover study of patients self-administering interferon beta-1b, use of autoinjectors was associated with a lower incidence of ISRs (32%) than manual injections performed without an autoinjector.18
The ExtaviJect® 30G autoinjector (Novartis Pharma AG, Basel, Switzerland; Figure 1) was developed to facilitate high-dose, high-frequency subcutaneous administration of Extavia® (interferon beta-1b; Novartis Pharma AG). This autoinjector has a thin, 30-gauge (30G) needle and includes features designed to make administration simpler and quicker than with some other autoinjectors, such as a needle cap that can remain in place while the syringe is being loaded. Therefore, it was hoped that patient satisfaction and adherence would be improved using ExtaviJect 30G, compared with using other devices or self-administration without an autoinjector.
Figure 1.

ExtaviJect® 30G autoinjector showing the thin 30-gauge needle.
Note: ExtaviJect® 30G autoinjector (Novartis Pharma AG, Basel, Switzerland).
The purpose of this 12-week European real-world observational study (EXCELLENT) conducted in patients with MS was to determine patient-reported compliance and satisfaction with treatment when using ExtaviJect 30G to self-administer interferon beta-1b subcutaneously. The study also captured the opinions of nurses who monitored patients using the device, as well as information about tolerability and health economic outcomes associated with use of the autoinjector.
Patients and methods
Patients aged 18–65 years and diagnosed with MS by McDonald criteria19 were recruited between November 2010 and December 2011 from nine European countries (Belgium, Bulgaria, France, Germany, Greece, Italy, Poland, Portugal, and Romania). A total of 110 centers were included. Patients who were able to provide written informed consent and who had been prescribed interferon beta-1b using the ExtaviJect 30G autoinjector as part of their routine clinical care were eligible to participate in the study. This included patients who were previously receiving an injectable disease-modifying therapy (including interferon beta-1b) or who were treatment-naïve. The study was evaluated and approved by an ethics committee and/or national regulatory authority in all participating countries, depending upon local requirements. The study was conducted in accordance with national guidelines and the expected standards of Good Clinical Practice, including the principles of the Declaration of Helsinki. This was a single-cohort, multicenter, noninterventional, observational study with a follow-up period of 12 weeks. A noninterventional approach was used to obtain a representative, real-world study population. All patients self-administered interferon beta-1b at the dose prescribed by their physician using the ExtaviJect 30G autoinjector. Participants attended a baseline visit and a maximum of two follow-up visits at approximately 6 (±2) weeks and/or approximately 12 (±2) weeks. Follow-up visits were conducted when patients attended the physician’s office as part of their routine clinical care and were therefore held in accordance with standard clinical practice in each country.
Treatment compliance was determined at weeks 6 and 12, and for the whole study. At weeks 6 and 12, the number of patient-reported missed injections during the 2 weeks before each visit was expressed as a percentage of the total number of injections prescribed in the same period (seven injections). For the whole study, the number of missed injections during the 2 weeks preceding both study visits was expressed as a percentage of the number of injections prescribed for those 4 weeks (14 injections). No missed injections were imputed at 12 weeks for patients who discontinued after 6 weeks. Patients reporting at least 20% of missed injections were defined as noncompliant.20 Patient and nurse evaluation of autoinjector use was assessed through questionnaires and rating scales at week 12. Patients completed the Multiple Sclerosis Treatment Concern Questionnaire and the Patient User Trial Questionnaire. Nurses completed the Trainer User Trial Questionnaire. All adverse events, including ISP or ISRs, were recorded. For ISP and ISRs, the severity levels reported by patients were collated for each type of reaction (itching/erythema, redness, swelling/inflammation, bruising, pain/stinging, and/or burning).
Patient satisfaction was assessed at the follow-up visits using the three scales of the Treatment Satisfaction Questionnaire for Medication-9 (TSQM-9) assessment, covering: effectiveness (three questions); convenience (three questions); and global satisfaction (three questions). The scores in each domain of the TSQM-9 were calculated as recommended by the instrument’s authors.21,22 Health-related quality of life at baseline and at week 12 was analyzed using both the EuroQol-5 Dimension (EQ-5D) instrument23 and the Patient-Reported Indices for MS (PRIMUS) questionnaire.24,25 Assessment of generic health outcomes using the EQ-5D instrument included the descriptive system (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and the self-rating visual analog scale measure of current health-related quality of life. Validated translations of the TSQM-9 were obtained from Quintiles (Reading, UK). Validated translations of questionnaire EQ-5D were obtained from EuroQol (Rotterdam, Netherlands). Translations of PRIMUS were provided by the sponsor in French, German, and Italian, and use was therefore confined to France, Germany, and Italy. PRIMUS was validated linguistically by forward and reverse translation and by cognitive debriefing; only two of the three independent assessment scales in the validated PRIMUS questionnaire were used (covering activity limitations and health-related quality of life). Evaluation of health care resource utilization included the number of hospitalizations since the last visit and the number of contacts with nurses and other health professionals during the course of the study due to MS.
Statistical analysis
A sample size of 580 patients was calculated to be suitably powered to allow estimation of different occurrence rates (from 10% to 50%) with a maximum 95% confidence interval width of ±0.041 points (when P=0.05). All patients enrolled in the study were included in the analysis population. Data analysis was performed using the statistics software package SAS version 9.1 (SAS Institute Inc, Cary, NC, USA). For between-group comparisons, an α-value of 0.05 was set as the significance level for all statistical tests. To analyze the relationship between different variables, the appropriate bivariate analysis was performed. For normally distributed continuous variables, strata were compared by Student’s t-test or analysis of variance models. Otherwise, continuous variables were analyzed using the nonparametric Mann–Whitney or Kruskal–Wallis test. For categorical variables, the differences between strata were analyzed using the Chi-square or Fisher’s Exact test.
Results
Baseline demographic and clinical characteristics
Of 582 patients enrolled, 568 (97.6%) received at least one injection and attended the first follow-up visit at 6 weeks, and 542 (93.1%) attended the second follow-up visit at 12 weeks (Figure 2). A total of 15 patients (ten at 6 weeks, five at 12 weeks) discontinued treatment during the study; 12 following adverse events, one owing to a dislike of injections, one after receiving a change of therapy, and one after becoming pregnant.
Figure 2.
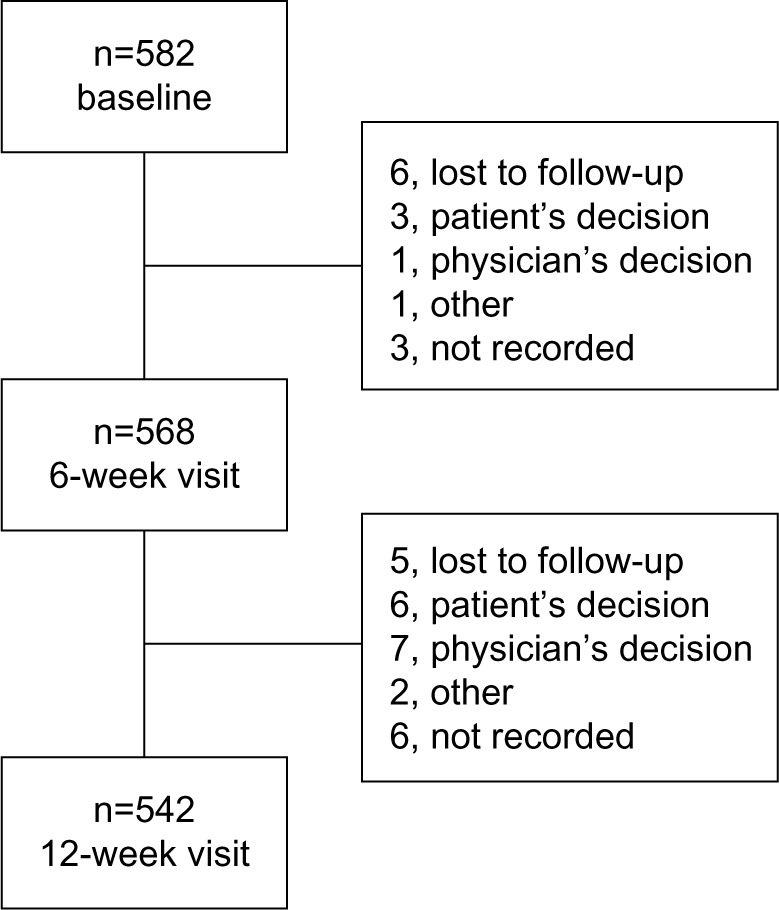
Patient flow.
Most patients (500 [85.9%]) had relapsing-remitting MS, the remainder either having secondary progressive MS or clinically isolated syndrome; baseline demographics and disease characteristics are summarized in Table 1. Mean (standard deviation [SD]) age was 40.1 (10.7) years and most patients (407 [69.9%]) were female. The mean (SD) time interval between diagnosis and the baseline visit was 66.9 (67.0) months. Most patients (374 [64.3%]) had experienced an MS relapse within the 2 years preceding the study, reporting a mean (SD) of 1.8 (1.0) relapses. Previous medical treatments for MS were reported at baseline for 468 patients (80.8%), and 111 (19.2%) patients were treatment-naïve (Table 1). A total of 314 (54.0%) patients were receiving Extavia at baseline.
Table 1.
Baseline demographic and disease characteristics
| Characteristic | n=582 |
|---|---|
| Gender [n (%)] | |
| Men | 175 (30.1) |
| Women | 407 (69.9) |
| Age, years [mean (SD)] | 40.1 (10.7) |
| Participants by country (n) | |
| Belgium | 15 |
| Bulgaria | 33 |
| France | 24 |
| Germany | 153 |
| Greece | 5 |
| Italy | 98 |
| Poland | 98 |
| Portugal | 8 |
| Romania | 148 |
| Time from first symptoms of MS, months [mean (SD)] (n=499) | 78.5 (70.3) |
| Time from MS diagnosis, months [mean (SD)] | 66.9 (67.0) |
| Subtype of MS [n (%)] | |
| Clinically isolated syndrome | 18 (3.1) |
| Relapsing-remitting MS | 500 (85.9) |
| Secondary progressive MS | 64 (11.0) |
| Patients with relapses in the last 2 years [n (%)] | 374 (64.3) |
| Relapses in the last 2 years [mean (SD)] (n=363) | 1.8 (1.0) |
| No previous treatment for MS [n (%)] (n=579) | 111 (19.2) |
| Previous medical treatment for MS [n (%)] (n=579) | 468 (80.8) |
| Type of previous treatment [n (%)]a | |
| Extavia | 327 (56.2) |
| Immune modulators (other than Extavia) | 270 (46.4) |
| Monoclonal antibodies | 7 (1.2) |
| Immunosuppressants | 21 (3.6) |
| NSAIDs | 57 (9.8) |
| Corticosteroids for acute relapses | 270 (46.4) |
| Corticosteroids for other reasons | 7 (1.2) |
| Other | 23 (4.0) |
| Type of current treatment (at baseline visit) [n (%)]a | |
| Extavia | 314 (54.0) |
| Immune modulators (other than Extavia) | 39 (6.7) |
| Monoclonal antibodies | 1 (0.2) |
| Immunosuppressants | 3 (0.5) |
| NSAIDs | 16 (2.7) |
| Corticosteroids for acute relapses | 43 (7.4) |
| Corticosteroids for other reasons | 1 (0.2) |
| Other | 14 (2.4) |
| Reasons to switch to new autoinjector [n (%)]a | |
| To treat patient with Extavia | 285 (49.0) |
| Availability of new autoinjector | 264 (45.4) |
| Compliance problems | 13 (2.2) |
| Patient not satisfied with treatment | 30 (5.2) |
| Other reasons | 24 (4.1) |
| Comorbidities at baseline visit (>20% frequency) [n (%)] | |
| Other neurologic disorder | 140 (24.1) |
| Other inflammatory disorder | 123 (21.1) |
| Other autoimmune disorder | 122 (21.0) |
| Cardiovascular and/or metabolic disease | 153 (26.3) |
| Osteoporosis | 120 (20.6) |
| Comorbidities related to MS at baseline visit (>20% frequency) [n (%)] | |
| Dysarthria | 132 (22.7) |
| Sensory disorders | 325 (55.8) |
| Ataxia | 255 (43.8) |
| Spasticity | 225 (38.7) |
| Nystagmus | 159 (27.3) |
| Visual disorders | 210 (36.1) |
| Bladder/urinary functional disorders | 200 (34.4) |
| Cognitive dysfunction | 151 (25.9) |
| Depression | 156 (26.8) |
| Fatigue | 281 (48.3) |
| Pain | 164 (28.2) |
Note:
Multiple response variable. Extavia® (Novartis Pharma AG, Basel, Switzerland).
Abbreviations: MS, multiple sclerosis; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
Treatment compliance
For the entire study period, 548 of 568 (96.5%) patients were treatment compliant (they had received more than 80% of their prescribed injections). Respective compliance levels at 6 weeks and 12 weeks were 97.4% (553/568) and 99.1% (537/542).
Injection-related questionnaires/rating scales
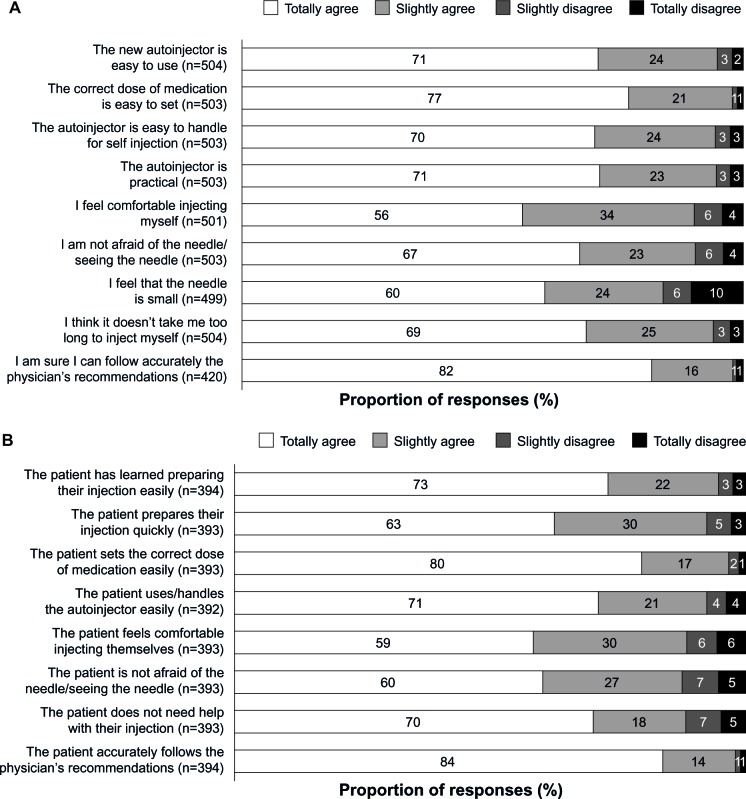
When asked to assess the practicability of the autoinjector, 93.6% (470/502) of patients considered it “easy” or “very easy” to use. The overall level of patient comfort with the autoinjector was also high (Figure 3A). Between 56% and 98% of patients agreed that the correct dose is easy to set and the device is easy to handle, that the needle is small, and they were neither afraid of it nor afraid of seeing it, that it did not take long to inject themselves, and that physician’s recommendations could be followed accurately. Furthermore, no significant differences in patient comfort level were seen comparing patients who were treatment-naïve at baseline with those previously receiving treatment for MS.
Figure 3.
Patient (A) and nurse (B) evaluations of the autoinjector.
Most nurses reported positive responses by patients relating to use of the autoinjector, with 59%–98% of nurses agreeing that patients prepared injections easily, quickly, and unaided, with the correct dose, felt comfortable injecting themselves without fear of the needle, and accurately followed the physician’s recommendations (Figure 3B). In agreement with patients’ evaluation of using the autoinjector, no significant differences in nurses’ assessments of their patients were seen when comparing patients who were treatment-naïve at baseline with those previously treated for MS.
Tolerability
In total, 227 of 582 (39%) patients experienced one or more adverse events, and 674 adverse events were recorded overall. Most (493 [73.3%]) were mild in severity; the severity of one adverse event was not reported. General disorders and administration site reactions (597 [88.7%]) were those most frequently documented. Nine serious adverse events were reported among eight patients, the most common being MS relapse (n=4). Other serious adverse events were optic neuritis (n=2), right limb hypoesthesia, critical ischemia of the second and third fingers of the right hand, and renal failure.
Eighteen adverse events leading to treatment discontinuation occurred in 12 patients. Nine patients discontinued treatment owing to tolerability concerns, the most common of which were fever (four events), ISP/ISRs (three events), and myalgia or pain in the limbs (three events). The reason for the remaining three discontinuations was given as “other”; these were an allergic reaction, Quincke’s edema, and a rash.
During follow-up visits, at least one ISR or report of ISP was recorded by 304 of 568 patients (53.5%, Table 2), and in most of these 304 patients (185 [60.9%]), ISP/ISRs were experienced only occasionally (defined as occurring after one of three injections) and were mild in severity. Among the 568 patients who received at least one injection, 222 (39.1%) experienced erythema, 206 (36.3%) pain, 95 (16.7%) itching, 84 (14.8%) swelling, and 13 (2.3%) phlebitis. Of the 504 reported ISRs, 429 (85.1%) did not require treatment, 48 (9.5%) were treated with nonsteroidal anti-inflammatory drugs, six (1.2%) with corticosteroids, and 21 (4.2%) with other medications.
Table 2.
Summary of highest levels of injection site reactions and/or pain
| Characteristic, n (%) | n=568 |
|---|---|
| Reaction and/or pain at the injection site | |
| No | 264 (46.5) |
| Yes | 304 (53.5) |
| At 6 weeks only | 69 (22.7) |
| At 12 weeks only | 35 (11.5 |
| At 6 weeks and at 12 weeks | 200 (65.8) |
| Type of reaction and/or paina | |
| Pain | 206 (36.3) |
| Itching | 95 (16.7) |
| Erythema | 222 (39.1) |
| Swelling with inflammation | 84 (14.8) |
| Phlebitis | 13 (2.3) |
| Other | 26 (4.6) |
| Frequency of reaction and/or pain, n=304 | |
| Always (after each injection) | 47 (15.5) |
| Often (after 2 of 3 injections) | 72 (23.7) |
| Occasionally (after 1 of 3 injections) | 185 (60.9) |
| Time to point of reaction and/or pain, n=304 | |
| Immediately | 130 (42.8) |
| After 30 minutes | 65 (21.4) |
| After 60 minutes | 31 (10.2) |
| >60 minutes | 78 (25.7) |
| Use of medication for treatment of reaction and/or pain, n=504b | |
| No | 429 (85.1) |
| Yes | 75 (14.9) |
| Type of medication used, n=75 | |
| NSAIDs | 48 (64.0) |
| Corticosteroids | 6 (8.0) |
| Other | 21 (28.0) |
| Frequency of medication, n=75 | |
| Always | 22 (29.3) |
| Often | 20 (26.7) |
| Occasionally | 33 (44.0) |
| Highest reported injection site reaction and pain | |
| Itching/erythema, n=518 | |
| None | 303 (58.5) |
| Mild | 143 (27.6) |
| Moderate | 61 (11.8) |
| Severe | 11 (2.1) |
| Redness, n=527 | |
| None | 184 (34.9) |
| Mild | 190 (36.1) |
| Moderate | 108 (20.5) |
| Severe | 45 (8.5) |
| Swelling/inflammation, n=514 | |
| None | 328 (63.8) |
| Mild | 107 (20.8) |
| Moderate | 63 (12.3) |
| Severe | 16 (3.1) |
| Bruising, n=517 | |
| None | 280 (54.2) |
| Mild | 130 (25.1) |
| Moderate | 78 (15.1) |
| Severe | 29 (5.6) |
| Pain, stinging and/or burning, n=525 | |
| None | 217 (41.3) |
| Mild | 160 (30.5) |
| Moderate | 107 (20.4) |
| Severe | 41 (7.8) |
Notes:
Multiple response question
number of reactions during the follow-up period.
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
Patient satisfaction and health-related quality of life
Comparing TSQM-9 assessment scores in the whole patient population between weeks 6 and 12, there was a significant increase in score in the convenience domain (P=0.0009), and nonsignificant increases in effectiveness and global satisfaction scores (Table 3). Significant increases in convenience score during this period were also seen among patients previously receiving any treatment (P=0.0030) and among patients previously treated with Extavia (P<0.0001). Increases were seen in all other domains and subgroups between weeks 6 and 12, but were not significant.
Table 3.
Treatment Satisfaction Questionnaire for Medication-9 scores at weeks 6 and 12
| TSQM-9 score
|
P-valuea | ||
|---|---|---|---|
| 6 weeks | 12 weeks | ||
| All patients, n=568 | |||
| Convenience | 52.1 (15.6) n=510 |
55.0 (16.1) n=503 |
0.0009 |
| Effectiveness | 48.0 (18.3) n=505 |
49.7 (18.6) n=497 |
0.0708 |
| Global satisfaction | 45.9 (19.7) n=508 |
47.3 (19.6) n=502 |
0.4314 |
| Treatment-naïve patients, n=111 | |||
| Convenience | 52.7 (15.2) n=100 |
55.9 (15.4) n=99 |
0.1207 |
| Effectiveness | 49.1 (17.7) n=98 |
51.1 (20.8) n=96 |
0.0681 |
| Global satisfaction | 47.1 (18.6) n=100 |
49.6 (20.4) n=99 |
0.1255 |
| Previously treated patients, n=468 | |||
| Convenience | 52.0 (15.6) n=409 |
54.8 (16.3) n=404 |
0.0030 |
| Effectiveness | 47.6 (18.3) n=406 |
49.3 (18.1) n=401 |
0.1022 |
| Global satisfaction | 45.6 (19.9) n=407 |
46.8 (19.4) n=403 |
0.9185 |
| Patients previously treated but not with Extavia, n=141 | |||
| Convenience | 53.0 (17.3) n=111 |
54.5 (20.1) n=107 |
0.9141 |
| Effectiveness | 43.4 (19.7) n=108 |
47.4 (18.9) n=106 |
0.2124 |
| Global satisfaction | 40.1 (23.9) n=109 |
42.6 (22.5) n=107 |
0.6509 |
| Patients previously treated with Extavia, n=327 | |||
| Convenience | 51.6 (15.0) n=298 |
54.9 (14.7) n=297 |
<0.0001 |
| Effectiveness | 49.1 (17.6) n=298 |
50.0 (17.7) n=295 |
0.2175 |
| Global satisfaction | 47.6 (17.9) n=298 |
48.3 (18.0) n=296 |
0.8828 |
Notes:
Comparison of scores at 6 and 12 weeks. Data are presented as the mean (standard deviation). Extavia® (Novartis Pharma AG, Basel, Switzerland).
Abbreviation: TSQM-9, Treatment Satisfaction Questionnaire for Medication-9.
Patient health-related quality of life, measured using the descriptive EQ-5D system (Table 4), found that, overall, the proportion reporting no problem in each of the five domains was numerically greater at week 12 than at baseline, and in four of the five domains more patients reported no more problems at week 12 than at baseline, despite a reduction in overall patient numbers. Of those patients who reported no health-related quality of life problems in each domain, a greater proportion was treatment-naïve than in those previously receiving treatment for MS. This difference was significant (P<0.01) at baseline in all domains except “anxiety/depression”. At 12 weeks, the proportions of previously treated patients in all domains reporting no problems had increased from baseline, so only the domains “mobility” and “usual activities” continued to be significantly different compared with treatment-naïve patients (P<0.05).
Table 4.
Descriptive EuroQol-5 Dimension system scores at baseline and week 12
| Overall
|
Previously treated
|
Treatment-naïve
|
P-valuea
|
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline, n (%) | 12 weeks, n (%) | Baseline, n (%) | 12 weeks, n (%) | Baseline, n (%) | 12 weeks, n (%) | Baseline | 12 weeks | |
| Patients reporting no problems | ||||||||
| Mobility | 314 (58.4) n=538 |
324 (63.8) n=508 |
232 (53.7) n=432 |
251 (61.2) n=410 |
82 (77.4) n=106 |
73 (74.5) n=98 |
<0.0001 | 0.0403 |
| Self-care | 462 (85.4) n=541 |
436 (85.8) n=508 |
360 (82.8) n=435 |
344 (84.1) n=409 |
102 (96.2) n=106 |
92 (92.9) n=99 |
0.0003 | 0.0751 |
| Usual activities | 325 (60.1) n=541 |
329 (64.9) n=507 |
246 (56.6) n=435 |
255 (62.5) n=408 |
79 (74.5) n=106 |
74 (74.7) n=99 |
0.0026 | 0.0441 |
| Pain/discomfort | 225 (41.7) n=540 |
273 (53.7) n=508 |
161 (37.1) n=434 |
211 (51.6) n=409 |
64 (60.4) n=106 |
62 (62.6) n=99 |
<0.0001 | 0.0899 |
| Anxiety/depression | 282 (52.1) n=541 |
320 (63.0) n=508 |
217 (49.9) n=435 |
250 (61.1) n=409 |
65 (61.3) n=106 |
70 (70.7) n=99 |
0.1117 | 0.1281 |
Note:
Previously treated versus treatment-naïve patients.
Among patients previously receiving treatment for MS, there were no significant differences in any of the descriptive EQ-5D domains between those previously receiving Extavia and those previously receiving another therapy. Analysis of the relationship at week 12 between patient satisfaction with treatment (assessed for the three TSQM-9 domains) and health-related quality of life (assessed for the five descriptive EQ-5D domains) found that patients reporting no health-related quality of life problems awarded significantly higher scores (P<0.0001) in all TSQM-9 domains than those experiencing health-related quality of life problems.
There was a significant increase in mean (SD) visual analog score between baseline and 12 weeks (3.0 [15.0]; P<0.0001), representing an improvement in patient-reported health-related quality of life. There was no significant difference when comparing the change from baseline score at week 12 between treatment-naïve and previously treated patients, or when comparing patients previously treated with Extavia with those previously receiving a different therapy. Stratification of visual analog score by severity of ISP demonstrated that increases in ISP correlated significantly with reductions in visual analog score and therefore with health-related quality of life (P<0.0001). No significant changes between baseline and week 12 were seen in either of the PRIMUS assessment scales applied.
Health care utilization
Of the 568 patients who received at least one injection, 40 (7.0%) contacted an MS nurse by telephone once or more during the follow-up period. Of these individuals, ten (25.0%) called because of ISRs, eight (20.0%) because of difficulty using the autoinjector, six (15.0%) because of ISP, and 16 (40.0%) for other reasons. Visits to the MS nurse were made by 18 (3.2%) patients, of whom four (22.2%) visited because of ISRs, four (22.2%) because of ISP, two (11.1%) because of autoinjection difficulties, and eight (44.4%) for other reasons.
Physicians were contacted by telephone by nine (1.6%) patients, all for reasons other than ISRs, ISP, or difficulty using the autoinjector, and 26 (4.6%) patients visited physicians for ISRs (three [11.5%]), ISP (one [3.8%]), or for other reasons (22 [84.6%]). Two patients (0.4%) visited an outpatient clinic and eight (1.4%) were hospitalized; mean hospitalization duration was 9.7 days.
Discussion
In this prospective, observational, real-world clinical study of patients with MS using the ExtaviJect 30G autoinjector for administration of interferon beta-1b, there was a high level of treatment compliance, and both patients and nurses regarded the device as quick and easy to use. Improvements in convenience scores and health-related quality of life were recorded during the study, and relatively few patients contacted a physician or nurse, suggesting little associated burden on the health care system.
Self-injection can be uncomfortable and inconvenient for many patients; fear of needles is common, and patients can perceive injections to be one of the burdens of their disease rather than a means of managing it.26 Autoinjectors may mitigate some of these issues by reducing the incidence of ISRs compared with manual self-injection,14,18 potentially leading to improvements in compliance. In one study, use of an autoinjector for interferon beta-1b was the strongest predictor of therapy adherence over 2 years.17 Treatment compliance is important because it is associated with better clinical and economic outcomes, including lower risks for MS relapse and MS-related hospitalization, and with lower MS-related medical costs.27 In our study, more than 95% of patients were compliant overall and at each visit, a level of adherence that is even higher than that reported recently for another autoinjector (88%) in a 12-week study that used the same compliance criteria.28
Several studies show that administration of interferon formulations using an autoinjector can increase satisfaction and/or convenience in patients with MS.12,13,15,29 Convenience and ease of use are recognized by patients as notable benefits of autoinjectors.16,28 This is echoed in our study, where questionnaires completed by patients revealed that almost all found ExtaviJect 30G “easy” or “very easy” to use and, importantly, almost all agreed they felt confident that they were following their physician’s recommendations. Additionally, almost all nurses in our study agreed that their patients were accurately following these recommendations. These findings of ease of use corroborate those of an earlier study30 in which patients with MS were asked to compare the ExtaviJect 30G autoinjector with the BetaJect® Comfort autoinjector (Bayer Schering Pharma AG). About 70% of patients said they would prefer to use the ExtaviJect 30G device, believing it to be easier to load and to handle.30 Such considerations are very important among patients who are commonly affected by loss of manual dexterity and, as summarized in Figure 3A, more than 90% of patients agreed that the ExtaviJect 30G auto-injector was practical and easy to handle for self-injection, and that it was easy to set the correct dose.
The safety and tolerability of treatment with interferon beta-1b is long established among patients with MS.7 Consistent with this, very few serious adverse events were recorded in our study, most of which were either disease-related (MS relapse) or may have been disease-related (optic neuritis and right limb hypoesthesia). Approximately half of patients reported ISRs or ISP of generally mild severity, and mostly requiring no treatment and not leading to discontinuation of therapy. Patients commonly experience ISRs or ISP when self-administering parenteral therapy.8,11 About half of the patients in our 12-week study experienced ISP/ISRs during follow-up, with erythema, pain, and itching being the most common symptoms. However, this is a considerable improvement on that seen in a study that evaluated self-assessed ISP among patients receiving interferon beta-1b, where a similar proportion (51%) of patients reported ISRs, with pain, itching, or erythema after only 6 weeks.11 Patients in the same study reported more pain-free injections when using a 29–30G needle compared with using a larger 25–27G needle,11 and similar findings have been reported elsewhere,12 so the needle gauge of ExtaviJect 30G may also be a factor determining this relatively low rate of ISP/ISR.
Improvements in the convenience of therapy were seen during our study, based on the TSQM-9 assessment, and there was a general trend toward improvement in the effectiveness and general satisfaction domains of the instrument. Improvement across the study was also seen in health-related quality of life and in most of the descriptive scales of the EQ-5D assessment, with more patients reporting no problems at the end of the study than at enrollment. There may also be benefits for health care providers associated with the use of ExtaviJect 30G, given that fewer than 10% of patients contacted their physician or nurse during the study. The main reason for contacting a health care provider was an ISR or ISP, which might be expected in the context of parenteral therapy.
Our study has several limitations: treatment compliance was patient-reported, so has the potential for recall bias and therefore overestimation; a longer follow-up period may have improved the accuracy of the compliance assessment, although a period of 12 weeks has been reported elsewhere;28 and it would have been interesting to include a TSQM-9 assessment at baseline because this may have revealed significant changes in all of the domains.
Building on these findings of the importance to patients of convenience and ease of use of autoinjectors, a new device (the ExtaviPro™ 30G, Novartis Pharma AG) is in development for subcutaneous administration of interferon beta-1b (Extavia). This new autoinjector has been ergonomically designed to sit comfortably in the hand to allow one-handed operation. It is hoped that this design could lead to improvements in convenience and support even higher rates of compliance among patients in future.
Conclusion
This prospective, observational study in a real-world setting indicates that use of the ExtaviJect 30G autoinjector to facilitate administration of interferon beta-1b (Extavia) results in a high level of compliance with therapy, accompanied by a high degree of patient satisfaction.
Acknowledgments
The authors would like to thank the patients and physicians who participated in this study and Kirstin Stricker (Novartis Pharma AG) for critical review of the manuscript.
Footnotes
Disclosure
This study was funded by Novartis Pharma AG. Sònia Rojas-Farreras is an employee of IMS Health, which was funded by Novartis Pharma AG to conduct statistical analyses of the data and to prepare the study report. Mark Tomlinson receives funding as a consultant to Novartis Pharma AG. Writing and editorial support was provided by Oxford PharmaGenesis™ Ltd and was funded by Novartis Pharma AG.
References
- 1.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 2.Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- 3.Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352:1491–1497. [No authors listed] [PubMed] [Google Scholar]
- 4.Panitch H, Miller A, Paty D, Weinshenker B. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788–1795. doi: 10.1212/01.wnl.0000146958.77317.3e. [DOI] [PubMed] [Google Scholar]
- 5.Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 6.Goodin DS, Ebers GC, Cutter G, et al. Cause of death in MS: long-term follow-up of a randomised cohort, 21 years after the start of the pivotal IFNbeta-1b study. BMJ Open. 2012;2:pii:e001972. doi: 10.1136/bmjopen-2012-001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reder AT, Ebers GC, Traboulsee A, et al. Cross-sectional study assessing long-term safety of interferon-beta-1b for relapsing-remitting MS. Neurology. 2010;74:1877–1885. doi: 10.1212/WNL.0b013e3181e240d0. [DOI] [PubMed] [Google Scholar]
- 8.Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012;18:932–946. doi: 10.1177/1352458511433302. [DOI] [PubMed] [Google Scholar]
- 9.Girouard N, Theoret G. Management strategies for improving the tolerability of interferons in the treatment of multiple sclerosis. Can J Neurosci Nurs. 2008;30:18–25. [PubMed] [Google Scholar]
- 10.Smith B, Carson S, Fu R, et al. Drug class review: Disease-modifying drugs for multiple sclerosis: Final Update 1 Report Portland, OR: Oregon Health and Science University; 2010Available from http://www.ncbi.nlm.nih.gov/books/NBK50570/Accessed August 5, 2013 [PubMed] [Google Scholar]
- 11.Baum K, O’Leary C, Coret Ferrer F, et al. Comparison of injection site pain and injection site reactions in relapsing-remitting multiple sclerosis patients treated with interferon beta-1a or 1b. Mult Scler. 2007;13:1153–1160. doi: 10.1177/1352458507079291. [DOI] [PubMed] [Google Scholar]
- 12.Jaber A, Bozzato GB, Vedrine L, et al. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. doi: 10.1186/1471-2377-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wray S, Armstrong R, Herrman C, et al. Results from the single-use autoinjector for self-administration of subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis (MOSAIC) study. Expert Opin Drug Deliv. 2011;8:1543–1553. doi: 10.1517/17425247.2011.628656. [DOI] [PubMed] [Google Scholar]
- 14.Mikol D, Lopez-Bresnahan M, Taraskiewicz S, Chang P, Rangnow J. A randomized, multicentre, open-label, parallel-group trial of the tolerability of interferon beta-1a (Rebif) administered by autoinjection or manual injection in relapsing-remitting multiple sclerosis. Mult Scler. 2005;11:585–591. doi: 10.1191/1352458505ms1197oa. [DOI] [PubMed] [Google Scholar]
- 15.Devonshire V, Arbizu T, Borre B, et al. Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb study. BMC Neurol. 2010;10:28. doi: 10.1186/1471-2377-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devonshire VA, Verdun di Cantogno E. Review of subcutaneous interferon beta-1a, delivered via the electronic self-injection device RebiSmart, for the treatment of multiple sclerosis. Ther Deliv. 2011;2:1455–1465. doi: 10.4155/tde.11.116. [DOI] [PubMed] [Google Scholar]
- 17.Pozzilli C, Schweikert B, Ecari U, Oentrich W. Supportive strategies to improve adherence to IFN beta-1b in multiple sclerosis – results of the betaPlus observational cohort study. J Neurol Sci. 2011;307:120–126. doi: 10.1016/j.jns.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Brochet B, Lemaire G, Beddiaf A. Reduction of injection site reactions in multiple sclerosis (MS) patients newly started on interferon beta 1b therapy with two different devices. Revue Neurologique. 2006;162:735–740. doi: 10.1016/s0035-3787(06)75071-8. French. [DOI] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 20.Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care. 2003;9:S136–S143. [PubMed] [Google Scholar]
- 21.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(Suppl 1):S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 24.Doward LC, McKenna SP, Meads DM, Twiss J, Eckert BJ. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS) Mult Scler. 2009;15:1092–1102. doi: 10.1177/1352458509106513. [DOI] [PubMed] [Google Scholar]
- 25.McKenna SP, Doward LC, Twiss J, et al. International development of the patient-reported outcome indices for multiple sclerosis (PRIMUS) Value Health. 2010;13:946–951. doi: 10.1111/j.1524-4733.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen BA, Rieckmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract. 2007;61:1922–1930. doi: 10.1111/j.1742-1241.2007.01561..x. [DOI] [PubMed] [Google Scholar]
- 27.Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011;28:51–61. doi: 10.1007/s12325-010-0093-7. [DOI] [PubMed] [Google Scholar]
- 28.Lugaresi A, Florio C, Brescia-Morra V, et al. Patient adherence to and tolerability of self-administered interferon beta-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC Neurol. 2012;12:7. doi: 10.1186/1471-2377-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cramer JA, Cuffel BJ, Divan V, Al-Sabbagh A, Glassman M. Patient satisfaction with an injection device for multiple sclerosis treatment. Acta Neurol Scand. 2006;113:156–162. doi: 10.1111/j.1600-0404.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 30.Kozubski W. Autoinjector improves injection-related tolerability issues in patients with multiple sclerosis. Exploring the new Extaviject 30G system for the injection of interferon beta-1b. Eur Neurol Rev. 2010;5:77–81. [Google Scholar]