Abstract
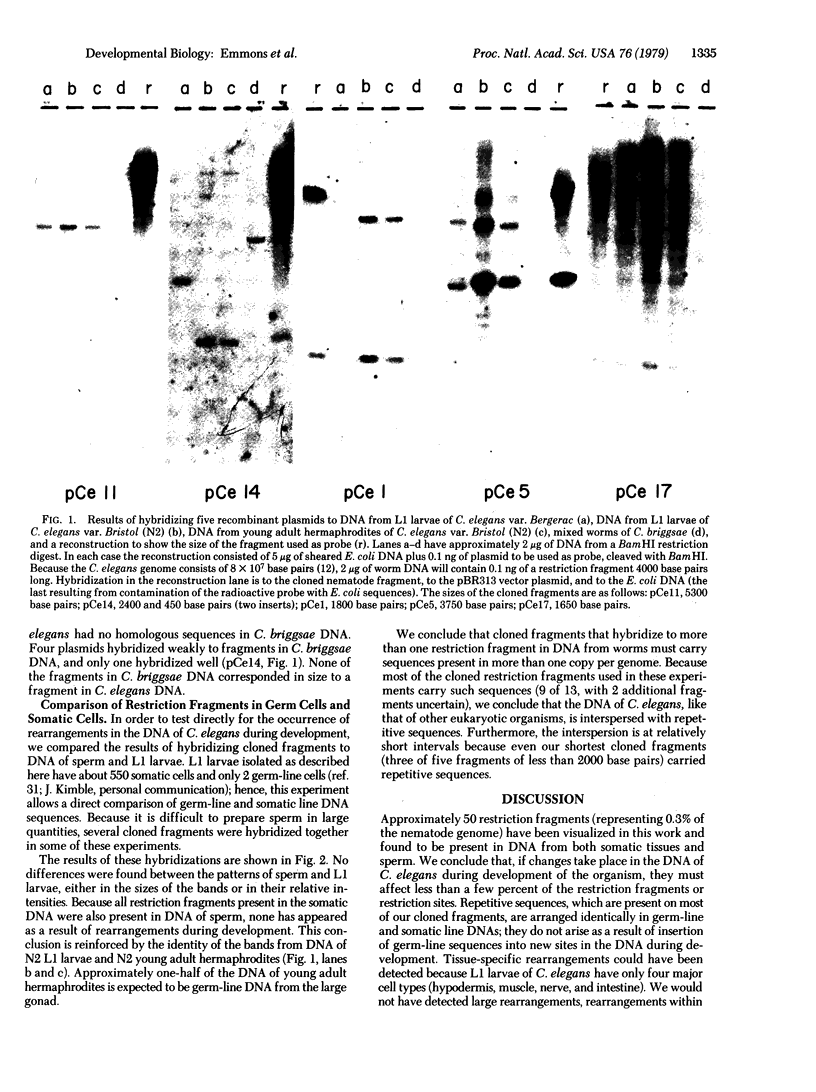
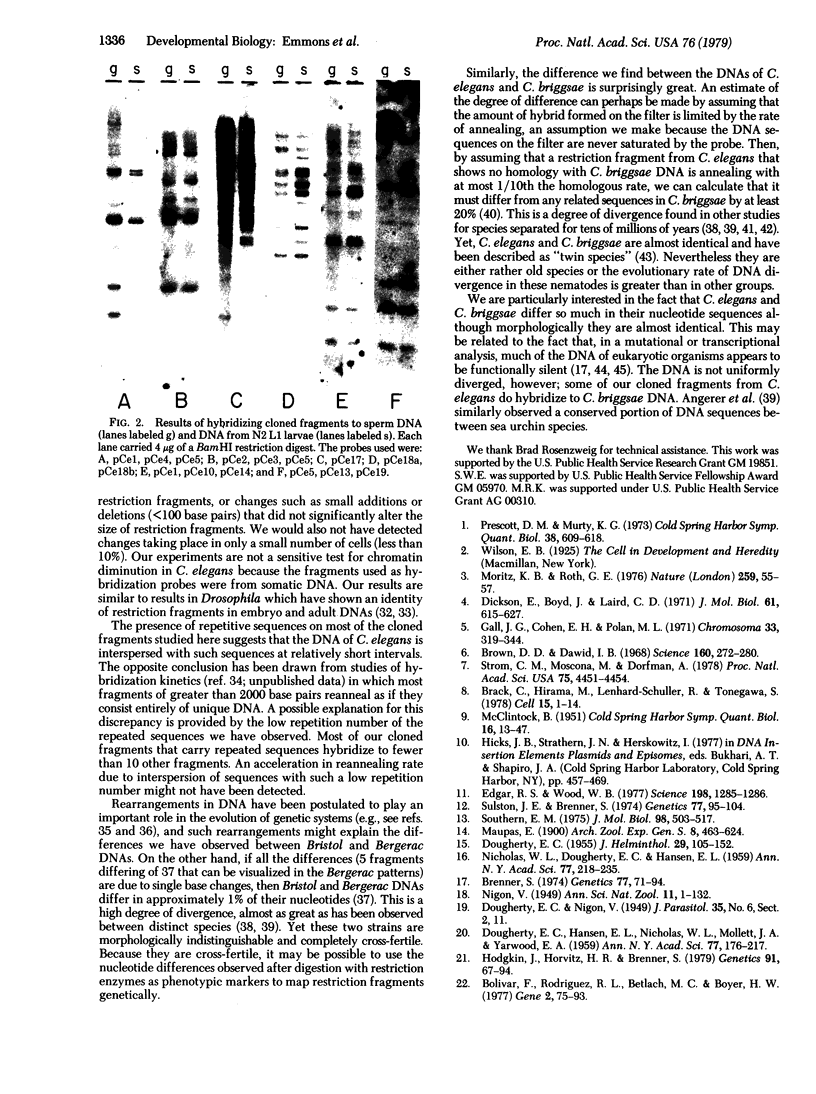
In order to test for the occurrence of rearrangements in DNA during development and to assess the rate of DNA divergence during evolution, we have compared restriction fragments derived from DNA from four sources: sperm cells and somatic tissues of one strain of the nematode Caenorhabditis elegans, somatic tissues of a second strain of the same species, and whole animals of a closely related species. Restriction fragments were detected by hybridizing radioactive cloned fragments to restriction digests that had been fractionated by size on agarose gels and transferred to nitrocellulose sheets. In this way, approximately 50 BamHI restriction fragments were visualized and compared. Fragments from sperm and somatic DNAs were found to be identical; 15% differed in size between the two strains. Little cross homology was found between the two species. We conclude that, if rearrangements occur in C. elegans DNA during development, they must affect fewer than a few percent of the restriction fragments or restriction sites. The difference found between the two strains and the two species is surprisingly great.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer R. C., Davidson E. H., Britten R. J. Single copy DNA and structural gene sequence relationships among four sea urchin species. Chromosoma. 1976 Jul 8;56(3):213–226. doi: 10.1007/BF00293186. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. DNA sequence arrangement and preliminary evidence on its evolution. Fed Proc. 1976 Aug;35(10):2151–2157. [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHERTY E. C. The genera and species of the subfamily Rhabditinae Micoletzky, 1922 (Nematoda): a nomenclatorial analysis, including an addendum on the composition of the family Rhabditidae Orley, 1880. J Helminthol. 1955;29(3):105–152. [PubMed] [Google Scholar]
- Davidson E. H., Klein W. H., Britten R. J. Sequence organization in animal DNA and a speculation on hnRNA as a coordinate regulatory transcript. Dev Biol. 1977 Jan;55(1):69–84. doi: 10.1016/0012-1606(77)90320-7. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Botchan P. Sequences homologous to ribosomal insertions occur in the Drosophila genome outside the nucleolus organizer. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4233–4237. doi: 10.1073/pnas.74.10.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Boyd J. B., Laird C. D. Sequence diversity of polytene chromosome DNA from Drosophila hydei. J Mol Biol. 1971 Nov 14;61(3):615–627. doi: 10.1016/0022-2836(71)90067-2. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. The nematode Caenorhabditis elegans: a new organism for intensive biological study. Science. 1977 Dec 23;198(4323):1285–1286. doi: 10.1126/science.929205. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Cohen E. H., Polan M. L. Reptitive DNA sequences in drosophila. Chromosoma. 1971;33(3):319–344. doi: 10.1007/BF00284948. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Horvitz H. R., Brenner S. Nondisjunction Mutants of the Nematode CAENORHABDITIS ELEGANS. Genetics. 1979 Jan;91(1):67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd B. H., Shen M. W., Kaufman T. C. The anatomy and function of a segment of the X chromosome of Drosophila melanogaster. Genetics. 1972 May;71(1):139–156. doi: 10.1093/genetics/71.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C. D., McConaughy B. L., McCarthy B. J. Rate of fixation of nucleotide substitutions in evolution. Nature. 1969 Oct 11;224(5215):149–154. doi: 10.1038/224149a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCLINTOCK B. Chromosome organization and genic expression. Cold Spring Harb Symp Quant Biol. 1951;16:13–47. doi: 10.1101/sqb.1951.016.01.004. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Moritz K. B., Roth G. E. Complexity of germline and somatic DNA in Ascaris. Nature. 1976 Jan 1;259(5538):55–57. doi: 10.1038/259055a0. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGON V., DOUGHERTY E. C. Reproductive patterns and attempts at reciprocal crossing of Rhabditis elegans Maupas, 1900, and Rhabditis briggsae Dougherty and Nigon, 1949 (Nematoda: Rhabditidae). J Exp Zool. 1949 Dec;112(3):485–503. doi: 10.1002/jez.1401120307. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Thomas C. A., Jr The two-dimensional fractionation of drosophila DNA. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1023–1031. doi: 10.1101/sqb.1978.042.01.102. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G. Chromosome structure in ciliated protozoans. Cold Spring Harb Symp Quant Biol. 1974;38:609–618. doi: 10.1101/sqb.1974.038.01.065. [DOI] [PubMed] [Google Scholar]
- Schachat F., O'Connor D. J., Epstein H. F. The moderately repetitive DNA sequences of Caenorhabditis elegans do not show short-period interspersion. Biochim Biophys Acta. 1978 Oct 24;520(3):688–692. doi: 10.1016/0005-2787(78)90154-5. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strom C. M., Moscona M., Dorfman A. Amplification of DNA sequences during chicken cartilage and neural retina differentiation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4451–4454. doi: 10.1073/pnas.75.9.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974 May;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977 Mar;56(1):110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]