Abstract
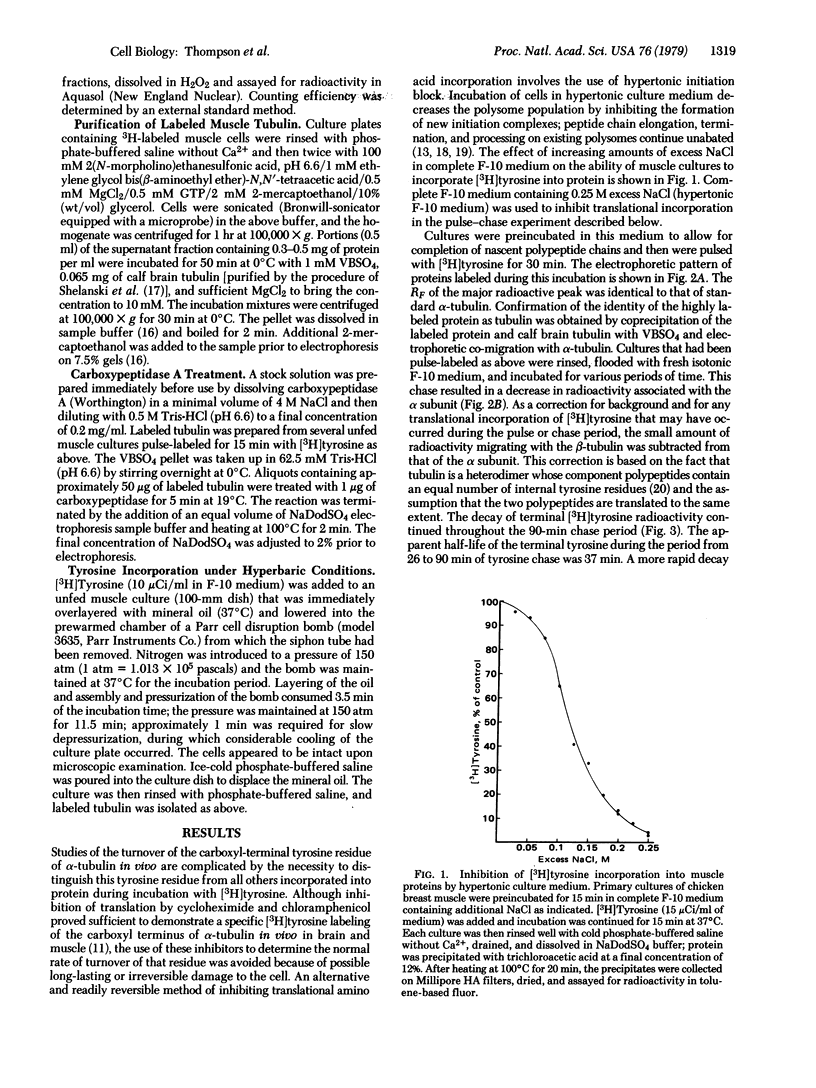
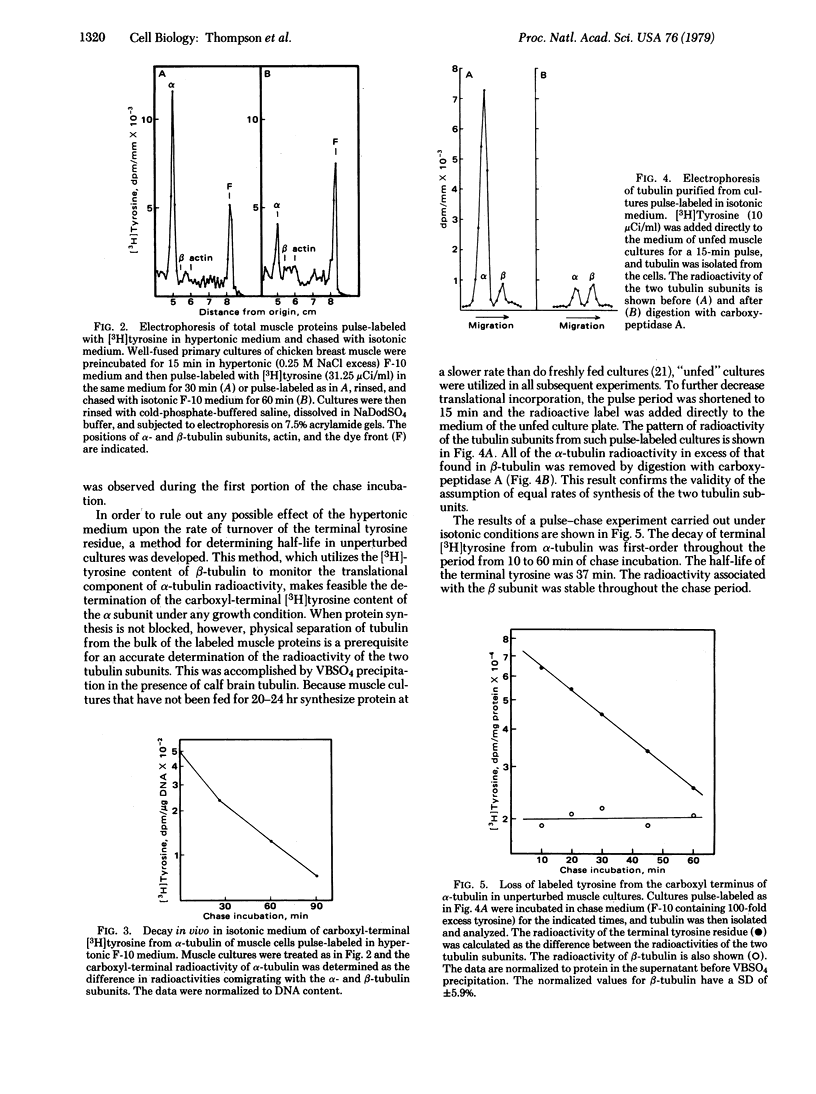
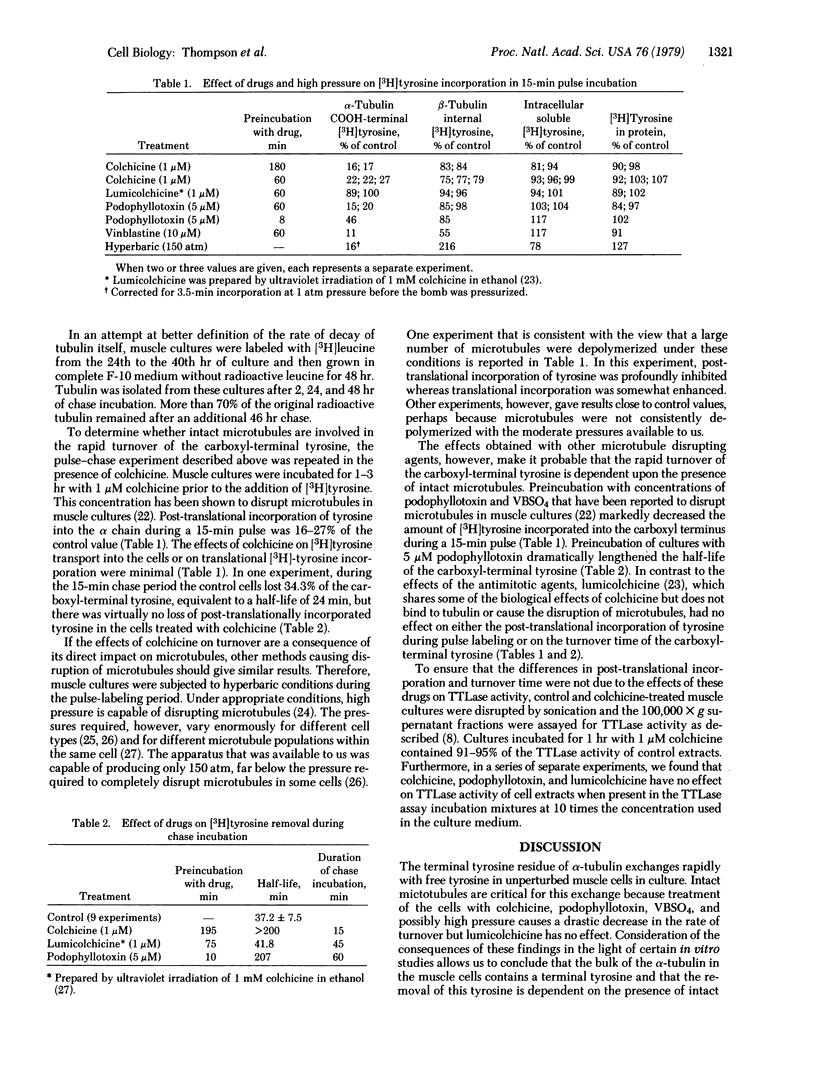
In cultured muscle cells the carboxyl-terminal tyrosine of alpha-tubulin was shown to exchange rapidly with free tyrosine. The rapid turnover of this residue was dependent upon the presence of intact microtubules. Half-life determinations were made by two methods: (i) the cells were pulse-labeled in hypertonic medium, in which the major tyrosine incorporation was post-translational, and then chased with isotonic medium; and (ii) the cells were pulsed and chased in isotonic medium, and the post-translational component of the radioactivity of purified alpha-tubulin was calculated. Both methods yielded a half-life of 37 min or less for the terminal tyrosine residue, whereas the half-life of tubulin itself was shown to be greater than 48 hr.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce C. A., Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Capability of tubulin and microtubules to incorporate and to release tyrosine and phenylalanine and the effect of the incorporation of these amino acids on tubulin assembly. J Neurochem. 1978 Jul;31(1):205–210. doi: 10.1111/j.1471-4159.1978.tb12449.x. [DOI] [PubMed] [Google Scholar]
- Arce C. A., Rodriguez J. A., Barra H. S., Caputo R. Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem. 1975 Nov 1;59(1):145–149. doi: 10.1111/j.1432-1033.1975.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. The effect of mitotic inhibitors in myogenesis in vitro. J Cell Biol. 1968 Jan;36(1):111–127. [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside B. The form and arrangement of microtubules: an historical, primarily morphological, review. Ann N Y Acad Sci. 1975 Jun 30;253:14–26. doi: 10.1111/j.1749-6632.1975.tb19189.x. [DOI] [PubMed] [Google Scholar]
- Deanin G. G., Gordon M. W. The distribution of tyrosyltubulin ligase in brain and other tissues. Biochem Biophys Res Commun. 1976 Jul 26;71(2):676–683. doi: 10.1016/0006-291x(76)90841-x. [DOI] [PubMed] [Google Scholar]
- Deanin G. G., Thompson W. C., Gordon M. W. Tyrosyltubulin ligase activity in brain, skeletal muscle, and liver of the developing chick. Dev Biol. 1977 May;57(1):230–233. doi: 10.1016/0012-1606(77)90370-0. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977 Feb 1;73(2):147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Variation in the relative synthesis of immunoglobulin G and non-immunoglobulin G proteins in cultured MPC-11 cells with changes in the overall rate of polypeptide chain initiation and elongation. J Mol Biol. 1976 Apr 15;102(3):601–612. doi: 10.1016/0022-2836(76)90337-5. [DOI] [PubMed] [Google Scholar]
- O'Connor T. M., Houston L. L., Samson F. Stability of neuronal microtubules to high pressure in vivo and in vitro. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4198–4202. doi: 10.1073/pnas.71.10.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybin D., Flavin M. An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1088–1095. doi: 10.1016/s0006-291x(75)80497-9. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry. 1977 May 17;16(10):2189–2194. doi: 10.1021/bi00629a023. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. Modification of tubulin by tyrosylation in cells and extracts and its effect on assembly in vitro. J Cell Biol. 1977 May;73(2):492–504. doi: 10.1083/jcb.73.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. A., Arce C. A., Barra H. S., Caputto R. Release of tyrosine incorporated as a single unit into rat brain protein. Biochem Biophys Res Commun. 1973 Sep 5;54(1):335–340. doi: 10.1016/0006-291x(73)90927-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. A., Borisy G. G. Modification of the C-terminus of brain tubulin during development. Biochem Biophys Res Commun. 1978 Jul 28;83(2):579–586. doi: 10.1016/0006-291x(78)91029-x. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Goode D., Maugel T. K., Bonar D. B. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J Cell Biol. 1976 May;69(2):443–454. doi: 10.1083/jcb.69.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E. D. Pressure-induced depolymerization of spindle microtubules. I. Changes in birefringence and spindle length. J Cell Biol. 1975 Jun;65(3):603–614. doi: 10.1083/jcb.65.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman K., Essien F., Heywood S. M. Polysomes from cultured muscle cells: the cell-free synthesis of myosin. J Cell Physiol. 1975 Dec;86(3 Pt 1):553–560. doi: 10.1002/jcp.1040860312. [DOI] [PubMed] [Google Scholar]
- Thompson W. C. Post-translational addition of tyrosine to alpha tubulin in vivo in intact brain and in myogenic cells in culture. FEBS Lett. 1977 Aug 1;80(1):9–13. doi: 10.1016/0014-5793(77)80395-5. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Hiramoto Y., Marsland D. Studies on the microtubules in heliozoa. 3. A pressure analysis of the role of these structures in the formation and maintenance of the axopodia of Actinosphaerium nucleofilum (Barrett). J Cell Biol. 1966 Apr;29(1):77–95. doi: 10.1083/jcb.29.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. H., Brunside B. Microtubules in cone myoid elongation in the teleost retina. J Cell Biol. 1978 Jul;78(1):247–259. doi: 10.1083/jcb.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. H. Microtubular organization in elongating myogenic cells. J Cell Biol. 1974 Nov;63(2 Pt 1):550–566. doi: 10.1083/jcb.63.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Medium hypertonicity and polyribosome structure in Hela cells. The influence of hypertonicity of the growth medium on polyribosomes in Hela cells. Eur J Biochem. 1972 May;27(1):162–173. doi: 10.1111/j.1432-1033.1972.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]


