Abstract
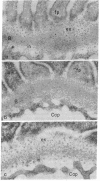
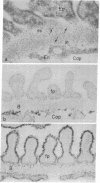
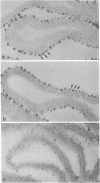
The glomerular basement membrane was subjected to digestion with specific enzymes to determine the chemical nature (sialoglycoproteins, collagenous peptides, or glycosaminoglycans) of the anionic sites previously demonstrated in the laminae rarae. Enzyme digestion was carried out both in situ and in vitro. Kidneys were perfused in situ with enzyme solutions followed by perfusion with fixative containing the cationic dye, ruthenium red, to detect the anionic sites. Glomerular basement membranes were isolated by detergent treatment of glomeruli and incubated with enzyme solutions, followed by incubation with cationized ferritin (pI 7.3-7.5) to label the anionic sites. Only highly purified enzymes free of proteolytic activity were used. The findings were the same both in situ and in vitro. The anionic sites were unaffected by treatment with neuraminidase, chondroitinase ABC, and testicular or leech hyaluronidase. However, they could no longer be demonstrated after digestion with crude heparinase, purified heparitinase, or Pronase or after nitrous acid oxidation. The results demonstrate that the sites contain heparan sulfate since they are removed by treatment with heparitinase and by nitrous acid oxidation—procedures specific for heparan sulfate; and that sialoglycoproteins or other glycosaminoglycans do not represent major components of these sites since the latter are not affected by digestion with neuraminidase and other glycosaminoglycan-specific enzymes. Identical findings were obtained on basement membranes in other locations (Bowman's capsule, tubule epithelium, and endothelium of peritubular capillaries). The presence of heparan sulfate in the glomerular basement membrane is discussed in relation to the charge-selective properties of the glomerular filter and in relation to its potential involvement in various types of glomerular injury.
Keywords: glycosaminoglycans, anionic sites, glomerular filtration
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau E. B., Haas J. E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973 Apr;28(4):477–481. [PubMed] [Google Scholar]
- Brenner B. M., Bohrer M. P., Baylis Ch, Deen W. M. Determinants of glomerular permselectivity: Insights derived from observations in vivo. Kidney Int. 1977 Oct;12(4):229–237. doi: 10.1038/ki.1977.107. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Root M. Enzymatic degradation of heparin-related mucopolysaccharides from the surface of endothelial cell cultures. Biochim Biophys Acta. 1975 Mar 14;385(1):1–10. doi: 10.1016/0304-4165(75)90067-7. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Doyle B. B., Hukins D. W., Hulmes D. J., Miller A., Woodhead-Galloway J. Collagen polymorphism: its origins in the amino acid sequence. J Mol Biol. 1975 Jan 5;91(1):79–99. doi: 10.1016/0022-2836(75)90373-3. [DOI] [PubMed] [Google Scholar]
- Hata R., Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal Biochem. 1972 Feb;45(2):462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- Hay E. D., Meier S. Glycosaminoglycan synthesis by embryonic inductors: neural tube, notochord, and lens. J Cell Biol. 1974 Sep;62(3):889–898. doi: 10.1083/jcb.62.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsing K. Immune reactions in polysaccharide media. The effect of hyaluronate, chondroitin sulphate and chondroitin sulphate-protein complex on the precipitin reaction. Biochem J. 1969 May;112(4):475–481. doi: 10.1042/bj1120475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. B. Mucosubstances of the glomerulus. Lab Invest. 1969 Aug;21(2):119–125. [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. II. Acid-soluble and -precipitable species of different cell lines. Biochemistry. 1971 Apr 13;10(8):1445–1451. doi: 10.1021/bi00784a027. [DOI] [PubMed] [Google Scholar]
- Latta H., Johnston W. H., Stanley T. M. Sialoglycoproteins and filtration barriers in the glomerular capillary wall. J Ultrastruct Res. 1975 Jun;51(3):354–376. doi: 10.1016/s0022-5320(75)80100-6. [DOI] [PubMed] [Google Scholar]
- Latta H., Johnston W. H. The glycoprotein inner layer of glomerular capillary basement membrane as a filtration barrier. J Ultrastruct Res. 1976 Oct;57(1):65–67. doi: 10.1016/s0022-5320(76)80055-x. [DOI] [PubMed] [Google Scholar]
- Laurent T. C. Interaction between proteins and glycosaminoglycans. Fed Proc. 1977 Jan;36(1):24–27. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The uses of degradative enzymes as tools for identification and structural analysis of glycosaminoglycans. Fed Proc. 1977 Jan;36(1):43–46. [PubMed] [Google Scholar]
- MANDL I., MACLENNAN J. D., HOWES E. L. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953 Dec;32(12):1323–1329. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezan E., Hjelle J. T., Brendel K., Carlson E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec 1;17(11):1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mohos S. C., Skoza L. Glomerular sialoprotein. Science. 1969 Jun 27;164(3887):1519–1521. doi: 10.1126/science.164.3887.1519. [DOI] [PubMed] [Google Scholar]
- RINEHART J. F., ABUL-HAJ S. K. An improved method for histologic demonstration of acid mucopolysaccharides in tissues. AMA Arch Pathol. 1951 Aug;52(2):189–194. [PubMed] [Google Scholar]
- Rennke H. G., Patel Y., Venkatachalam M. A. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int. 1978 Apr;13(4):278–288. doi: 10.1038/ki.1978.41. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schurer J. W., Kalicharan D., Hoedemaeker P. J., Molenaar I. The use of polyethyleneimine for demonstration of anionic sites in basement membranes and collagen fibrils. J Histochem Cytochem. 1978 Aug;26(8):688–689. doi: 10.1177/26.8.690407. [DOI] [PubMed] [Google Scholar]
- Seiler M. W., Rennke H. G., Venkatachalam M. A., Cotran R. S. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest. 1977 Jan;36(1):48–61. [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Human glomerular basement membrane. Preparation and composition. Biochemistry. 1970 Sep 15;9(19):3837–3846. doi: 10.1021/bi00821a025. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol. 1975 Dec;67(3):660–674. doi: 10.1083/jcb.67.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]