Abstract
Background
A secondary rise of intracellular Ca2+ (Cai) and an upregulation of IKAS are characteristic findings of failing ventricular myocytes. We hypothesize that apamin, a specific IKAS blocker, may induce torsades de pointes (TdP) ventricular arrhythmia from failing ventricles exhibiting secondary rises of Cai.
Objectives
To test the hypothesis that small conductance Ca2+ activated apamin sensitive K+ current (IKAS) maintains repolarization reserve and prevents ventricular arrhythmia in a rabbit model of heart failure (HF).
Methods
We performed Langendorff perfusion and optical mapping studies in 7 hearts with pacing-induced HF and in 5 normal control rabbit hearts. Atrioventricular (AV) block was created by cryoablation to allow pacing at slow rates.
Results
The left ventricular ejection fraction reduced from 69.1 [95% confidence interval 62.3–76.0]% pre-pacing to 30.4 [26.8–34.0]% (N=7, p<0.001) post-pacing. The QTc in failing ventricles was 337 [313–360] ms at baseline and 410 [381–439] ms after applying 100 nmol/L of apamin (p=0.01). Apamin induced early afterdepolarizations (EADs) in 6 ventricles, premature ventricular beats (PVBs) in 7 ventricles and polymorphic ventricular tachycardia consistent with TdP in 4 ventricles. The earliest activation site of the EADs and PVBs always occurred at the site with long APD and large amplitude of the secondary rises of Cai. Apamin induced secondary rises of Cai in 1 non-failing ventricles, but no EAD or TdP were observed.
Conclusion
In HF ventricles, apamin induces EADs, PVBs and TdP from areas with secondary rises of Cai. IKAS is important in maintaining repolarization reserve and preventing TdP in HF ventricles.
Keywords: Action potential duration, apamin, optical mapping, potassium channels, torsades de pointes
Ventricular arrhythmia is a major cause of death in patients with heart failure (HF).1 Multiple randomized clinical trials2–4 conducted in patients with HF documented increased ventricular arrhythmias or mortality in patients randomized to the drug treatment arm, suggesting that HF predisposes patients to drug-induced arrhythmia. A recent study confirmed that there is enhanced sensitivity to drug-induced QT interval lengthening in patients with HF due to left ventricular systolic dysfunction.5 The mechanisms by which HF increases the risk of drug-induced arrhythmia and reduces drug safety remain poorly understood. Previous studies showed that HF is associated with down-regulation of multiple K+ currents (Ito, IKs, IKr, IK1 and IKATP)6,7 but upregulation of apamin-sensitive K+ current (IKAS) conducted through the small conductance Ca2+ activated K+ (SK) channels.8 Drugs that block these K+ currents may reduce repolarization reserve, prolong action potential duration (APD) and increase propensity of ventricular arrhythmia. The upregulation of IKAS in failing ventricles was recently reproduced by Bonilla et al in a canine model of HF.9 In addition to IKAS upregulation, failing ventricular myocytes are known to develop a slow secondary rise of intracellular Ca2+ (Cai) that prolongs the Cai transient duration.10 The prolonged availability of Ca2+ may activate the IKAS to counterbalance the downregulation of other K+ currents, thereby maintain repolarization reserve and prevent ventricular arrhythmias in HF. If this hypothesis is correct, then blocking the IKAS by apamin should reduce the repolarization reserve and promote ventricular arrhythmias in failing but not normal ventricles. This hypothesis has important implications in drug safety in HF because inadvertent blocking of IKAS by food or drugs may increase the incidence of ventricular arrhythmia and sudden cardiac death. The purpose of the present study was to perform optical mapping studies in failing rabbit ventricles to test the hypotheses that apamin, a specific IKAS blocker, induces early afterdepolarizations (EADs) and torsades de pointes (TdP) ventricular arrhythmias from areas with secondary rises of Cai in failing ventricles.
Methods
Surgical preparation
Rapid pacing protocol was conducted to induce HF in 7 New Zealand white rabbits.8,11 Five normal rabbits were also studies as controls. Echocardiography was performed before and after high-rate pacing. The hearts were harvested and Langendorff perfused with oxygenated 37°C Tyrode’s solution that includes (in mmol/L): NaCl 125, KCl 4.5, NaHCO3 24, NaH2PO4 1.8, CaCl2 1.8, MgCl2 0.5, dextrose 5.5 and bovine serum albumin 100 mg/L with a pH of 7.40. Cryoablation of atrioventricular (AV) node was then performed to reduce ventricular rate. We performed simultaneous membrane potential (Vm) and Cai optical mapping according to methods published elsewhere.12,13 More detailed descriptions are included in an Online Supplement.
Experimental Protocol
Pseudoelectrocardiogram (pECG) was monitored using 2 electrodes placed at the left atrium and the right ventricle, respectively. A bipolar electrode was used to pace the right ventricle with an output at 2.5 times the diastolic pacing threshold. Dynamic pacing protocol14 was performed and the optical signals were mapped at different pacing cycle length (PCL). We started to acquire optical mapping signal after at least 30 paced beats at the same PCL. A S1/S2/S3 short-long-short pacing protocol (S1 30 beats with S1-S1 300 ms, a long S1–S2 of 1000 or 2000 ms and a S2–S3 starting from 300 ms and gradually shortened to the ventricular effective refractory period) was used to simulate the ECG characteristics that initiate the TdP ventricular tachycardia in humans.15 Apamin (100 nmol/L) was then added to the perfusate and the protocol was repeated 30 minutes later. Nifedipine (2 μmol/L) was then added in 4 of the 7 failing rabbit hearts to determine if it prevents the development of TdP. The same protocol was also done in 5 normal rabbit hearts for comparison.
Data Analysis
APD80 was measured at the level of 80% repolarization of APD and mean APD80 was calculated for all available ventricular pixels. A secondary rise of Cai is defined as the spontaneous increase of the Cai at the downslope of the primary Cai released.10 Continuous variables are expressed as mean [the 95% confident interval]. Paired Student’s t-tests were used to compare continuous variables measured at baseline and during apamin infusion. Comparison of prevalence of EADs inducibility between baseline and during apamin infusion was performed using paired McNemar test. A p ≤ 0.05 was considered statistically significant.
Results
Induction of Heart Failure
All 7 rabbits developed significant symptoms and signs of HF, including tachypnea, poor appetite, cardiomegaly and pleural effusion. The left ventricular (LV) ejection fraction reduced from 69.1 [62.3 – 76.0]% pre-pacing to 30.4 [26.8 – 34.0]% (p < 0.001) post-pacing. LV end-diastolic diameter increased from 12.3 [11.5 – 13.12] mm to 18.1 [16.6 – 20.0] mm (p < 0.001) and the LV end-systolic diameter from 7.9 [7.0 – 8.8] mm to 15.7 [14.3 – 17.2] mm (p < 0.001). The Langendorff perfused rabbit hearts had sinus rhythm with sinus cycle length 405 [341 – 469] ms and normal 1:1 AV conduction before AV node cryoablation. All rabbits developed complete AV block after 1 to 3 attempts of cryoablation, with average ventricular escape cycle lengths of 1757 [1217 – 2297] ms. After addition of apamin, the average spontaneous ventricular escape rate did not change significantly (1757 [1176 – 2338] ms, p = 0.99). The sinus (atrial) cycle length did not change significantly after cryoablation (457 [366 – 549] ms, p = 0.33) or after adding apamin (434 [323 – 545] ms, p = 0.62).
Effects of Apamin on QT Interval and Ventricular Arrhythmias in Failing Ventricles
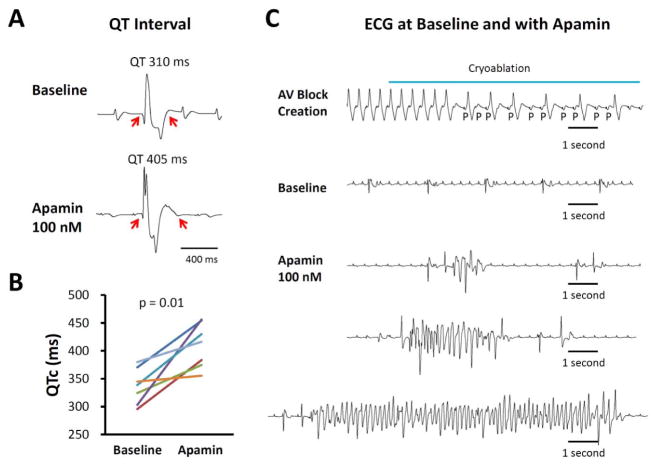
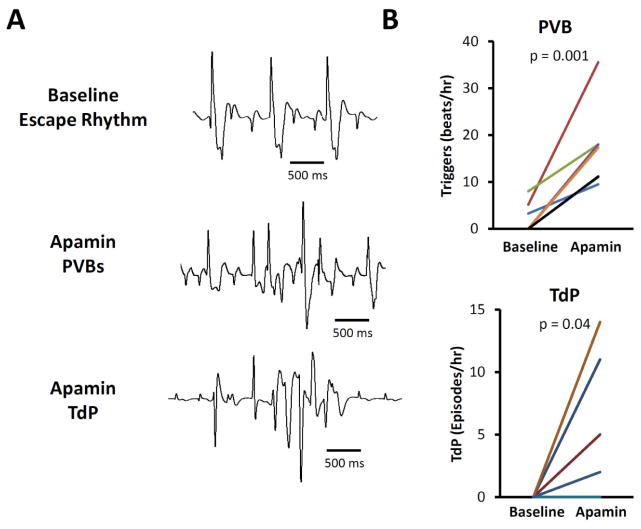
Apamin significantly prolonged corrected QT interval (QTc) during spontaneous escape rhythm. The QTc was 337 [313 – 360] ms at baseline and 410 [381 – 439] ms after applying 100 nmol/L of apamin (p = 0.01). Figure 1A shows an example of QT prolongation after apamin administration. Figure 1B shows the effects of apamin on QTc intervals in all 7 hearts studied. In addition to prolonging QTc intervals, apamin also led to the development of EADs in 6 ventricles. The ventricle without EAD had an average APD of 208 ms (at 500 ms PCL), which was within the range of APDs (188 ms–261 ms, at 500 ms PCL) in ventricles with EADs. Premature ventricular beats (PVBs) were observed in 7 ventricles and polymorphic ventricular tachycardia consistent with TdP in 4 ventricles. Figure 1C shows the creation of AV block with cryoablation. The hearts have stable escape rhythm without arrhythmias until the administration of apamin, when episodes of TdP developed spontaneously in 2 ventricles and after short-long-short pacing protocols in an additional 2 ventricles. Figure 2A shows additional examples of ventricular escape rhythm at baseline, PVBs and an episode of TdP in the presence of apamin. Apamin significantly increased frequency of PVB in the failing ventricles (from 2.35 [−0.05 – 4.74] beats/hour to 17.23 [10.65 – 23.8] beats/hour, p = 0.001, Fig 2B, upper subpanel). The frequency of spontaneous and induced TdP was also increased from 0 [0 – 0] episodes/hour to 2.88 [0.27 – 5.50] episodes/hour, p = 0.04, Fig 2B, lower subpanel).
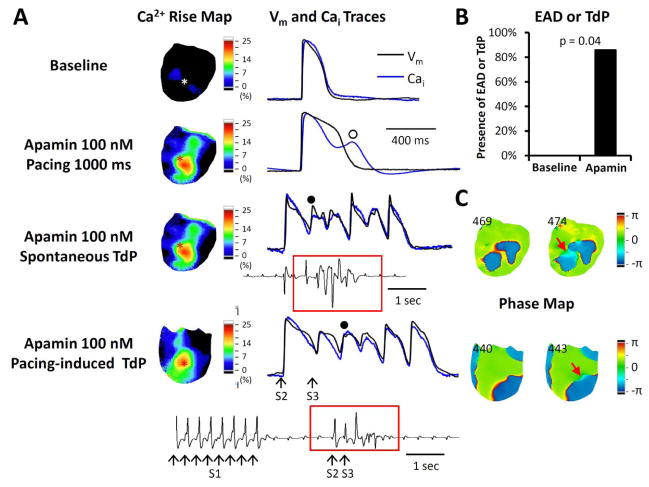
Figure 1.
Apamin effect on QT interval and arrhythmias in failing hearts. A. Representative pECG traces of QT interval in a failing heart with complete atrioventricular (AV) block before and after 100 nmol/l apamin. B. Paired dot plot shows QTc at baseline and in the presence of apamin 100 nmol/L. There was significant prolongation of QTc. C. Representative traces at baseline and in the presence of apamin. Top panel, complete AV block developed during AV node cryoablation. Second panel, no polymorphic ventricular tachycardia (VT) was recorded at baseline. However, several episodes of spontaneous torsade de pointes (TdP) polymorphic ventricular arrhythmia developed in the presence of apamin (bottom panels).
Figure 2.
Comparison of premature ventricular beats (PVBs), TdP and early afterdepolarizations (EADs) at baseline and during apamin perfusion in failing hearts. A. Representative examples of ventricular escape rhythm at baseline (top subpanel), a PVB (center subpanel), and an episode of TdP (bottom subpanel) in the presence of apamin. B. There was significant increase in PVBs and TdP during 100 nmol/l apamin perfusion.
Activation Cycle Length and the Effects of Apamin in Failing Ventricles
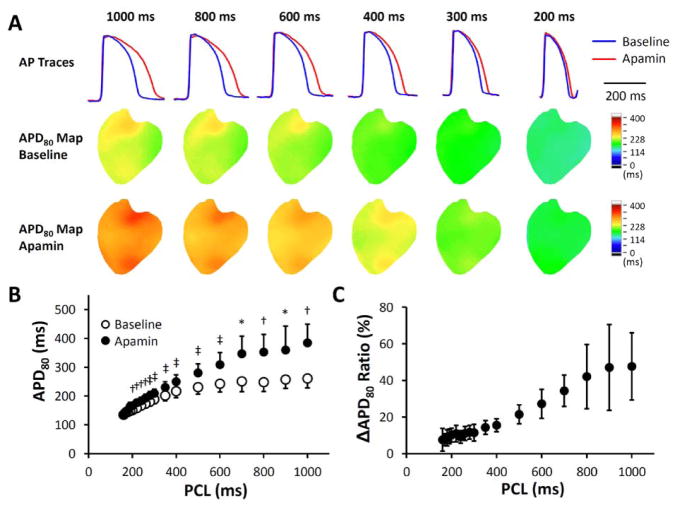
Apamin significantly prolonged APD80 at all pacing cycle lengths (PCLs). The ratio of APD prolongation was larger at long PCLs than at short PCLs. Figure 3A shows representative examples of APD prolongation at different PCLs. Figure 3B shows the average APD80 without and with apamin. The color APD80 maps show heterogeneous distribution of APD80 at long PCL, both before and after apamin. Figure 3C shows the ratio of delta APD and the baseline APD. Note that the delta APD ratio increases with the prolongation of PCL, indicating the importance of IKAS in repolarization at slow heart rates.
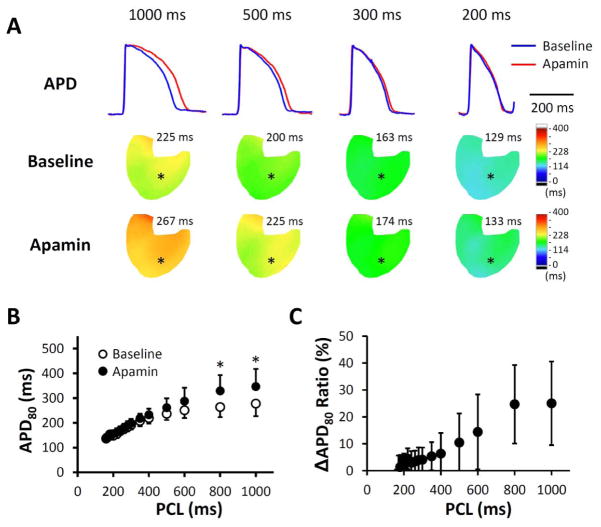
Figure 3.
Apamin effect on action potential duration (APD) at different pacing cycle lengths (PCLs) in failing hearts. A. Representative membrane potential (Vm) traces and APD80 maps at baseline and in the presence of apamin 100 nmol/l. The magnitude of APD prolongation was more prominent at long PCLs than at physiologic PCLs. B. Apamin significantly prolonged APD80 at all PCLs, and the prolongation was more prominent at longer PCLs. C. A plot of ΔAPD80 ratio ((APD80 after apamin − APD80 at baseline)/APD80 at baseline) versus PCL shows that apamin prolonged APD80 by around 50% at PCL 1000 ms but only by 10% at PCL 200 ms. *, p < 0.05; †, p < 0.01; ‡, p < 0.001.
Secondary Rises of Cai in Failing Ventricles Were Enhanced by Apamin
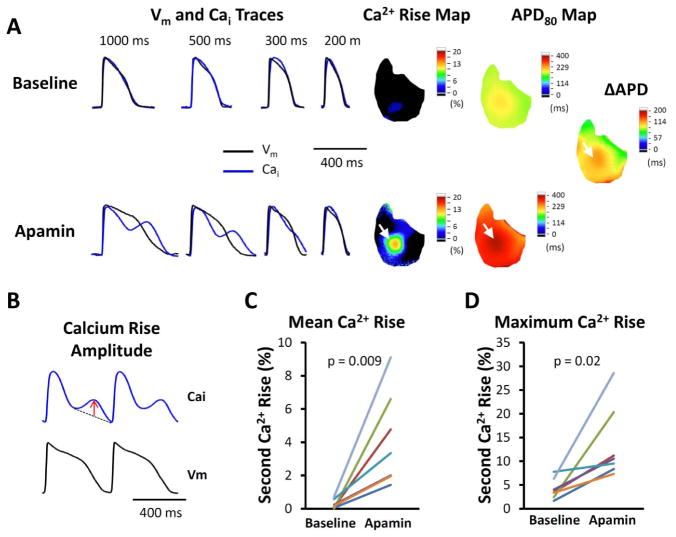
We observed secondary rise of Cai in all failing ventricles both at baseline and after apamin administration. The area occupied by secondary rises of Cai was 15.2 [0.25 – 27.8]% of the mapped region at baseline, and increased to 61.9 [50.6 – 73.1]% after apamin administration (p < 0.001). Figure 4 shows that the secondary rise of Cai was accentuated in the presence of apamin, along with APD prolongation. Figure 4A shows a representative Vm and Cai traces of a failing ventricle. At long PCL (500 or 1000 ms), apamin administration enhanced secondary Cai rises and Cai transient duration. The amplitude of secondary Cai rises is defined as the largest deviation from a line drawn between the onset and offset of the secondary Cai rise (Fig. 4B). Figure 4A, right panels show representative secondary Cai rises and APD80 maps without and with the presence of apamin. There were only minimal secondary Cai rises at baseline, but the secondary Cai rise became more apparent after adding apamin. The maximum secondary Cai rises co-localized with the areas with the longest APD (see white arrows, Fig. 4A, right panels) and sites of origin of EADs (Fig. 5). The secondary Cai rises were enhanced by apamin and may, but not always, trigger EADs and initiate an episode of TdP. We compared average (Fig. 4C) and the maximum (Fig. 4D) secondary Cai rises of all mapped pixels. The average secondary Cai rises of all pixels increased significantly (from 0.32 [−0.03 – 0.67]% to 3.5 [1.80 – 5.20]%, p = 0.014) in the presence of apamin. The maximum amplitude of secondary Cai rises was also increased (from 3.15 [1.10 – 5.20]% to 13.33 [7.16 – 19.50]%, p = 0.03) by apamin administration. These data indicate that apamin enhances the secondary Cai rises and promotes the development of EADs.
Figure 4.
Secondary rises of Cai in failing ventricles. A. Representative Vm, Cai traces, secondary rises of calcium and APD maps at different PCLs without and with 100 nmol/l apamin perfusion. Apamin prolonged APD, along with secondary rises of Cai, especially at longer PCLs. The areas with the secondary rises of Cai co-localized with areas with the most significant APD prolongation (white arrows). The delta APD indicates the difference of APD after and before apamin. B. The amplitude of secondary Cai rises is defined as the largest deviation from a line drawn between the onset and offset of the secondary Cai rises, as indicated by the red arrow. C. Comparison of average secondary rises of Cai amplitude among all available ventricular pixels at baseline and in the presence of 100 nmol/l apamin of all hearts studied. D. Comparison of maximal amplitude of secondary rises of Cai in each heart at baseline and in the presence of apamin of all hearts studied.
Figure 5.
Relationship between secondary rises of Cai and the development of EAD and PVBs in failing hearts with AV block. A. The Vm and Cai traces were taken from the site marked by an asterisk in the Cai rise map (map of secondary rise of Cai). In this pixel, the Cai tracing tracked the Vm tracing at baseline. Apamin prolonged APD and induced secondary rise of Cai (unfilled circle). Subsequently, spontaneous TdP developed from the same site with a secondary rise of Cai. In addition to spontaneous TdP, short-long-short (30 short S1S1 beats, a long S1S2 and a short S2S3 intervals) pacing protocol also induced TdP in this ventricle, as shown in the bottom tracing. B. Apamin increased EADs and/or TdP inducibility in failing hearts. None of the hearts had EADs at baseline and 6 of 7 hearts developed EADs during apamin perfusion. C. Phase maps of corresponding TdP beats (filled circles in panel A) in the spontaneous and the pacing-induced TdPs. Red arrows point to an area with light blue color (phase change), which is the earliest activation sites of the TdP beats. The numbers indicate frame.
Effects of Apamin on Early Afterdepolarization and Ventricular Arrhythmia
Figure 5A shows the representative examples of optical mapping traces at baseline and after addition of apamin. Without apamin, HF rabbit hearts did not develop TdP arrhythmia (Fig. 5A, Baseline) even with short-long-short pacing. Apamin massively prolonged the APD at 1000 ms PCL. The corresponding Cai trace shows a secondary rise of Cai at the same pixel (Fig 5A, second subpanel, blue trace). None of the HF rabbit hearts had EADs without apamin and 6 (86%) hearts developed EADs or TdP in the presence of apamin (p = 0.04, Fig. 5B). Figure 5C shows phase maps of corresponding EAD beats in the spontaneous and the pacing-induced TdPs. The earliest activation sites of the EAD beats co-localized with the highest secondary rises of Cai regions. We analyzed a total of 19 EAD episodes in 6 failing hearts (2.71 [1.53 – 3.90] episodes per heart). Among them, the earliest activation site of the EADs and PVBs always occurred at the site with long APD and large amplitude of the secondary rises of Cai. However, not all secondary rises of Cai resulted in EADs or PVBs. Figure 6A shows two different traces of spontaneous beats, one without (top subpanel) and the other with (second subpanel) PVB. Both tracings were from the same pixel indicated by the black asterisk in Fig. 6B in a failing ventricles after apamin administration. Both episodes had secondary rises of Cai (green and red lines, respectively). When these two traces were superimposed on a third subpanel, it is clear that the one with PVB (red trace) had higher secondary rise of Cai than the one without PVB (green trace). A corresponding p-ECG shows the first QRS complex is followed by a PVB (red arrow). Figure 6D shows the phase, Vm and Cai maps at the time of PVB onset. An arrow on the Vm map points to the initial propagation, which corresponding to the light blue (change of phase) in frame 617 of the phase map. Figure 6C shows the direction of propagation. The results further support an association between the amplitude of secondary Cai rises and the development of PVBs.
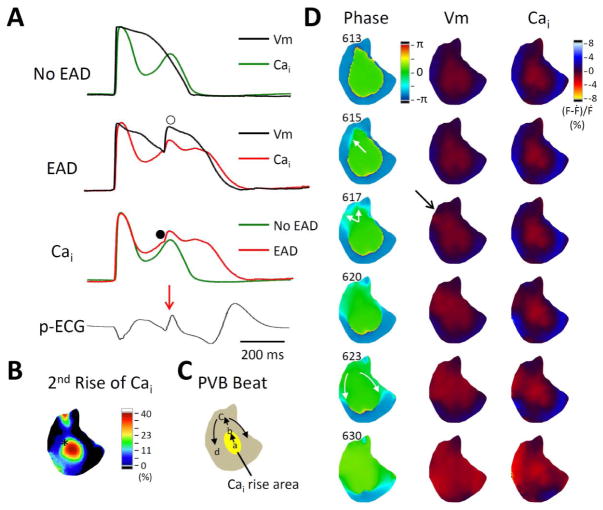
Figure 6.
Secondary rises of Ca2+ and the origin of PVB. A. Vm and Cai traces of an automatic beat not associated with EAD (top subpanel) and an automatic beat with EAD development (marked by unfilled circle, second subpanel) acquired from the same region (panel B, left subpanel, black asterisk). The third subpanel shows the overlaid Cai traces of these two beats. Note the red trace has higher amplitude than the green trace. The red trace has a hump (filled circle), indicate further SR Ca2+ release induced by a propagated PVB. B. Secondary rise of Cai map of the spontaneous beat without EAD. C. Schematic illustration of the EAD propagation. D. Phase, Vm and Cai maps of the EAD. The EAD developed from 10 o’clock site of long APD-high Cai area (see the light blue budding, which indicates the earliest activation, of frame 615 and 617). The arrows in frame 623 indicate the direction of propagation from that early site towards the apex.
Effects of Nifedipine
We tested the effects of nifedipine on apamin-enhanced secondary Cai rises in 4 of the 7 failing rabbit hearts. Nifedipine at 2 μmol/L shortened APD80, and reduced the slope of the primary rise of Cai (i.e., Ca release triggered by depolarization). It also nearly completely eliminated the secondary rises of Cai in all 4 hearts (Fig. 7). TdP ventricular arrhythmia and EADs were completely suppressed by nifedipine.
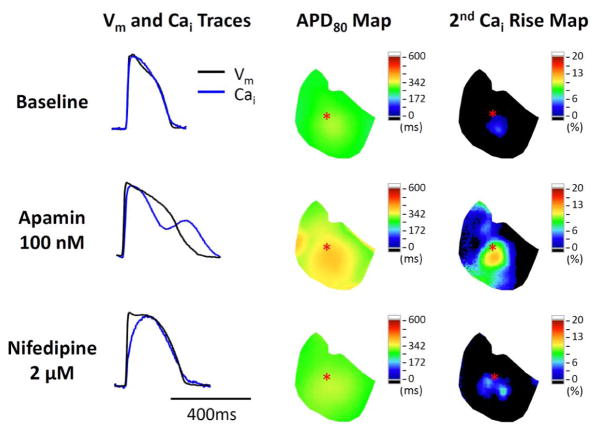
Figure 7.
Representative traces and maps in a failing heart at baseline, during apamin perfusion, and after adding nifedipine. Nifedipine shortened APD and ameliorated secondary rises of Cai. The Vm and Cai tracings were obtained from the site labeled by an asterisk on the APD80 map and secondary Cai rise map. Note that the latter two maps show the co-localization of the secondary rises of Cai and the prolonged APD80 in the same ventricle.
Effects of Apamin on Non-failing Rabbit Ventricles
We also tested apamin effect on 5 non-failing (control) rabbit hearts with AV block. Apamin did not prolong APD80 significantly at short (200 ms and 300 ms) PCLs (Fig. 8A and 8B), but it increased APD80 by 25% at long PCLs (278 [227 – 328] ms to 346 [275 – 427] ms at PCL 1000 ms, p = 0.04). The magnitude of APD80 prolongation (Figure 8B) was much smaller than in the failing ventricles (Figure 3B). The delta APD80 ratio (Figure 8B) is also less than that in failing ventricles (Figure 3C). Apamin induced secondary rises of Cai in 1 out of 5 non-failing ventricles at 500 ms PCL. No EADs or TdP were observed in that or other ventricles either before or after apamin.
Figure 8.
Apamin effect on APD and secondary rises of Cai in non-failing ventricles. A. Representative membrane potential (Vm) traces and APD80 maps at baseline and in the presence of apamin 100 nmol/l. The results show that apamin prolonged APD at long PCLs, but not at physiologic PCLs. B. Apamin significantly prolonged APD80 at PCL 1000 ms and 800 ms, but not at shorter PCLs. Asterisk indicates p values of < 0.05. C. A plot of ΔAPD80 ratio ((APD80 after apamin − APD80 at baseline)/APD80 at baseline) versus PCL.
Discussion
Importance of Ventricular Rate on the IKAS
As previously reported, apamin did not significantly prolong APD at 300 ms PCL.9,16,17 However, when the PCL is lengthened to 1000 ms, even normal ventricles showed significant APD prolongation after apamin administration. According to modeling and experimental studies,18 longer diastolic intervals are associated with higher availability of L-type Ca2+ current (ICa,L) and longer Cai transient duration. The persistent trans-sarcolemmal Ca2+ flow through L-type Ca2+ channels may facilitate the SK channel activation. Therefore, blocking SK channels at long PCL prolongs APD.
Secondary Rises of Cai
The reduced initial phase of Ca2+ transient, the slowed decay of Cai transient and secondary rises of Cai are commonly observed in cardiomyocytes from failing ventricles,10 but may also be present during bradycardia.19 HF reduces the initial phase of Ca2+ transient, which in turn reduces Ca2+-induced inactivation of the ICa,L. As repolarization continues, the driving force for Ca2+ entry increases, which promotes greater Ca2+ entry through already opened L-type Ca2+ channels, leading to additional SR Ca2+ release during the latter phase of the plateau. The increased Ca2+ can activate sodium-calcium exchangers to prolong APD and to promote EADs. IKAS in failing ventricles serves to counterbalance the APD prolonging effects of the secondary rises of Cai. IKAS blockade, especially during bradycardia, removes this built-in counterbalance, leading to excessively prolonged APD, PVBs and TdP arrhythmia.
IKAS and Drug Safety
IKAS upregulation is a mechanism by which failing ventricles maintain repolarization reserve and prevent afterdepolarizations especially in myocytes with a secondary rise of Cai and prolonged Cai transient duration. The importance of IKAS in human ventricular repolarization is supported by our recent study20 that showed apamin prolonged APD in failing human ventricular cells by an average of 11.8%. In addition to apamin, previous studies have shown that anesthetic agents, such as butanol, ethanol, ketamine, lidocaine, and methohexital block recombinant SK2 channel currents.21 In addition, quinine, quinidine, d-tubocurarine, tetraethylammonium chloride and 4-aminopyridine are also IKAS blockers.22 Preliminary investigation from our laboratory indicates that amiodarone is an effective IKAS blocker.23 It is possible that further investigations will discover the SK blocking action of many other drugs used in treating patients with HF. Systematic study is necessary to determine the effects of drugs or chemicals on IKAS to improve the drug safety in patients with HF.
Study Limitations
In addition to blocking SK currents, apamin also blocks the fetal ICa,L. 24 If there is significant blockade of ICa,L in rabbit ventricles, then apamin should have suppressed EADs. The fact that ventricular arrhythmias are induced by apamin suggests that either apamin is not a significant ICa,L blocker, or that the EAD is not a mechanism of these arrhythmias. Therefore, we added nifedipine, which eliminated EADs due to its impact on ICa,L. These findings further support that EAD is a mechanism of apamin-induced arrhythmia. Other than ICa,L blockade, apamin is thought to be a highly selective blocker of SK currents.25–27 We propose that the results of the present study are best explained by the SK current inhibition.
Summary and Clinical Significance
HF is a major risk factor for drug-induced ventricular arrhythmias.28 IKAS, the only K current known to be upregulated in HF,8 may have a protective role in the failing ventricle, and that efforts (intentional or secondary to off-target drug actions) to suppress this current may have pro-arrhythmic consequences. We showed in the present study that IKAS blockade in HF ventricles results in EADs, PVBs and TdP from areas with secondary rises of Cai. These findings indicate that IKAS is important in maintaining repolarization reserve and preventing TdP in HF ventricles. Better understanding the drug effects on IKAS may be important in the prevention of sudden death in this patient population.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported in part by NIH Grants P01HL78931, R01HL71140, R21HL106554, the Kawata and Laubisch Endowments (J.N.W.), a Medtronic-Zipes Endowment (P.-S.C.) and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
We thank Nicole Courtney, Lei Lin and Jessica Warfel for their assistance.
Abbreviation list
- APD
action potential duration
- AV
atrioventricular
- Cai
intracellular calcium
- EAD
early afterdepolarization
- HF
heart failure
- ICa,L
L-type Ca2+ current
- IKAS
apamin-sensitive K+ current
- LV
left ventricle
- PCL
pacing cycle length
- pECG
Pseudoelectrocardiogram
- PVB
premature ventricular beat
- SK
small conductance Ca2+ activated K+
- TdP
torsades de pointes
- Vm
membrane potential
Footnotes
Conflict of Interest Disclosures
Medtronic, St Jude and Cyberonics Inc. donated research equipment to Dr Chen’s laboratory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 2.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 3.Waldo AL, Camm AJ, deRuyter H, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol [see comments] [published erratum appears in Lancet 1996 Aug 10;348(9024):416] Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 4.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 5.Tisdale JE, Overholser BR, Wroblewski HA, et al. Enhanced sensitivity to drug-induced QT interval lengthening in patients with heart failure due to left ventricular systolic dysfunction. Journal of clinical pharmacology Sep. 2012;52:1296–1305. doi: 10.1177/0091270011416939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol Jan. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nattel S, Maguy A, Le BS, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 8.Chua SK, Chang PC, Maruyama M, et al. Small-Conductance Calcium-Activated Potassium Channel and Recurrent Ventricular Fibrillation in Failing Rabbit Ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla IM, Long VL, Vargas-Pinto P, et al. Calcium-activated potassium current modulates ventricular (but not atrial) repolarization in chronic heart failure. Circulation. 2012;126:A16846. doi: 10.1371/journal.pone.0108824. (Abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piacentino V, III, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa M, Morita N, Tang L, et al. Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm. 2009;6:784–792. doi: 10.1016/j.hrthm.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruyama M, Joung B, Tang L, et al. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of Purkinje fibers and triggered activity. Circ Res. 2010;106:399–408. doi: 10.1161/CIRCRESAHA.109.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YS, Maruyama M, Chang PC, et al. Ryanodine receptor inhibition potentiates the activity of Na channel blockers against spontaneous calcium elevations and delayed afterdepolarizations in Langendorff-perfused rabbit ventricles. Heart rhythm. 2012;9:1125–1132. doi: 10.1016/j.hrthm.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. American Journal of Physiology. 1998;275:H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 15.Kay GN, Plumb VJ, Arciniegas JG, Henthorn RW, Waldo AL. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. Journal of the American College of Cardiology. 1983;2:806–817. doi: 10.1016/s0735-1097(83)80226-5. [DOI] [PubMed] [Google Scholar]
- 16.Nagy N, Szuts V, Horvath Z, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009;47:656–663. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan A, Shiferaw Y, Sato D, et al. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophysical journal. 2008;94:392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JJ, Nemec J, Papp R, Strongin R, Abramson JJ, Salama G. Bradycardia alters Ca2+ dynamics which enhances dispersion of repolarization and arrhythmia risk. Am J Physiol Heart Circ Physiol. 2013;304:848–860. doi: 10.1152/ajpheart.00787.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang P-C, Turker I, Lopshire JC, et al. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. JAHA. 2013;1:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreixler JC, Jenkins A, Cao YJ, Roizen JD, Houamed KM. Patch-clamp analysis of anesthetic interactions with recombinant SK2 subtype neuronal calcium-activated potassium channels. Anesthesia and analgesia Mar. 2000;90:727–732. doi: 10.1097/00000539-200003000-00040. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Kakehata S, Yamada T, Saito T, Saito H, Akaike N. Effects of potassium channel blockers on the acetylcholine-induced currents in dissociated outer hair cells of guinea pig cochlea. Neuroscience letters. 1997;236:79–82. doi: 10.1016/s0304-3940(97)00749-0. [DOI] [PubMed] [Google Scholar]
- 23.Turker I, Chang P, Chen Z, Chen P-S, Ai T. Amiodarone Inhibits Small Conductance Ca2+-Activated K+ (SK2) Channels Expressed in HEK-293 Cells. Circulation. 2011;124:A17232. (abstract) [Google Scholar]
- 24.Bkaily G, Sculptoreanu A, Jacques D, Economos D, Menard D. Apamin, a highly potent fetal L-type Ca2+ current blocker in single heart cells. Am J Physiol. 1992;262:H463–471. doi: 10.1152/ajpheart.1992.262.2.H463. [DOI] [PubMed] [Google Scholar]
- 25.Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci Feb. 1989;12:59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- 26.Adelman JP, Maylie J, Sah P. Small-Conductance Ca(2+)-Activated K(+) Channels: Form and Function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 27.Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem. 1997 Sep 12;272:23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- 28.Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing and clinical electrophysiology: PACE. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.