Abstract
Salmonella typhimurium strain TM677 was mutagenized with aflatoxin B1 (AFB1) in liquid suspension culture in the presence of a rat liver postmitochondrial supernatant. Forward mutation to 8-azaguanine resistance was measured in the treated cultures and was found to increase linearly with AFB1 concentration. DNA purified from mutagenized cells was analyzed for AFB1 adduct formation by high-pressure liquid chromatography after adduct liberation. AFB1 exposures at 0.16 and 0.32 micrometer for 35 min produced 15 and 22 AFB1--DNA adducts per genome, respectively, and induced 8-azaguanine-resistant fractions of 4.9 X 10(-4) and 9.6 X 10(-4). Approximately 70% of the AFB1 bound to DNA was chromatographically identical to 2,3-dihydro-2-(N7-guanyl)-3-hydroxyaflatoxin B1 at the two AFB1 levels used.
Full text
PDF
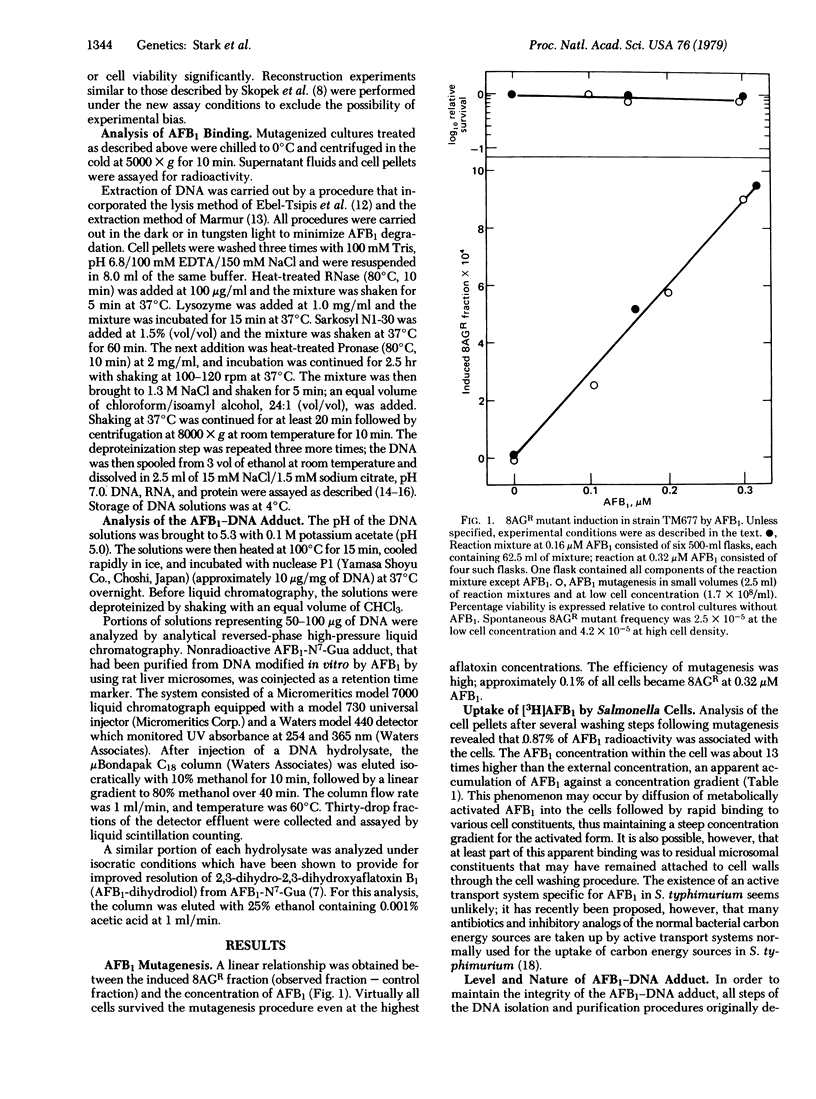
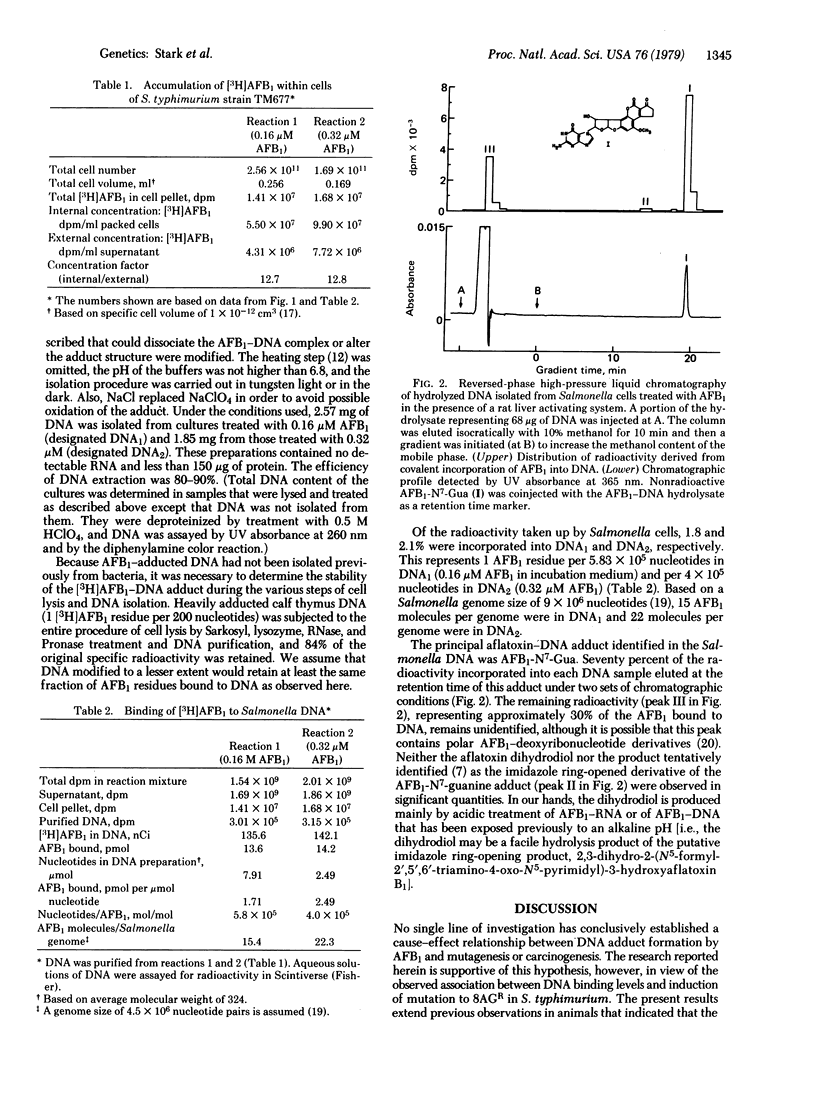


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adye J. C., Gots J. S. Further studies on genetically altered purine nucleotide pyrophosphorylases of Salmonella. Biochim Biophys Acta. 1966 May 5;118(2):344–350. doi: 10.1016/s0926-6593(66)80043-7. [DOI] [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Benson C. E., Gots J. S. Genetic modification of substrate specificity of hypoxanthine phosphoribosyltransferase in Salmonella typhimurium. J Bacteriol. 1975 Jan;121(1):77–82. doi: 10.1128/jb.121.1.77-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Croy R. G., Essigmann J. M., Reinhold V. N., Wogan G. N. Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1745–1749. doi: 10.1073/pnas.75.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Comparative rates of spontaneous mutation. Nature. 1969 Mar 22;221(5186):1132–1132. doi: 10.1038/2211132a0. [DOI] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Botstein D., Fox M. S. Generalized transduction by phage P22 in Salmonella typhimurium. I. Molecular origin of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):433–448. doi: 10.1016/0022-2836(72)90361-0. [DOI] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner R. C., Wright C. M. Binding of [-14C]aflatoxin B1 to cellular macromolecules in the rat and hamster. Chem Biol Interact. 1975 Aug;11(2):121–131. doi: 10.1016/0009-2797(75)90019-8. [DOI] [PubMed] [Google Scholar]
- LARK K. G., MAALOE O. Nucleic acid synthesis and the division cycle of Salmonella typhimurium. Biochim Biophys Acta. 1956 Sep;21(3):448–458. doi: 10.1016/0006-3002(56)90181-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin J. K., Miller J. A., Miller E. C. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977 Dec;37(12):4430–4438. [PubMed] [Google Scholar]
- Martin C. N., Garner R. C. Aflatoxin B -oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature. 1977 Jun 30;267(5614):863–865. doi: 10.1038/267863a0. [DOI] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Kaden D. A., Thilly W. G. Relative sensitivities of forward and reverse mutation assays in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4465–4469. doi: 10.1073/pnas.75.9.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Krolewski J. J., Thilly W. G. Quantitative forward mutation assay in Salmonella typhimurium using 8-azaguanine resistance as a genetic marker. Proc Natl Acad Sci U S A. 1978 Jan;75(1):410–414. doi: 10.1073/pnas.75.1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson D. H., Lin J. K., Miller E. C., Miller J. A. Aflatoxin B1-2,3-oxide as a probable intermediate in the covalent binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal RNA in vivo. Cancer Res. 1977 Jan;37(1):172–181. [PubMed] [Google Scholar]
- Swenson D. H., Miller E. C., Miller J. A. Aflatoxin B1-2,3-oxide: evidence for its formation in rat liver in vivo and by human liver microsomes in vitro. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1036–1043. doi: 10.1016/0006-291x(74)90417-3. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]



