Abstract
Learning- and memory-related processes are thought to result from dynamic interactions in large-scale brain networks that include lateral and mesial structures of the temporal lobes. We investigate the impact of incidental and intentional learning of verbal episodic material on functional brain networks that we derive from scalp-EEG recorded continuously from 33 subjects during a neuropsychological test schedule. Analyzing the networks' global statistical properties we observe that intentional but not incidental learning leads to a significantly increased clustering coefficient, and the average shortest path length remains unaffected. Moreover, network modifications correlate with subsequent recall performance: the more pronounced the modifications of the clustering coefficient, the higher the recall performance. Our findings provide novel insights into the relationship between topological aspects of functional brain networks and higher cognitive functions.
Introduction
During the last years evidence has accumulated suggesting that an improved characterization of time-variant interactions between different regions within the complex network brain can be achieved with graph-theoretical approaches (see [1]–[6] for an overview). Within this framework a network (or graph) is considered as a set of nodes (or vertices) and a set of links (or edges) connecting the nodes. Functional brain networks can be derived from direct or indirect measurements of neural activity (e.g., electroencephalogram (EEG), magnetoencephalogram (MEG), or functional magnetic resonance imaging (fMRI) data). The sampled brain regions are usually considered as network nodes, and network links represent interactions between pairs of brain regions that can be assessed by evaluating interdependencies (see [7]–[11] for an overview) between their neural activities. The resulting connection schemes can then be characterized by network metrics [12] such as the average shortest path length or the clustering coefficient. The average shortest path length 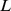 is the mean number of steps along the shortest paths for all possible pairs of nodes. The clustering coefficient
is the mean number of steps along the shortest paths for all possible pairs of nodes. The clustering coefficient  is the mean of the local clustering coefficients of all nodes and quantifies the tendency of nodes to form local clusters. The local clustering coefficient of a node is the fraction of triangles among all connected triples with the node as their center. Large values of both
is the mean of the local clustering coefficients of all nodes and quantifies the tendency of nodes to form local clusters. The local clustering coefficient of a node is the fraction of triangles among all connected triples with the node as their center. Large values of both 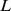 and
and  are characteristic for an ordered, lattice-like structure; low values of
are characteristic for an ordered, lattice-like structure; low values of 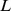 and
and  are typically observed for random networks. When analyzing functional brain networks, the clustering coefficient is often interpreted as a measure of the local efficiency of information transfer, and/or of the robustness of the network to deletion of individual nodes. Similarly, the average shortest path length is often interpreted as a measure of the global efficiency of a network to transfer information between nodes [2], [5].
are typically observed for random networks. When analyzing functional brain networks, the clustering coefficient is often interpreted as a measure of the local efficiency of information transfer, and/or of the robustness of the network to deletion of individual nodes. Similarly, the average shortest path length is often interpreted as a measure of the global efficiency of a network to transfer information between nodes [2], [5].
Higher cognitive functions are thought to result from dynamic interactions of distributed brain areas operating in large-scale networks [13]–[18]. Using graph-theoretical approaches, previous studies provided evidence that cognitive tasks–such as motor learning or (n-back) working memory–specifically modify functional brain networks derived from recordings of neural activities (EEG, MEG, fMRI) acquired during the tasks [19]–[35] and that these modifications appear to be associated with task performance [19], [24], [29], [35]. Other studies reported on associations between properties of functional brain networks recorded during a resting state condition and cognitive performance metrics assessed independently during neuropsychological evaluation [36]–[39].
Most of the aforementioned studies investigated cognitive functions that mainly involve prefrontal areas [40] and primary association cortices but only rarely temporolateral neocortex as well as important structures of the mediotemporal lobe, namely hippocampus and surrounding perirhinal cortex and parahippocampal gyri. The lateral and mesial temporal lobe structures are known to be involved in learning- and memory-related processes [41]–[49], and their functionality has frequently been investigated with fMRI [50]–[53], intracranial electrophysiological techniques [54]–[59], or via non-invasively recorded event-related electric potentials or magnetic fields [60].
Here we investigate whether incidental and intentional learning of verbal episodic material differentially modifies functional brain networks and whether modifications are related to subsequent recall performance. To this end, we assess–in a time-resolved manner–global statistical characteristics (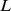 and
and  ) of functional brain networks that we derive from ongoing multichannel EEG data recorded non-invasively from healthy subjects and from epilepsy patients during a neuropsychological test schedule. Chronic epilepsy and its treatment is known to impair cognitive processes and to induce functional reorganization and behavioral compensation [61]–[63]. Investigating both healthy subjects and epilepsy patients thus provides a spectrum of recall performances that is required to study possible relationships between learning-induced network modifications and memory.
) of functional brain networks that we derive from ongoing multichannel EEG data recorded non-invasively from healthy subjects and from epilepsy patients during a neuropsychological test schedule. Chronic epilepsy and its treatment is known to impair cognitive processes and to induce functional reorganization and behavioral compensation [61]–[63]. Investigating both healthy subjects and epilepsy patients thus provides a spectrum of recall performances that is required to study possible relationships between learning-induced network modifications and memory.
Materials and Methods
Ethics statement
The study was approved by the local Ethical Committees, and all subjects gave written informed consent.
Subjects
Thirteen patients (age mean of 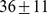 years; 8 females; mean duration of epilepsy
years; 8 females; mean duration of epilepsy 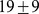 years) with pharmacoresistant epilepsies of suspected temporal or extratemporal neocortical origin as well as twenty healthy controls (age mean of
years) with pharmacoresistant epilepsies of suspected temporal or extratemporal neocortical origin as well as twenty healthy controls (age mean of 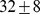 years; 10 females) were included in the study. All patients had been submitted for pre-surgical evaluation at the University of Bonn Epilepsy Program [64]. Seven patients were diagnosed as having a unilateral temporal lobe epilepsy (mesial seizure-onset zone in four patients and lateral seizure-onset zone in three patients). In five patients the seizure-onset zone was located in neocortical structures (central, parietal, parieto-central, parieto-occipital), and one patient was diagnosed as having multiple seizure-onset zones. Surgery was performed in eleven patients (selective amygdalohippocampectomy; extended lesionectomy) and led to complete seizure control in six patients.
years; 10 females) were included in the study. All patients had been submitted for pre-surgical evaluation at the University of Bonn Epilepsy Program [64]. Seven patients were diagnosed as having a unilateral temporal lobe epilepsy (mesial seizure-onset zone in four patients and lateral seizure-onset zone in three patients). In five patients the seizure-onset zone was located in neocortical structures (central, parietal, parieto-central, parieto-occipital), and one patient was diagnosed as having multiple seizure-onset zones. Surgery was performed in eleven patients (selective amygdalohippocampectomy; extended lesionectomy) and led to complete seizure control in six patients.
Neuropsychological test schedule and EEG recordings
Before participating in the neuropsychological test schedule, subjects were informed about its general outline. They will be asked to learn a set of words (learning task) and later on, to remember the learned words (retrieval task). They will also be asked to perform an extra control task during which they just have to listen to words, however they were not informed about a subsequent retrieval task. The test schedule thus consisted of two blocks of tasks which involved incidental (block 1) or intentional (block 2) learning and retrieval of verbal material (cf. Figure 1):
Figure 1. Neuropsychological test schedule.
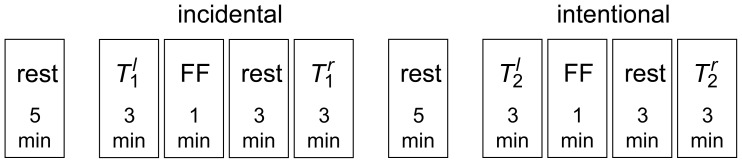
The schedule consists of two blocks of tasks which involve incidental (block 1) or intentional (block 2) learning and retrieval of verbal material. Each block is preceded by a rest phase of 5 minutes duration. Learning tasks (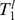 and
and 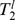 , 3 minutes duration) are followed by a figural fluency task (FF, 1 minutes duration) and a rest phase of 3 minutes duration. Retrieval tasks (
, 3 minutes duration) are followed by a figural fluency task (FF, 1 minutes duration) and a rest phase of 3 minutes duration. Retrieval tasks (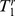 and
and 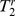 , 3 minutes duration) consist of a free recall of words listened to/memorized during the respective learning task.
, 3 minutes duration) consist of a free recall of words listened to/memorized during the respective learning task.
Block 1: during a learning task (
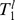 ) subjects were orally presented 15 highly frequent German nouns (cf. [65]) five times in randomized order. During the retrieval task (
) subjects were orally presented 15 highly frequent German nouns (cf. [65]) five times in randomized order. During the retrieval task (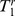 ) subjects were asked to freely recall words they listened to during
) subjects were asked to freely recall words they listened to during 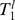 .
.Block 2: during a learning task (
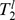 ) subjects were required to learn 15 highly frequent German nouns (different from those presented during
) subjects were required to learn 15 highly frequent German nouns (different from those presented during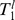 ) that were orally presented five times in randomized order. During the retrieval task (
) that were orally presented five times in randomized order. During the retrieval task (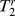 ) subjects were asked to freely recall words memorized during
) subjects were asked to freely recall words memorized during 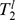 .
.
Learning and retrieval tasks lasted 3 minutes each and were separated by a figural fluency task (to minimize recency effects, to discourage verbal rehearsal, and to avoid interference of any verbal information with the learned verbal material) of 1 minute duration followed by a rest phase of 3 minutes duration. Blocks were separated by a rest phase (with eyes open) of 5 minutes duration, and a baseline recording (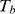 ) of 5 minutes was performed with eyes open preceding the experiment. In order to minimize possible order effects, we balanced the sequence of the blocks over subjects.
) of 5 minutes was performed with eyes open preceding the experiment. In order to minimize possible order effects, we balanced the sequence of the blocks over subjects.
The number of correctly retrieved words (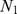 and
and 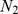 ; excluding perseverations) during the retrieval tasks served as measure of verbal memory (recall performance). Control subjects recalled 9.1±3.5 (range: 3–14) words (
; excluding perseverations) during the retrieval tasks served as measure of verbal memory (recall performance). Control subjects recalled 9.1±3.5 (range: 3–14) words (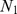 ) during
) during 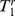 , and 11.5±2.8 (range: 6–15) words (
, and 11.5±2.8 (range: 6–15) words (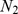 ) during
) during 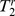 . Epilepsy patients recalled 5.6±3.6 (range: 0–11) words (
. Epilepsy patients recalled 5.6±3.6 (range: 0–11) words (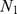 ) during
) during 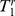 , and 7.7±3.5 (range: 3–13) words (
, and 7.7±3.5 (range: 3–13) words (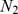 ) during
) during 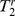 . The correlation between
. The correlation between 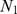 and
and 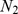 (Pearson's correlation coefficient; control subjects:
(Pearson's correlation coefficient; control subjects: 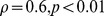 ; epilepsy patients:
; epilepsy patients: 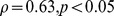 ) as well as the difference between the distributions of performance measures (t-tests; both groups:
) as well as the difference between the distributions of performance measures (t-tests; both groups: 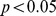 ) proved significant. Control subjects performed significantly better than epilepsy patients (t-tests; both tasks:
) proved significant. Control subjects performed significantly better than epilepsy patients (t-tests; both tasks: 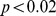 ), and both groups showed a significantly increased recall performance during
), and both groups showed a significantly increased recall performance during 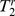 (t-tests; both groups:
(t-tests; both groups: 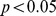 ).
).
During the whole examination procedure EEG data were acquired continuously at a sampling rate of 254.31 Hz (16 bit A/D conversion) within a bandwidth of 0–50 Hz from 29 electrodes, and right mastoid served as physical reference. Locations and nomenclature of these electrodes are standardized by the American Electroencephalographic Society [66]. In addition, we recorded right horizontal and vertical electrooculogram. In order to minimize the influence of technical (e.g., amplifier resets) and physiologic artifacts (e.g., eye movements/blinks or head movements) we applied a wavelet-based correction scheme as described in detail elsewhere [67].
Patients were investigated, on average, seven days prior to pre-surgical evaluation with blood levels of the anticonvulsant medication within the therapeutic range.
Construction of functional networks
To construct functional networks from EEG data we associated network nodes with EEG electrodes (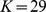 ) and inferred network links by estimating interdependencies between EEG time series from pairs of brain regions, regardless of their anatomical connectivity. For this purpose, and motivated by an increasing evidence for (phase) synchronization between distant brain areas to play an important role in learning- and memory-related processes [49], [68], [69], we employed the concept of time-variant phase coherence [70] which is an established method for studying time-variant changes in phase synchronization. The mean phase coherence [71]
) and inferred network links by estimating interdependencies between EEG time series from pairs of brain regions, regardless of their anatomical connectivity. For this purpose, and motivated by an increasing evidence for (phase) synchronization between distant brain areas to play an important role in learning- and memory-related processes [49], [68], [69], we employed the concept of time-variant phase coherence [70] which is an established method for studying time-variant changes in phase synchronization. The mean phase coherence [71]
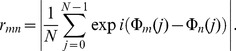 |
(1) |
is the temporal average of the differences of the instantaneous phases 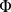 of time series from nodes
of time series from nodes  and
and  , and
, and 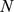 denotes the number of data points. By definition,
denotes the number of data points. By definition, 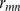 is confined to the interval [0,1] where
is confined to the interval [0,1] where 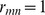 indicates fully phase-synchronized systems. We used the analytic signal approach [72], [73] with which instantaneous phases are obtained from the Hilbert transform of a time series. An important property of the analytic signal approach is that the the instantaneous frequency–in case of two or more superimposed oscillatory components–relates to the predominant frequency in the Fourier spectrum [74], [75] which may be subject to fluctuations in the time series. In such a case the instantaneous frequency varies rhythmically around the predominant frequency resulting in spurious estimates of the instantaneous phase. However, such effects can be reduced by taking the temporal average (cf. Eq. 1) or by applying adaptive approximation methods proposed recently [76]. From an electrophysiological point of view, we consider it more reasonable to look adaptively (via the Hilbert transform) at synchronization between predominant rhythms in the EEG than to look at synchronization in some a priori fixed frequency bands (e.g., via wavelet) for which there is no power in the time series (cf. Refs. [75], [77], [78]).
indicates fully phase-synchronized systems. We used the analytic signal approach [72], [73] with which instantaneous phases are obtained from the Hilbert transform of a time series. An important property of the analytic signal approach is that the the instantaneous frequency–in case of two or more superimposed oscillatory components–relates to the predominant frequency in the Fourier spectrum [74], [75] which may be subject to fluctuations in the time series. In such a case the instantaneous frequency varies rhythmically around the predominant frequency resulting in spurious estimates of the instantaneous phase. However, such effects can be reduced by taking the temporal average (cf. Eq. 1) or by applying adaptive approximation methods proposed recently [76]. From an electrophysiological point of view, we consider it more reasonable to look adaptively (via the Hilbert transform) at synchronization between predominant rhythms in the EEG than to look at synchronization in some a priori fixed frequency bands (e.g., via wavelet) for which there is no power in the time series (cf. Refs. [75], [77], [78]).
For further analyses and to allow an interval-based estimation of the mean phase coherence, we split the EEG time series into consecutive time intervals of 16.1 s duration each (corresponding to 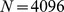 data points) and estimated, for each time interval, the elements
data points) and estimated, for each time interval, the elements 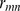 of the phase synchronization matrix R. From this matrix we constructed binary networks using a thresholding approach, and set the non-diagonal elements of the adjacency matrix A to
of the phase synchronization matrix R. From this matrix we constructed binary networks using a thresholding approach, and set the non-diagonal elements of the adjacency matrix A to 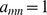 if the corresponding entry
if the corresponding entry 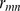 of R exceeded a threshold
of R exceeded a threshold  , and to
, and to 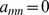 otherwise (
otherwise (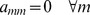 ). The choice of a threshold is not trivial, and there is currently no commonly accepted method to infer links from estimators of signal interdependence. However, since the number of nodes and the link density can affect network characteristics [79]–[85] precautions must be taken to minimize such influences when comparing between different networks. In order to determine
). The choice of a threshold is not trivial, and there is currently no commonly accepted method to infer links from estimators of signal interdependence. However, since the number of nodes and the link density can affect network characteristics [79]–[85] precautions must be taken to minimize such influences when comparing between different networks. In order to determine  we requested that the networks are sparse (
we requested that the networks are sparse (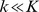 ) and that they do not disintegrate into unconnected network components as this leads to infinite values for the average shortest path length. Connectedness is guaranteed for mean degrees
) and that they do not disintegrate into unconnected network components as this leads to infinite values for the average shortest path length. Connectedness is guaranteed for mean degrees 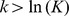 , at least for random networks [86], and with the fixed number of nodes
, at least for random networks [86], and with the fixed number of nodes 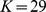 we obtained a mean degree
we obtained a mean degree 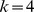 , such that
, such that 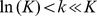 holds.
holds.
Computation of statistical network characteristics
Here we considered the average shortest path length 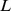 and the clustering coefficient
and the clustering coefficient  as global statistical characteristics of a functional brain network. We used an algorithm proposed in Ref. [87] to determine the shortest paths between all pairs of nodes from the adjacency matrix A. The length of the shortest path
as global statistical characteristics of a functional brain network. We used an algorithm proposed in Ref. [87] to determine the shortest paths between all pairs of nodes from the adjacency matrix A. The length of the shortest path 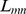 between two nodes
between two nodes  and
and  is the minimum number of links to traverse in order to get from
is the minimum number of links to traverse in order to get from  to
to  (or vice versa). With this definition we derived the average shortest path length
(or vice versa). With this definition we derived the average shortest path length 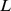 of the network by taking the average of all
of the network by taking the average of all 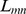 (
(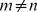 ) with
) with 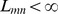 . The clustering coefficient
. The clustering coefficient 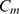 for node
for node  can be defined as (see, e.g., [1]):
can be defined as (see, e.g., [1]):
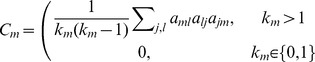 |
(2) |
where 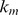 denotes the degree, i.e., the number of in- or out-going connections of node
denotes the degree, i.e., the number of in- or out-going connections of node  . We averaged
. We averaged 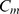 over all nodes to obtain the clustering coefficient
over all nodes to obtain the clustering coefficient  of a network.
of a network.
In the following, we consider the baseline recording (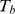 ) and the learning tasks (
) and the learning tasks (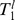 and
and 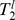 ) only. We omitted the retrieval tasks (
) only. We omitted the retrieval tasks (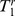 and
and 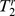 ) and the figural fluency task in order to avoid the influence of movement-related artifacts. With the interval-based estimation of interdependencies we obtained for each subject 11 intervals for
) and the figural fluency task in order to avoid the influence of movement-related artifacts. With the interval-based estimation of interdependencies we obtained for each subject 11 intervals for 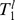 and for
and for 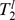 and 18 intervals for
and 18 intervals for 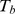 . Averaging over intervals resulted in mean network characteristics which we denote as
. Averaging over intervals resulted in mean network characteristics which we denote as 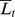 and
and 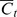 (the suffix
(the suffix  denotes task).
denotes task).
Results
Task-related modifications of functional brain networks
In Figure 2 we show, for an exemplary epilepsy patient and an exemplary control subject, temporal evolutions of the network characteristics during different neuropsychological tasks. Independent of tasks, both characteristics exhibited considerable fluctuations over time, and the patient's fluctuations were more pronounced. In both subjects, the learning tasks (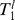 and
and 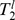 ) did not appear to modify the average shortest path length of the functional brain networks, as we could not observe clear-cut differences in
) did not appear to modify the average shortest path length of the functional brain networks, as we could not observe clear-cut differences in 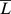 between any tasks. The same holds true for the clustering coefficient of the patient's functional brain network. For the control subject however, the intentional learning task (
between any tasks. The same holds true for the clustering coefficient of the patient's functional brain network. For the control subject however, the intentional learning task (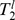 ) led to a significant increase of the clustering coefficient
) led to a significant increase of the clustering coefficient 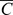 (when compared to both
(when compared to both 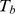 and
and 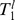 ; t-tests;
; t-tests; 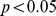 ). Both network characteristics attained slightly higher values in the control subject during all tasks. When comparing the characteristics' distributions from the patient group and the control group however, we could not observe any significant differences between the groups despite their significantly different recall performances. For the binary networks considered here, this observation is in line with our previous results [67]. In the following, we present our findings obtained from merged groups.
). Both network characteristics attained slightly higher values in the control subject during all tasks. When comparing the characteristics' distributions from the patient group and the control group however, we could not observe any significant differences between the groups despite their significantly different recall performances. For the binary networks considered here, this observation is in line with our previous results [67]. In the following, we present our findings obtained from merged groups.
Figure 2. Exemplary temporal evolutions of global statistical network characteristics during different neuropsychological tasks.
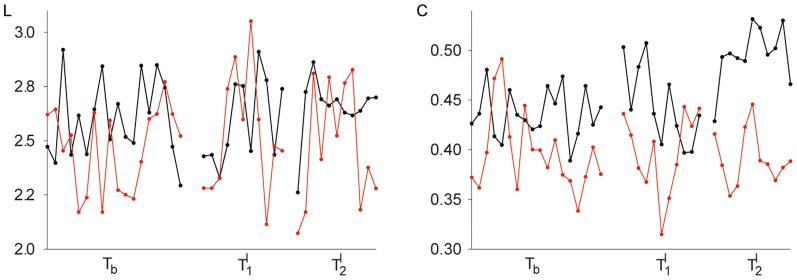
Time courses of average shortest path length 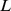 (left) and clustering coefficient
(left) and clustering coefficient  (right) from an epilepsy patient (red lines) and a control subject (black lines). Baseline recording (
(right) from an epilepsy patient (red lines) and a control subject (black lines). Baseline recording (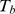 ); incidental learning task (
); incidental learning task (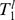 ); intentional learning task (
); intentional learning task (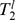 ). Lines are for eye-guidance only.
). Lines are for eye-guidance only.
In order to investigate whether the aforementioned influences of neuropsychological tasks on network characteristics extend beyond exemplary data, we estimated–for all subjects and each task–statistical properties of the distributions of 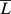 and
and 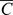 and evaluated possible differences between tasks (ANOVA with Greenhouse-Geisser and Huynh-Feldt corrections for departure from sphericity;
and evaluated possible differences between tasks (ANOVA with Greenhouse-Geisser and Huynh-Feldt corrections for departure from sphericity; 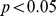 ). The distributions differed between tasks for the clustering coefficient but not for the average shortest path length. From the box-plots shown in Figure 3 it can be deduced that both learning tasks did not significantly modify the average shortest path length
). The distributions differed between tasks for the clustering coefficient but not for the average shortest path length. From the box-plots shown in Figure 3 it can be deduced that both learning tasks did not significantly modify the average shortest path length 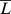 of the functional networks. The intentional learning task
of the functional networks. The intentional learning task 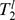 , however, led to a small but significant increase of the networks' clustering coefficient (
, however, led to a small but significant increase of the networks' clustering coefficient (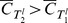 and
and 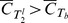 ; t-tests;
; t-tests; 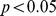 ).
).
Figure 3. Comparison of the distributions of global statistical network characteristics from 33 subjects for different neuropsychological tasks.

Box-plots of the average shortest path length 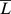 (left) and the clustering coefficient
(left) and the clustering coefficient 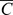 (right) for the baseline recording (
(right) for the baseline recording (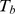 ) and for the incidental (
) and for the incidental (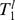 ) and the intentional learning task (
) and the intentional learning task (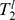 ). Bottom and top of a box are the first and third quartiles, and the band inside a box is the median. The ends of the whiskers represent the minimum and maximum of the data.
). Bottom and top of a box are the first and third quartiles, and the band inside a box is the median. The ends of the whiskers represent the minimum and maximum of the data.
Relationship between network modifications and recall performance
We investigated whether network modifications induced during the learning tasks (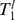 and
and 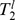 ) are related to the number of words (
) are related to the number of words (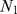 and
and 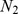 ) subjects recalled during the subsequent retrieval tasks (
) subjects recalled during the subsequent retrieval tasks (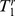 and
and 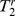 ). Given the high interindividual variability (cf. Figure 3) we focused on the relative deviation of the average shortest path length and of the clustering coefficient during the learning tasks from the respective values during the baseline recording
). Given the high interindividual variability (cf. Figure 3) we focused on the relative deviation of the average shortest path length and of the clustering coefficient during the learning tasks from the respective values during the baseline recording 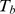 , i.e.
, i.e. 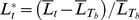 and
and 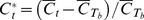 , where
, where  denotes
denotes 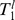 and
and 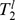 , respectively.
, respectively.
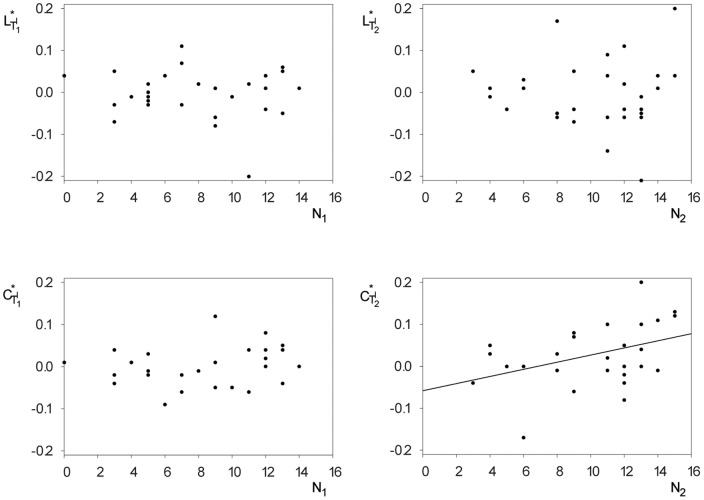
For the whole group of subjects, both characteristics deviated up to 20% from the baseline (cf. Figure 4). On average, however, deviations of the average shortest path length were close to zero (0.3% for 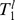 ; 0.2% for
; 0.2% for 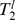 ; no significant difference), while those of the clustering coefficient amounted to 2.9% for
; no significant difference), while those of the clustering coefficient amounted to 2.9% for 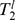 , being significantly higher than the deviation for
, being significantly higher than the deviation for 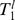 (0.2%; t-test;
(0.2%; t-test; 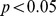 ). Interestingly, only for the intentional learning task–for which subjects were explicitly instructed to learn the presented material–could we observe a significant positive correlation (Pearson's correlation coefficient
). Interestingly, only for the intentional learning task–for which subjects were explicitly instructed to learn the presented material–could we observe a significant positive correlation (Pearson's correlation coefficient 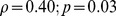 ; cf. Figure 5) between the relative clustering coefficient
; cf. Figure 5) between the relative clustering coefficient 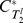 and the number
and the number 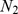 of subsequently retrieved words. Due to the aforementioned significant correlation between the recall performances,
of subsequently retrieved words. Due to the aforementioned significant correlation between the recall performances, 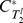 was also positively correlated to the number of words
was also positively correlated to the number of words 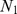 acquired during
acquired during 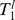 (Pearson's correlation coefficient
(Pearson's correlation coefficient 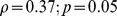 ). Of note, there were no significant correlations between the recall performances and the characteristics
). Of note, there were no significant correlations between the recall performances and the characteristics  and
and  of the networks from the baseline recording
of the networks from the baseline recording  .
.
Figure 4. Comparison of the distributions of task-induced modifications of global statistical network characteristics.
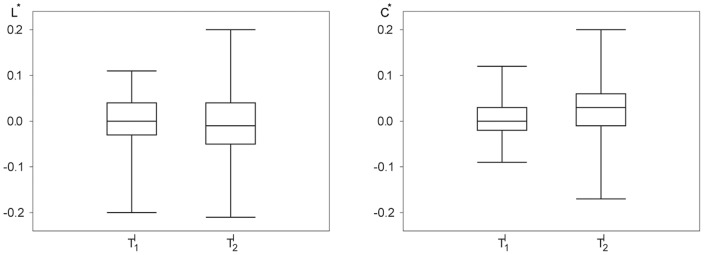
Box-plots (see Figure 3 for details) of the relative deviation of the average shortest path length 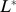 (left) and of the clustering coefficient
(left) and of the clustering coefficient 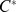 (right) during the learning tasks (
(right) during the learning tasks (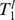 and
and 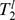 ) from the respective values during the baseline recording
) from the respective values during the baseline recording 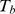 .
.
Figure 5. Relationships between task-induced modifications of global statistical network characteristics and recall performances.
Scatterplots of relative deviations of the average shortest path length 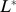 (top) and of the clustering coefficient
(top) and of the clustering coefficient 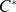 (bottom) during incidental
(bottom) during incidental 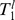 (left) and intentional learning
(left) and intentional learning 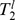 (right) from the respective values during the baseline recording and subsequent recall performances
(right) from the respective values during the baseline recording and subsequent recall performances 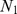 and
and 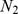 . A significant correlation could only be observed between the relative clustering coefficient
. A significant correlation could only be observed between the relative clustering coefficient 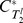 and the number
and the number 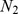 of recalled words during
of recalled words during 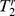 (linear regression is represented with a solid black line).
(linear regression is represented with a solid black line).
Conclusions
We investigated whether functional brain networks are differentially modified during incidental and intentional learning of verbal episodic material and whether modifications are related to subsequent recall performance. We assessed the two global statistical characteristics–clustering coefficient and average shortest path length–of functional brain networks that we derived from ongoing multichannel EEG data recorded from 33 subjects during a neuropsychological test schedule. Despite the fact that learning and memory-related processes involve structures of the lateral and mesial temporal lobes, whose dynamics may not be directly accessible with non-invasive EEG recordings, we observed differential learning-related modifications of the networks' global statistical properties. While there were no detectable modifications of the average shortest path length, networks attained a significantly higher clustering coefficient during intentional learning as compared to incidental learning and to the resting state. Interestingly, modifications during intentional learning even allowed us to predict the subsequent recall performance: the more pronounced the modifications of the clustering coefficient the higher the recall performance. An elevated clustering coefficient might be indicative of an increased occurrence of small but tightly connected groups of network nodes. Identification of these groups (or communities) using appropriate analysis techniques [88], [89] may aid in further exploration of the biological relevance of communities [29] and may provide novel insights into the relationship between the topology of functional brain networks and cognitive functions.
Our findings are in line with some previous reports on task-related modifications of global properties of functional brain networks and their relationship to task performance (e.g. [19], [24], [29], [35]) and support the view that higher cognitive functions–such as learning- and memory-related processes–result from dynamic interactions of distributed brain areas operating in large-scale networks. The differences seen for intentional and for incidental learning, however, indicate that task-induced topological changes of functional brain networks may not only reflect the cognitive process per se but also attentional effects [90]–[92]. Exploring the interactions between attention-related networks and those important for learning and memory remains a challenging task.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (Grant No. SFB-TR3 sub-project A2 to KL and HH; Grant Nos. LE660/4-1 and LE660/4-2 to KL) and by the Bundesministerium für Bildung und Forschung (Grant No. 01GQ0702 “Bernstein Group Magdeburg” to HH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang DU (2006) Complex networks: Structure and dynamics. Phys Rep 424: 175–308. [Google Scholar]
- 2. Reijneveld JC, Ponten SC, Berendse HW, Stam CJ (2007) The application of graph theoretical analysis to complex networks in the brain. Clin Neurophysiol 118: 2317–2331. [DOI] [PubMed] [Google Scholar]
- 3. Ioannides AA (2007) Dynamic functional connectivity. Curr Opin Neurobiol 17: 161–170. [DOI] [PubMed] [Google Scholar]
- 4. Arenas A, Díaz-Guilera A, Kurths J, Moreno Y, Zhou C (2008) Synchronization in complex networks. Phys Rep 469: 93–153. [Google Scholar]
- 5. Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198. [DOI] [PubMed] [Google Scholar]
- 6. Bullmore E, Sporns O (2013) The economy of brain network organization. Nat Rev Neurosci 13: 336–349. [DOI] [PubMed] [Google Scholar]
- 7. Pikovsky AS, Rosenblum MG, Kurths J (2001) Synchronization: A universal concept in nonlinear sciences. Cambridge, UK: Cambridge University Press.
- 8. Pereda E, Quian Quiroga R, Bhattacharya J (2005) Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol 77: 1–37. [DOI] [PubMed] [Google Scholar]
- 9. Hlaváčková-Schindler K, Paluš M, Vejmelka M, Bhattacharya J (2007) Causality detection based on information-theoretic approaches in time series analysis. Phys Rep 441: 1–46. [Google Scholar]
- 10. Lehnertz K, Bialonski S, Horstmann MT, Krug D, Rothkegel A, et al. (2009) Synchronization phenomena in human epileptic brain networks. J Neurosci Methods 183: 42–48. [DOI] [PubMed] [Google Scholar]
- 11.Lehnertz K, Krug D, Staniek M, Glusenkamp D, Elger CE (2011) Preictal directed interactions in epileptic brain networks. In: Osorio I, Zaveri H, Frei M, Arthurs S, In:editors, Epilepsy: The Intersection of Neurosciences, Biology, Mathematics, Engineering and Physics, Boca Raton, FL: CRC Press. pp. 265–272. [Google Scholar]
- 12. da F Costa L, Rodrigues FA, Travieso G, Boas PRV (2007) Characterization of complex networks: A survey of measurements. Adv Phys 56: 167–242. [Google Scholar]
- 13. Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 14. Varela FJ, Lachaux JP, Rodriguez E, Martinerie J (2001) The brain web: Phase synchronization and large-scale integration. Nat Rev Neurosci 2: 229–239. [DOI] [PubMed] [Google Scholar]
- 15. Robertson LC (2003) Binding, spatial attention and perceptual awareness. Nat Rev Neurosci 4: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6: 285–296. [DOI] [PubMed] [Google Scholar]
- 17. Bressler SL, Menon V (2010) Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14: 277–290. [DOI] [PubMed] [Google Scholar]
- 18. Dehaene S, Changeux JP (2011) Experimental and theoretical approaches to conscious processing. Neuron 70: 200–227. [DOI] [PubMed] [Google Scholar]
- 19. Weiss S, Rappelsberger P (2000) Long-range EEG synchronization during word encoding correlates with successful memory performance. Cogn Brain Res 9: 299–312. [DOI] [PubMed] [Google Scholar]
- 20. Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E (2006) Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA 103: 19518–19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, et al. (2006) Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res 87: 60–66. [DOI] [PubMed] [Google Scholar]
- 22. Astolfi L, Fallani FDV, Cincotti F, Mattia D, Bianchi L, et al. (2008) Neural basis for brain responses to TV commercials: A high-resolution EEG study. IEEE Trans Neural Syst Rehabil Eng 16: 522–531. [DOI] [PubMed] [Google Scholar]
- 23. Valencia M, Martinerie J, Dupont S, Chavez M (2008) Dynamic small-world behavior in functional brain networks unveiled by an event-related networks approach. Phys Rev E 77: 050905 (R).. [DOI] [PubMed] [Google Scholar]
- 24. Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, et al. (2009) Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci USA 106: 11747–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dimitriadis SI, Laskaris NA, Tsirka V, Vourkas M, Micheloyannis S, et al. (2010) Tracking brain dynamics via time-dependent network analysis. J Neurosci Methods 193: 145–155. [DOI] [PubMed] [Google Scholar]
- 26. Palva S, Monto S, Palva JM (2010) Graph properties of synchronized cortical networks during visual working memory maintenance. NeuroImage 49: 3257–3268. [DOI] [PubMed] [Google Scholar]
- 27. Palva JM, Monto S, Kulashekhar S, Palva S (2010) Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci USA 107: 7580–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang H, Douw L, Hernández JM, Reijneveld JC, Stam CJ, et al. (2010) Effect of tumor resection on the characteristics of functional brain networks. Phys Rev E 82: 021924. [DOI] [PubMed] [Google Scholar]
- 29.Bassett DS (2010) Clinical applications of complex network analysis. In: Sporns O, editor, Short Course III - Analysis and Function of Large-Scale Brain Networks, Washington, DC, USA: Society for Neuroscience. pp. 55–63. [Google Scholar]
- 30. Kitzbichler MG, Henson RNA, Smith ML, Nathan PJ, Bullmore ET (2011) Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci 31: 8259–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moussa MN, Vechlekar CD, Burdette JH, Steen MR, Hugenschmidt CE, et al. (2011) Changes in cognitive state alter human functional brain networks. Front Hum Neurosci 5: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schinkel S, Zamora-López G, Dimigen O, Sommer W, Kurths J (2011) Functional network analysis reveals differences in the semantic priming task. J Neurosci Methods 197: 333–339. [DOI] [PubMed] [Google Scholar]
- 33. Tsirka V, Simos PG, Vakis A, Kanatsouli K, Vourkas M, et al. (2011) Mild traumatic brain injury: Graph-model characterization of brain networks for episodic memory. Int J Psychophysiol 79: 89–96. [DOI] [PubMed] [Google Scholar]
- 34. Vlooswijk MCG, Vaessen MJ, Jansen JFA, de Krom MCFTM, Majoie HJM, et al. (2011) Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology 77: 938–944. [DOI] [PubMed] [Google Scholar]
- 35. Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET (2013) Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci 33: 5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P (2007) Small-world networks and functional connectivity in Alzheimer's disease. Cereb Cortex 17: 92–99. [DOI] [PubMed] [Google Scholar]
- 37. van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE (2009) Efficiency of functional brain networks and intellectual performance. J Neurosci 29: 7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Douw L, Schoonheim MM, Landi D, van der Meer ML, Geurts JJG, et al. (2011) Cognition is related to resting-state small-world network topology: An magnetoencephalographic study. Neuroscience 175: 169–177. [DOI] [PubMed] [Google Scholar]
- 39. Langer N, Pedroni A, Gianotti LRR, Hanggi J, Knoch D, et al. (2012) Functional brain network efficiency predicts intelligence. Hum Brain Mapp 33: 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buckner RL, Wheeler ME (2001) The cognitive neuroscience of remembering. Nat Rev Neurosci 2: 624–634. [DOI] [PubMed] [Google Scholar]
- 41. Alvarez P, Squire LR (1994) Memory consolidation and the medial temporal-lobe – A simple network model. Proc Natl Acad Sci USA 91: 7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Helmstaedter C, Grunwald T, Lehnertz K, Gleissner U, Elger CE (1997) Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: Evidence from temporal lobe epilepsy. Brain Cogn 35: 110–131. [DOI] [PubMed] [Google Scholar]
- 43. Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- 44. Brown MW, Aggleton JP (2001) Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51–61. [DOI] [PubMed] [Google Scholar]
- 45. Eichenbaum H, Yonelinas AP, Ranganath C (2007) The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Squire LR, Wixted JT, Clark RE (2007) Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci 8: 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henke K (2010) A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci 11: 523–532. [DOI] [PubMed] [Google Scholar]
- 48. Norman KA (2010) How hippocampus and cortex contribute to recognition memory: Revisiting the complementary learning systems model. Hippocampus 20: 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fell J, Axmacher N (2011) The role of phase synchronization in memory processes. Nat Rev Neurosci 12: 105–118. [DOI] [PubMed] [Google Scholar]
- 50. Cabeza R, Nyberg L (2000) Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- 51. Brewer JB, Moghekar A (2002) Imaging the medial temporal lobe: exploring new dimensions. Trends Cogn Sci 6: 217–223. [DOI] [PubMed] [Google Scholar]
- 52. Stark CEL, Okado Y (2003) Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci 23: 6748–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carr VA, Rissman J, Wagner AD (2010) Imaging the human medial temporal lobe with high-resolution fMRI. Neuron 65: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elger CE, Grunwald T, Lehnertz K, Kutas M, Helmstaedter C, et al. (1997) Human temporal lobe potentials in verbal learning and memory processes. Neuropsychologia 35: 657–667. [DOI] [PubMed] [Google Scholar]
- 55. Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, et al. (1999) Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science 285: 1582–1585. [DOI] [PubMed] [Google Scholar]
- 56. Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, et al. (2001) Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci 4: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 57. Engel AK, Moll CKE, Fried I, Ojemann GA (2005) Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci 6: 35–47. [DOI] [PubMed] [Google Scholar]
- 58. Mormann F, Osterhage H, Andrzejak RG, Weber B, Fernandez G, et al. (2008) Independent delta/theta rhythms in the human hippocampus and entorhinal cortex. Front Hum Neurosci 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE (2012) High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol 98: 279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rugg MD, Curran T (2007) Event-related potentials and recognition memory. Trends Cogn Sci 11: 251–257. [DOI] [PubMed] [Google Scholar]
- 61. Elger CE, Helmstaedter C, Kurthen M (2004) Chronic epilepsy and cognition. Lancet Neurol 3: 663–672. [DOI] [PubMed] [Google Scholar]
- 62. Helmstaedter C, Elger CE (2009) Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain 132: 2822–2830. [DOI] [PubMed] [Google Scholar]
- 63. Bell B, Lin JJ, Seidenberg M, Hermann B (2011) The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol 7: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kral T, Clusmann H, Urbach J, Schramm J, Elger CE, et al. (2002) Preoperative evaluation for epilepsy surgery (Bonn Algorithm). Zentralbl Neurochir 63: 106–110. [DOI] [PubMed] [Google Scholar]
- 65. Helmstaedter C, Durwen HF (1990) VLMT: Verbaler Lern- und Merkfähigkeitstest. Ein praktikables und differenziertes Instrumentarium zur Prüfung der verbalen Gedächtnisleistungen. Schweizer Archiv für Neurologie und Psychiatrie 141: 21–30. [PubMed] [Google Scholar]
- 66. Sharbrough F, Chatrian GE, Lesser RP, Lüders H, Nuwer M, et al. (1991) American Electroencephalographic Society guidelines for standard electrode position nomenclature. J Clin Neurophysiol 8: 200–202. [PubMed] [Google Scholar]
- 67. Horstmann MT, Bialonski S, Noennig N, Mai H, Prusseit J, et al. (2010) State dependent properties of epileptic brain networks: Comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clin Neurophysiol 121: 172–185. [DOI] [PubMed] [Google Scholar]
- 68. Weiss S, Mueller HM (2003) The contribution of EEG coherence to the investigation of language. Brain Lang 85: 325–343. [DOI] [PubMed] [Google Scholar]
- 69. Buzsáki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- 70. Schack B, Rappelsberger P, Weiss S, Moller E (1999) Adaptive phase estimation and its application in EEG analysis of word processing. J Neurosci Methods 93: 49–59. [DOI] [PubMed] [Google Scholar]
- 71. Mormann F, Lehnertz K, David P, Elger CE (2000) Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D 144: 358–369. [Google Scholar]
- 72. Gabor D (1946) Theory of communication. J I Electr Eng III 93: 429–441. [Google Scholar]
- 73.Panter P (1965) Modulation, Noise, and Spectral Analysis. New York: McGraw-Hill.
- 74.Boashash B (1992) Time frequency signal analysis: methods and applications. Longman Cheshire, Melbourne.
- 75. Frei MG, Zaveri HP, Arthurs S, Bergey GK, Jouny C, et al. (2010) Controversies in epilepsy: Debates held during the Fourth International Workshop on Seizure Prediction. Epilepsy Behav 19: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wacker M, Witte H (2011) Adaptive phase extraction: Incorporating the Gabor transform in the matching pursuit algorithm. IEEE Trans Biomed Eng 58: 2844–2851. [DOI] [PubMed] [Google Scholar]
- 77. Bruns A (2004) Fourier-, Hilbert- and wavelet-based signal analysis: Are they really different approaches? J Neurosci Methods 137: 321–332. [DOI] [PubMed] [Google Scholar]
- 78. Osterhage H, Mormann F, Staniek M, Lehnertz K (2007) Measuring synchronization in the epileptic brain: A comparison of different approaches. Int J Bifurcation Chaos Appl Sci Eng 17: 3539–3544. [Google Scholar]
- 79. Anderson BS, Butts C, Carley K (1999) The interaction of size and density with graph–level indices. Soc Networks 21: 239–267. [Google Scholar]
- 80. Bialonski S, Horstmann MT, Lehnertz K (2010) From brain to earth and climate systems: Small-world interaction networks or not? Chaos 20: 013134. [DOI] [PubMed] [Google Scholar]
- 81. van Wijk BCM, Stam CJ, Daffertshofer A (2010) Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 5: e13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ansmann G, Lehnertz K (2011) Constrained randomization of weighted networks. Phys Rev E 84: 026103. [DOI] [PubMed] [Google Scholar]
- 83. Bialonski S, Wendler M, Lehnertz K (2011) Unraveling spurious properties of interaction networks with tailored random networks. PLoS ONE 6: e22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ansmann G, Lehnertz K (2012) Surrogate-assisted analysis of weighted functional brain networks. J Neurosci Methods 208: 165–172. [DOI] [PubMed] [Google Scholar]
- 85. Bialonski S, Lehnertz K (2013) Assortative mixing in functional brain networks during epileptic seizures. Chaos 23: 033139. [DOI] [PubMed] [Google Scholar]
- 86. Albert R, Barabási AL (2002) Statistical mechanics of complex networks. Rev Mod Phys 74: 47–97. [Google Scholar]
- 87. Dijkstra EW (1959) A note on two problems in connexion with graphs. Numer Math 1: 269–271. [Google Scholar]
- 88. Fortunato S (2010) Community detection in graphs. Phys Rep 486: 75–174. [Google Scholar]
- 89. Newman MEJ (2012) Communities, modules and large-scale structure in networks. Nat Phys 8: 25–31. [Google Scholar]
- 90. Posner MI, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- 91. Chun MM, Turk-Browne NB (2007) Interactions between attention and memory. Curr Opin Neurobiol 17: 177–184. [DOI] [PubMed] [Google Scholar]
- 92. Muzzio IA, Kentros C, Kandel E (2009) What is remembered? Role of attention on the encoding and retrieval of hippocampal representations. J Physiol 587: 2837–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]