Abstract
Background
U.S. state AIDS Drug Assistance Programs (ADAPs) are federally funded to provide antiretroviral therapy (ART) as the payer of last resort to eligible persons with HIV infection. States differ regarding their financial contributions to and ways of implementing these programs, and it remains unclear how this interstate variability affects HIV treatment outcomes.
Methods
We analyzed data from HIV-infected individuals who were clinically-eligible for ART between 2001 and 2009 (i.e., a first reported CD4+ <350 cells/uL or AIDS-defining illness) from 14 U.S. cohorts of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Using propensity score matching and Cox regression, we assessed ART initiation (within 6 months following eligibility) and virologic suppression (within 1 year) based on differences in two state ADAP features: the amount of state funding in annual ADAP budgets and the implementation of waiting lists. We performed an a priori subgroup analysis in persons with a history of injection drug use (IDU).
Results
Among 8,874 persons, 56% initiated ART within six months following eligibility. Persons living in states with no additional state contribution to the ADAP budget initiated ART on a less timely basis (hazard ratio [HR] 0.73, 95% CI 0.60–0.88). Living in a state with an ADAP waiting list was not associated with less timely initiation (HR 1.12, 95% CI 0.87–1.45). Neither additional state contributions nor waiting lists were significantly associated with virologic suppression. Persons with an IDU history initiated ART on a less timely basis (HR 0.67, 95% CI 0.47–0.95).
Conclusions
We found that living in states that did not contribute additionally to the ADAP budget was associated with delayed ART initiation when treatment was clinically indicated. Given the changing healthcare environment, continued assessment of the role of ADAPs and their features that facilitate prompt treatment is needed.
Introduction
Reducing HIV-related health disparities is a priority of the United States (U.S.) National HIV/AIDS Strategy (NHAS) [1]. Many U.S. studies have demonstrated marked disparities in HIV health care use and outcomes by factors such as race/ethnicity [2], insurance status [3], and transmission risk [4], [5]. For example, people with HIV infection who use illicit drugs have been found to be less likely to receive antiretroviral therapy (ART) [6], [7], although gaps have been decreasing in more recent years [8]/ Furthermore, geographic variation has been linked with differences in treatment initiation [7], [9], hospitalizations [10], [11], and mortality [12] in HIV-infected people. State policy differences likely contribute to geographic disparities; individuals infected with HIV are often dependent on public health care services [13], whose guiding policies are largely determined at the state level.
In particular, differences by state response to the Ryan White CARE Act Part B AIDS Drug Assistance Programs (ADAPs), which are used by about one-quarter of HIV-infected individuals in care in the United States [13], may affect the timeliness of obtaining treatment, as well as the benefits of such treatment. State ADAPs act as the “payer of last resort” in providing ART and other prescription medications to eligible people with HIV infection [14]. People are eligible for ADAP services if they do not have their own prescription drug coverage and do not qualify for coverage through Medicare or their own state's Medicaid program (i.e., the inadequately insured, the less sick, and/or the working poor). While ADAPs receive federal funding annually through the Ryan White HIV/AIDS Program, each state administers its program independently. As a result, ADAPs differ in many ways, including the additional criteria used to define who is eligible for ADAP assistance, the comprehensiveness of the state ADAP drug formulary, and the procurement of additional funding by the ADAP through sources such as state general revenue [14]. This last factor is relevant because federal allocations may not cover the full needs of a state, and therefore many states supplement the ADAP budget using monies from state funds, which in Fiscal Year 2011 made up 16% of the national ADAP budget [15]. Additionally, some state ADAPs over the years have instituted enrollment waiting lists, an action that has been particularly scrutinized, since these lists may delay people from receiving ART, which in turn prevents them from benefiting clinically from timely ART [16], [17]. Waiting lists reached peak use in 2011, when 14 states had an active waiting list, representing 9,298 people who had applied for ADAP services but were not yet able to access medications through their states' programs [18].
The published research on the clinical consequences of specific features of ADAPs, primarily based on mathematical modeling, has found the overall program to be cost-effective [19], and that more generous state ADAPs are associated with better health outcomes, including a lower incidence of opportunistic illnesses and lower mortality [20]–[22]. Empirical data from observational studies offer an opportunity to corroborate these findings and better understand potential barriers to ADAP enrollment and therefore timely initiation of treatment. Such information is important as states manage their programs under increasingly greater client demand and limited resources [18], [23].
To understand the association between state ADAP policies and treatment outcomes, we assessed differences in ART initiation and viral load suppression among newly treatment-eligible participants in U.S. cohorts of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of prospective cohort studies of HIV-infected individuals in the U.S. and Canada, between 2001 and 2009. We compared these outcomes based on two potentially unfavorable ADAP circumstances: not having additional state funding in the annual ADAP budget and the use of waiting lists. Our research question was whether individuals living in states under each of these circumstances were less likely to have timely ART initiation and virologic suppression, compared with similar individuals not living in states under the same circumstances. A secondary question was whether these differences were more pronounced among those with a history of injection drug use. We hypothesized that effects would be greater in this population, owing to their greater needs with respect to engagement in care and starting treatment [24], [25].
Methods
Data source and study population
NA-ACCORD is a collaboration of single- and multi-site HIV cohorts that includes over 100,000 individuals from more than 100 research sites in the U.S. and Canada [26]. At least annually, each participating NA-ACCORD cohort submits standardized data regarding enrolled participants' demographic characteristics, prescribed antiretroviral therapies, laboratory tests, clinical diagnoses, and vital status to a centralized Data Management Core, where the data undergo quality control for completeness and accuracy before being combined into harmonized analysis files.
The source population for our analyses consisted of HIV-infected individuals in the NA-ACCORD who were newly eligible to initiate ART between 2001 and 2009, based on existing treatment guidelines during this period (an incident AIDS-defining event or CD4+ lymphocyte [CD4+] count recorded <350 cells/uL) [27] from 14 U.S. cohorts. Inclusion criteria included known residence within a U.S. state, no prior CD4+ counts <350 cells/uL or AIDS-defining illnesses documented, at least two CD4+ counts in the study period, and no prior use of ART documented.
Because we were interested in answering the question of whether individuals would have had different outcomes if they did not live in a state without a particular ADAP characteristic, we limited certain analyses to a subset of individuals who lived in states with that particular feature in place at the time of ART eligibility, and similar individuals who lived in states without that feature.
In a secondary analysis, we examined individuals with a documented history of injection drug use (IDU). To account for potential underreporting of IDU, we also included individuals without a documented history of IDU but with a diagnosis of hepatitis C infection recorded in the absence of either a report of hemophilia, contact with blood products, or among men, sex with men. While this may have included some individuals without a history of IDU, we conducted sensitivity analyses excluding these additional individuals.
Ethics: The activities of the NA-ACCORD have been reviewed and approved by the local institutional review boards (IRBs) for each site. This study was determined to not qualify as human subjects research by the Johns Hopkins Bloomberg School of Public Health IRB.
Outcomes of interest
Our first outcome of interest was time to ART initiation, using the date of ART eligibility (i.e., the first date that an incident AIDS-defining illness or a CD4+ count <350 cells/uL was recorded) as the time origin. Time to ART initiation was defined as the duration between the date of eligibility and the date an ART regimen was prescribed (denoted in the medical record), or if this was not available, when a regimen was started (denoted by self-report). Time was censored at six months after eligibility to focus on more timely treatment initiation. ART regimens comprised at least three active antiretroviral agents, including a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, an entry inhibitor, or an integrase strand transfer inhibitor; or three nucleoside reverse transcriptase inhibitors, including abacavir or tenofovir. Ritonavir in the presence of another protease inhibitor was not included in this definition.
The other outcome of interest was time from ART eligibility to viral load (VL) suppression (within one year). Suppression was based on a laboratory result report of an HIV-1 RNA level ≤500 copies/mL. This threshold was used to account for differences in detection limits of commercial assays over the study period [28].
Variables of interest
For each individual in our study, the two state ADAP features in place on the date of ART eligibility were assessed and stratified into dichotomous categories that could be classified as more cost-containing versus less cost-containing: (1) amount of state funding provided to the annual ADAP budget (none vs. any); and (2) use of waiting lists in the state (yes vs. no). Information on state ADAP features was derived from the results of surveys conducted by the National Alliance of State and Territorial AIDS Directors (NASTAD) and published in annual reports [15]. We initially considered two additional state ADAP features, financial eligibility criteria for ADAP enrollment and inclusiveness of the state ADAP drug formulary with respect to commercially available antiretroviral drugs, but found limited variation in these variables across states (Table 1) (e.g., most states have a comprehensive ART formulary), restricting our ability to assess their impact on the outcomes of interest. State-level ADAP variables were linked to individuals by their state of residence at the time of ART eligibility (i.e., these values varied by time). For three multi-site cohorts, the state of residence was not available, and the state of the clinic site was used instead as a proxy. We hypothesized that living in a state with a less generous ADAP feature would be associated with delayed ART initiation and virologic suppression (i.e., a hazard ratio less than one).
Table 1. Comparison of demographic characteristics and AIDS Drug Assistance Program features of U.S. states represented in study, 2001 and 2009 data.
| Characteristic | 2001 | 2009 | ||||||
| All U.S. states* | 34 states* in study | All U.S. states* | 34 states* in study | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Demographic variables | ||||||||
| Population density (per square mile) | 90 | 42–221 | 137 | 63–274 | 100 | 43–230 | 150 | 66–282 |
| % of population that is of black race | 7.2 | 2.3–15.8 | 10.9 | 4.1–19.8 | 7.6 | 3.1–16.3 | 11.5 | 5.3–19.7 |
| Annual household income (current U.S. dollars, thousands) | 51,004 | 46,473–58,205 | 51,663 | 47,095–56,861 | 49,909 | 45,455–56,568 | 49,271 | 45,036–56,853 |
| % of population living below FPL | 10.5 | 8.5–14.1 | 11.1 | 8.5–14.2 | 13.3 | 10.9–15.8 | 13.9 | 11.7–16.6 |
| State Medicaid HIV spending per capita | N/A | N/A | N/A | N/A | 18,757 | 15,768–22,710 | 19,621 | 16,417–23,088 |
| AIDS Drug Assistance Program (ADAP) features | ||||||||
| % state contribution to total ADAP budget expenditures | 9 | 0–21 | 14 | 3–28 | 11 | 0–25 | 19 | 5–31 |
| States contributing to total ADAP budget, by percentage (N, %) | ||||||||
| 0% | N = 15 | 29% | N = 6 | 18% | N = 17 | 33% | N = 8 | 24% |
| Less than 20% | N = 22 | 43% | N = 17 | 50% | N = 15 | 29 | N = 9 | 26% |
| 20% or more | N = 14 | 27% | N = 11 | 32% | N = 19 | 37% | N = 17 | 50% |
| % of all available antiretroviral drugs on formulary | 100 | 100–100 | 100 | 100–100 | 100 | 97–100 | 100 | 97–100 |
| Financial eligibility threshold as % of FPL | 300 | 230–350 | 300 | 281–370 | 300 | 300–400 | 300 | 300–400 |
| States with waiting list at least once during study (N, %) | - | - | - | - | N = 20 | 39% | N = 11 | 32% |
Including the District of Columbia.
FPL = federal poverty level, IQR = interquartile range, N/A = not available. State demographic variables from annual U.S. Census population estimates and the Current Population Survey [31], [33]. State Medicaid spending from the Kaiser Commission on Medicaid and the Uninsured and the Urban Institute [32]. ADAP features from the National Alliance of State and Territorial AIDS Directors (NASTAD) [15].
Other variables
Other individual-level variables assessed at the time of ART eligibility and included as potential confounders were age, race/ethnicity (black; Hispanic; white or other), sex and transmission risk (men who have sex with men; male IDU; female IDU; male heterosexual or other risk, female heterosexual or other risk), CD4+ count, HIV viral load, calendar year, and documented histories of drug abuse, alcohol abuse, and mental illness. Drug abuse, alcohol abuse, and mental illness were categorized on the basis of more specific diagnoses derived from electronic medical record diagnoses and chart reviews. As potential psychosocial barriers to ART initiation, they were grouped as a single ordinal variable, representing the number of barriers experienced [29].
To account for differences in ART initiation influenced by characteristics of the cohorts or clinics themselves, we categorized cohorts into the following categories: multi-site clinical cohort, single-site clinical cohort, and interval cohort. Interval cohorts differ from clinical cohorts in both timing and data collection; individuals are followed at specified intervals (e.g., every six months) that are unrelated to health care visits, and data are collected according to defined protocols [30]. We also included two variables representing specific mechanisms undertaken by individual clinics to assist with access to ART drugs. This information came from the results of a standardized questionnaire given in September 2011 to all clinical cohorts contributing data to this study. Mechanisms were divided into those performed by clinic staff and those done after referral to entities outside the clinic. Additional information about the questionnaire is in File S1.
State-specific characteristics related to population demographics and Medicaid spending may also affect decisions on how ADAPs are run, as well as ART initiation. To account for these potential confounding differences, we included the following state variables, linked to individuals by the year of ART eligibility and categorized into quartiles: population density [31], the percentage of the population who are of black race, the percentage of the population living below the federal poverty line, median household income, and per capita Medicaid spending on enrollees with HIV. Medicaid data came from the Henry J. Kaiser Family Foundation and were available for the years 2007–2009 only [32]. All other data were available from all years (i.e., these values varied by time) and came from annual U.S. Census population estimates [31] and the Current Population Survey [33].
Statistical methods
To estimate the effect of each ADAP characteristic on treatment outcomes, we used propensity score matching to account for potential differences between persons living in a state with a specific ADAP characteristic (“exposed” participants) and persons living in a state without that characteristic (“unexposed” participants). Details of the use of this method are included in File S1. Briefly, for each characteristic, we developed a multivariable logistic regression model to estimate the predicted probability of living in a state with that feature, controlling for individual- and clinic-level variables that might confound the relationship between the exposure and the outcomes of interest. We then matched exposed participants to comparable unexposed participants based on the propensity of exposure, using 1∶3 nearest neighbor matching (i.e., matching to the unexposed participant with the most similar propensity score), with replacement. Balance on potential confounders between exposed and unexposed participants was evaluated quantitatively and graphically. Propensity score matching was performed using the MatchIt package [34] in R 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria). After matching, we used Cox regression to examine differences in the time to ART initiation and time to VL suppression by each ADAP feature. Models were adjusted for the propensity score and any additional variables with residual imbalance, and weighted to account for matching with replacement. The resulting inferences are generalizable to persons who are similar to those living in states with the less generous ADAP characteristic, maximizing internal validity in this subset of individuals [35].
We also performed analyses that did not use propensity score matching but rather conventional multivariable Cox regression analysis. Such models may be less able to adjust for known confounders if there is limited covariate overlap, but use the entire study population instead of a more limited subset. We also used conventional Cox regression analysis for our pre-specified subgroup analysis among IDU, because we could not get adequate balance on confounders in the propensity score model.
To further explore the relationship between a state contribution to the annual ADAP budget and increases in ART initiation, we looked for evidence of a “dose-response” trend in state funding. Because our propensity score models used logistic regression and thus require a dichotomous “treatment”, we used conventional Cox models to explore this relationship. We created three levels of state funding: 0% of the total ADAP budget (i.e., no state contribution), >0% but <20%, and 20% or more.
Finally, we performed several sensitivity analyses to examine assumptions about the relationship between state ADAP characteristics and the outcomes of interest. These included use of alternate statistical methods, modifications to the exposure definition, and additional subgroup analyses (see File S1 for details).
Results
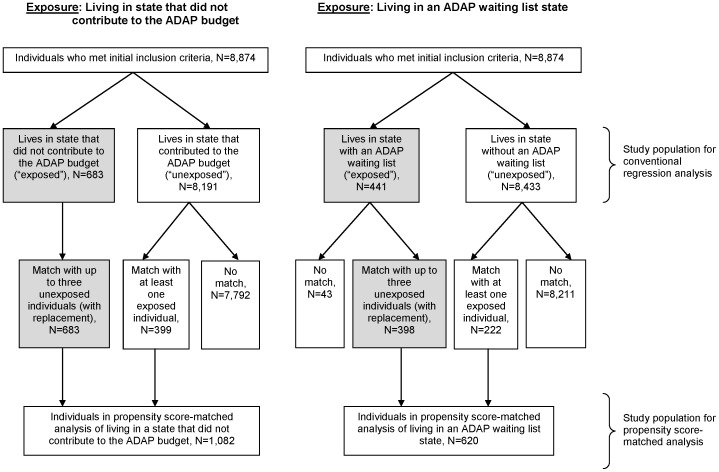
There were 8,874 individuals initially eligible between 2001 and 2009 for inclusion in this analysis. Figure 1 shows the study selection process used to identify individuals living in states with the ADAP characteristics under question, and similar individuals not living in these states, used in propensity score analyses. Overall, the median age was 40 years, and 74% were men (Table 2). Among men, 59% reported sex with men as a transmission risk factor, 14% reported IDU and 27% reported heterosexual transmission or other risk. Among women, 17% reported IDU and 83% reported heterosexual or other risk. Among all individuals, 44% were black, 33% were white, 18% were Hispanic, and 4% were Asian or of other race/ethnicity. The overall study population lived in 33 states and the District of Columbia (Figure 2).
Figure 1. Flow charts showing selection into each of the two analyses.
Gray indicates the population of interest for the propensity score-matched analyses.
Table 2. Characteristics of newly treatment-eligible HIV-infected U.S. residents in NA-ACCORD, 2001–2009.
| Overall (N = 8,874) | Included in analysis of state contribution to ADAP budget (N = 1,082) * | Included in analysis of state ADAP waiting lists (N = 620) * | ||||
| N | % | N | % | N | % | |
| Age at eligibility, years (median, IQR) | 40 | 33–46 | 41 | 34–47 | 37 | 31–44 |
| 18–29 | 1,555 | 18 | 139 | 13 | 131 | 21 |
| 30–39 | 2,869 | 32 | 343 | 32 | 236 | 38 |
| 40–49 | 2,989 | 34 | 397 | 37 | 196 | 32 |
| 50–59 | 1,216 | 14 | 181 | 17 | 47 | 8 |
| 60+ | 245 | 3 | 22 | 2 | 10 | 1.6 |
| Race/ethnicity | ||||||
| Black (non-Hispanic) | 3,937 | 44 | 617 | 57 | 272 | 44 |
| Hispanic | 1,631 | 18 | 57 | 5 | 40 | 7 |
| White (non-Hispanic) | 2,944 | 33 | 382 | 35 | 293 | 47 |
| Other (non-Hispanic) | 362 | 4 | 26 | 2.4 | 15 | 2.4 |
| Sex and transmission risk | ||||||
| Men who have sex with men | 3,839 | 43 | 368 | 34 | 282 | 46 |
| Male injection drug user | 946 | 11 | 210 | 19 | 46 | 7 |
| Male, heterosexual or other risk | 1,764 | 20 | 162 | 15 | 145 | 23 |
| Female injection drug user | 387 | 4 | 115 | 11 | 12 | 1.9 |
| Female, heterosexual or other risk | 1,938 | 22 | 227 | 21 | 135 | 22 |
| Eligibility criteria | ||||||
| CD4+ count 0–199 cell/uL | 3,118 | 35 | 274 | 25 | 224 | 36 |
| CD4+ count 200–349 cells/uL | 5,464 | 62 | 775 | 72 | 380 | 61 |
| Incident AIDS-defining illness (i.e., CD4+ count not <350 cells/uL) | 292 | 3 | 33 | 3 | 16 | 2.6 |
| Viral load at eligibility | ||||||
| 501–999 copies/mL | 152 | 1.7 | 12 | 1.1 | 6 | 1 |
| 1,000–9,999 copies/mL | 1,299 | 15 | 156 | 14 | 56 | 9 |
| 10,000–99,999 copies/mL | 3,743 | 42 | 464 | 43 | 248 | 40 |
| 100,000+ copies/mL | 2,588 | 29 | 261 | 24 | 162 | 26 |
| Missing | 1,092 | 12 | 189 | 18 | 148 | 24 |
ART = antiretroviral therapy, IQR = interquartile range. Percentages may not add up to 100 due to rounding.
See Figure 1 for details of study selection procedure.
Figure 2. Map of U.S. states represented in study.
In Table 1, we show state-level demographic and socioeconomic characteristics based on 2001 and 2009 data of the 34 jurisdictions represented by the overall study population, as well as a comparison to the U.S. overall. States in this study were more densely populated, more diverse with respect to black race, had a greater percentage of their population living below the federal poverty line, and spent more Medicaid dollars on HIV per capita. Table 1 also shows the distribution of selected ADAP features in these states. The percent state contribution to the state's total ADAP budget was not significantly associated with having an ADAP waiting list.
Regarding the mechanisms undertaken by individual clinics to assist with access to ART drugs (Table S1 in File S1), clinics on average had four procedures in place to directly assist their patients with accessing prescription drugs, including assisting patients with ADAP enrollment (91%), Medicare Part D, and Medicaid (both 86%), and pharmaceutical assistance programs (77%). 64% also had mechanisms in place to refer patients to other organizations for additional help.
Association between no state ADAP contribution and treatment outcomes
In the overall study population (N = 8,874), 56% of individuals initiated ART within six months of eligibility. Persons living in states not contributing to the ADAP budget were less likely to initiate ART within six months than persons living in states that did (39% vs. 58%). Table 3 shows crude and adjusted conventional Cox regression-based hazard ratios for the association between living in a state contributing to its ADAP budget and ART initiation (adjusted hazard ratio [HR] 0.80 [95% CI 0.69–0.93]). After propensity score matching, the association between living in a state with no additional state contribution to the ADAP budget and delayed ART initiation retained statistical significance (N = 1,082, HR 0.73, 95% CI 0.60–0.88). We also found a significant dose-response relationship: compared with living in a state with a 20% or greater state contribution, the HR for ART initiation when living in a state with more than 0% but less than <20% contribution was 0.90 (95% CI 0.82–0.99), and the HR for no contribution was 0.75 (95% CI 0.63–0.88) (ptrend<0.001). In the analysis limited to IDU, the adjusted hazard ratio for ART initiation was 0.67 (95% CI 0.47–0.95), and the dose-response effect persisted. Other sensitivity analyses examining alternative approaches or within different subgroups showed generally consistent findings with the base case results, although some of these associations did not reach statistical significance (Table S2 of File S1).
Table 3. Association between living in a state not contributing to the annual ADAP budget and ART initiation and virologic suppression, U.S. NA-ACCORD, 2001–2009.
| Outcome: 6-month ART initiation | Outcome: 1-year virologic suppression | |||
| HR | 95% CI | HR | 95% CI | |
| Overall | ||||
| No contribution (vs. any contribution) | ||||
| Crude (N = 8,874) | 0.56 | 0.49–0.63 | 0.75 | 0.67–0.83 |
| Regression-adjusted (N = 8,874) | 0.80 | 0.69–0.93 | 1.02 | 0.88–1.18 |
| Propensity score-matched (N = 1,082) * | 0.73 | 0.60–0.88 | 1.13 | 0.93–1.36 |
| Dose-response effect (Ptrend) (N = 8,874) | <0.001 | 0.25 | ||
| No contribution | 0.75 | 0.63–0.88 | 1.06 | 0.91–1.24 |
| Contribution <20% | 0.90 | 0.82–0.99 | 1.07 | 0.97–1.17 |
| Contribution >20% | 1.00 | Ref. | 1.00 | Ref. |
| Injection drug users only (N = 1,824) | ||||
| No contribution (vs. any contribution) | ||||
| Crude | 0.40 | 0.31–0.51 | 0.78 | 0.64–0.96 |
| Regression-adjusted | 0.67 | 0.47–0.95 | 1.14 | 0.82–1.59 |
| Dose-response effect (Ptrend) | 0.005 | 0.29 | ||
| No contribution | 0.58 | 0.40–0.86 | 1.21 | 0.83–1.74 |
| Contribution <20% | 0.81 | 0.63–1.04 | 1.10 | 0.85–1.42 |
| Contribution >20% | 1.00 | Ref. | 1.00 | Ref. |
ART = antiretroviral therapy, CI = confidence interval, HR = hazard ratio.
All analyses use Cox proportional hazards regression.
Hazard ratios obtained after 1∶3 matching (with replacement) 683 “exposed” to 399 “unexposed” individuals based on propensity of living in a state contributing to the ADAP budget.
Both regression-adjusted and propensity-score matched analyses account for the following variables: age; sex; race/ethnicity; transmission risk; CD4+ count and viral load at eligibility; history of alcohol abuse, substance abuse, and mental disorders; year of eligibility; type of cohort; clinic-specific mechanisms to help obtain ART; state-level population density, % population of black race, % population below poverty line, median household income, and per capita Medicaid spending on HIV.
Virologic suppression one year after ART eligibility among the entire study population was 58%, with those living in states not contributing to the ADAP budget less likely to have a suppressed viral load (51% versus 59%). In adjusted analyses, this association was not statistically significant (conventional Cox regression-adjusted HR 1.02, 95% CI 0.88–1.18; propensity score-matched HR 1.13, 95% CI 0.93–1.36).
Association between ADAP waiting lists and treatment outcomes
Among the overall study population (N = 8,874), ART initiation after six months was higher among those living in a state with an existing ADAP waiting list than those living in a state without a list (73% versus 55%). A similar pattern was observed in this overall population for one-year virologic suppression (71% versus 58%). In regression-adjusted analyses, the hazard ratio based on living in a waiting list state was 1.73 (95% CI 1.45–2.07) for ART initiation and 1.21 (95% CI 1.01–1.44) for virologic suppression (Table 4). After propensity score matching to improve exchangeability between groups, living in a waiting list state was no longer associated with delayed ART initiation (N = 620, HR 1.12, 95% CI 0.87–1.45) or virologic suppression (HR 1.05, 0.79–1.38). However, our analysis among IDU maintained the significant association between living in a waiting list state and ART initiation (HR 2.15, 95% CI 1.31–3.55).
Table 4. Association between living in an ADAP waiting list state and ART initiation and virologic suppression, U.S. NA-ACCORD, 2001–2009.
| Outcome: 6-month ART initiation | Outcome: 1-year virologic suppression | |||
| HR | 95% CI | HR | 95% CI | |
| Overall | ||||
| Living in a waiting list state (vs. not living in a waiting list state) | ||||
| Crude (N = 8,874) | 1.55 | 1.38–1.73 | 1.39 | 1.24–1.57 |
| Regression-adjusted (N = 8,874) | 1.73 | 1.45–2.07 | 1.21 | 1.01–1.44 |
| Propensity score-matched (N = 620) * | 1.12 | 0.87–1.45 | 1.05 | 0.79–1.38 |
| Injection drug users only (N = 1,824) | ||||
| Living in a waiting list state (vs. not living in a waiting list state) | ||||
| Crude | 1.59 | 1.19–2.11 | 1.49 | 1.10–2.03 |
| Regression-adjusted | 2.15 | 1.31–3.55 | 1.30 | 0.80–2.09 |
ART = antiretroviral therapy, CI = confidence interval, HR = hazard ratio.
All analyses use Cox proportional hazards regression.
Hazard ratios obtained after 1∶3 matching (with replacement) 398 “exposed” to 222 “unexposed” individuals based on propensity of living in a waiting list state.
Both regression-adjusted and propensity-score matched analyses account for the following variables: age; sex; race/ethnicity; transmission risk; CD4+ count and viral load at eligibility; history of alcohol abuse, substance abuse, and mental disorders; year of eligibility; type of cohort; clinic-specific mechanisms to help obtain ART; state-level population density, % population of black race, % population below poverty line, median household income, and per capita Medicaid spending on HIV.
We performed a sensitivity analysis to examine whether the non-significant association was maintained when shortening the time to ART initiation to 3 months after eligibility instead of 6 months. Here, the HR was 1.44 (95% CI 1.06–1.97) (Table S2 of File S1). When we did not account for clinic-specific mechanisms to obtain treatment for patients in the propensity score model, the associations between living in a waiting list state and our outcomes of interest were greater and reached statistical significance (HR 1.93, 95% CI 1.49–2.51 for ART initiation, HR 1.29, 95% CI 1.02–1.63 for virologic suppression) compared with the base case scenario. Thus, additional follow-up time and confounder control seemed to attenuate the association between living in a waiting list state and ART initiation.
Discussion
In this study of HIV-infected individuals in the United States who were newly clinically eligible to begin ART, we found that not having an additional state contribution to an ADAP's annual budget was associated with delayed ART initiation. This finding was robust to the type of statistical procedure used to account for known confounders, and furthermore was maintained when considering different assumptions, and when focusing on specific subpopulations, including those with a history of IDU.
Our findings are consistent with an ecologic analysis that suggested greater HIV inequities in some U.S. states as a result of lower state ADAP contributions [36]. Combining this information with our a priori hypothesis and the dose-response effect identified, we believe that we have described a plausible mechanism in delayed ART uptake. Several reports have noted the importance of state contributions to ADAP budgets [37], [38]. While discretionary federal funds to ADAP are proportionally allocated to states on the basis of HIV prevalence, in principle to guarantee distributional equity [39], this metric may not measure all aspects of need in individual states. The additional funding stream based on state general revenue may play a role in maintaining the core functions of the program or help to improve treatment uptake in the target population, such as the inadequately insured or the working poor. It could also result in more or better trained ADAP office staff to work with and follow up with clients or in better ancillary client services like adherence support.
We found that living in a state with an active ADAP waiting list was not associated with less timely ART initiation, and in fact, in some scenarios associated with more timely ART initiation. On the surface, this may seem paradoxical; we expected that living in a state with an ADAP waiting list would be associated with less timely ART initiation. However, this finding may reflect efforts at study sites contributing data to NA-ACCORD to get patients promptly treated when there is knowledge of existing structural barriers. For example, the more timely initiation related to waiting lists that we observed among IDU could reflect special efforts by sites to engage this high-need group into care, since it is known that IDU have lower levels of engagement in HIV care compared with other risk groups [25]. While we controlled for some clinic-level behaviors regarding assistance with procurement of ART, we may not have fully captured the scope of these efforts, since assessments were conducted retrospectively based on a survey of participating clinics. Furthermore, additional efforts occurring at state ADAP offices (e.g., efforts to help people sign up for pharmaceutical company prescription assistance programs when being placed on a waiting list, use of other cost-containment strategies when resources are low) were not assessed in our study. In our analysis, excluding clinic-based efforts from the propensity score model resulted in more pronounced and statistically significant associations between living in a waiting list state and ART initiation and virologic suppression, suggesting that we at least partially accounted for clinic-level factors related to ART initiation. Having additional information on the mechanisms that people use to access treatment would further inform this important data consideration.
We used propensity score matching methods to create comparable groups of “exposed” and “unexposed” individuals, capitalizing on the heterogeneity of policies across different states in the NA-ACCORD. This technique measures the “average treatment effect in the treated” population, which is different from the “average treatment effect” in the entire study population that conventional regression analyses assess. We can interpret our propensity-score matched estimates regarding state ADAP features as applicable to the subset of individuals with the same risk factor distribution as those living in those states with those features (i.e., no state contribution to the ADAP budget; presence of ADAP waiting lists) [40]. Thus, these findings may not necessarily apply to those with different risk factor distributions, or those who were not selected as a match. Nonetheless, when we ran conventional regression models that estimated effects among the entire study population, the results were generally similar to the propensity score-based results, lending further support to our conclusions.
We did not find significant associations between less generous ADAP features and less timely virologic suppression. One possibility for this is that the majority of HIV-infected individuals in our population were eventually treated (the percentage increasing to 65% overall after one year of eligibility), and once they began treatment, differences in the state ADAP features we examined may have played less of a role. In other words, the majority of people reached guideline-defined treatment goals, despite the delay in starting therapy that more limited state budgets may influence. This is encouraging, even though the additional efforts expended to procure treatment in light of these delays have costs.
Furthermore, we did not report on longer-term outcomes like sustained viral load suppression and mortality. Because our study is essentially an intent-to-treat analysis, we did not take into account changes in ADAP features over the course of an individuals' treatment trajectory. An analysis of time-updated ADAP changes could help to understand these processes better, especially considering the variability in coverage by some state ADAPs of medications for other health conditions relevant to HIV-infected individuals like hepatitis infection, cardiovascular disease, and mental health conditions [15], [41]–[43].
We originally hypothesized that our effect estimates would be greater among IDU owing to their increased needs with respect to care engagement and treatment initiation. While our data provide some evidence of this, the overall effects are not dramatically different from those overall, suggesting that on the whole, state-level differences in the ADAP features we examined may affect their target populations similarly with respect to ART initiation. However, it is possible that other differences in state ADAP formularies, such as coverage of hepatitis treatment or opioid dependency [23], [44], could influence outcomes more likely to affect IDU such as liver disease and drug overdose, and this is worth exploring.
Recent observational studies have taken other approaches to understand the influence of ADAP features, directly examining the benefits of ADAP enrollment itself on treatment utilization [42], [45], [46]. For example, the Women's Interagency HIV Study found increased use of ART among HIV-infected women enrolled in an ADAP versus those not enrolled, even after adjusting for insurance status [42]. A study from the 1917 Clinic in Alabama found that many ADAP enrollees, despite having ART available to them, still use ART suboptimally [45]. Because these studies focused on ADAP enrollment as an exposure itself, they examined pathways related to successful use of treatment as a consequence of enrollment. Our analysis complements these studies by providing information on earlier mechanisms that are a function of the state-related features of the ADAPs themselves, to address potential barriers to ADAP enrollment and therefore timely initiation of treatment. Our study is the largest to date examining the role of ADAP on treatment outcomes. Importantly, six of the ten states with the highest ADAP enrollments in the country were among the largest ten states represented in our study population (California, New York, Florida, Texas, Illinois, Pennsylvania) [18]. We also used consistent methods across states in our analysis, which would be more difficult to accomplish systematically using data from individual state programs [47]. However, our study has limitations. First, although NA-ACCORD sites are diverse and represent a variety of research settings [26], [48], many of the participating clinics are located at major academic centers, and therefore our inferences may be less generalizable to patients not seen at such clinics. However, we are reassured somewhat by the fact that many of these sites are responsible for the majority of HIV care in their respective catchment areas, and therefore are applicable to a large proportion of the general HIV-infected population. Second, because information on individual-level socioeconomic status or insurance status (including actual ADAP enrollment itself for each of the participants) was not available, our study population includes both people who are financially eligible for ADAP services (i.e., lower income) and people who may not qualify for assistance (i.e., higher income). Thus, we could not specifically study the subset of our population that was the true population at risk. Because of the ecologic nature of this analysis, the effects we estimated could be considered “contextual” effects, in that they apply to those living in the state during which a particular ADAP feature was in place, and not just those who were actually enrolled in an ADAP. Nonetheless, such contextual effects are useful since they suggest benefits from policies that go beyond the narrower population of ADAP enrollees.
Another limitation is that our exposures of interest were based on the results of annual surveys of state ADAP offices conducted by NASTAD over the study period and therefore are dependent on the quality of these findings. However, these results are publicly available and therefore allow for transparency should similar assessments be conducted by other investigators. Unmeasured confounding may have also affected our effect estimates. Both propensity score matching and conventional regression techniques are designed to account for observed confounders, but there may be other characteristics of patients, clinics, or the states themselves that we have not accounted for in our analysis. For example, we did not account for the diffusion of each state's ADAP program among its HIV-infected population, or more nuanced differences in state Medicaid eligibility or generosity beyond per capita HIV spending, which if important could lead to some bias in our conclusions. In sensitivity analyses, we controlled for state fixed effects to try to account for all of the unobserved characteristics of a particular state, but by doing so this technique may have over-adjusted for these effects, which may have been highly correlated with the exposure of interest.
Finally, the period of eligibility for this analysis ended in 2009, when at least two major changes occurred in the HIV epidemic in the United States: the adoption of clinical guidelines recommending starting treatment at a CD4+ count of 500 cells/uL or even higher [49], and a substantial rise in the number of people in the U.S. on ADAP waiting lists in 2010 and 2011, due to state-level economic crises [15], [18]. While better understanding of more recent changes is needed, our analysis nonetheless covered a significant portion of the history of the ADAP program. Future work should monitor ongoing changes to the healthcare funding landscape [50].
In conclusion, our study found an association between living in a state that does not provide an additional contribution to ADAP funding and delays in ART initiation. The importance of timely ART initiation when clinically indicated is well-established [17]. Many factors complicate the healthcare environment for people with HIV infection in the United States, including competition for resources as more people are tested and treated earlier [51], [52] and evolving trends in health insurance coverage [53], which will likely further change as Medicaid eligibility expands with the implementation of the Patient Protection and Affordable Care Act [54]–[56]. Because of these changes, more research on the impact of budgetary differences on the effectiveness of state ADAPs in providing timely therapies is clearly warranted, particularly for the groups that need this assistance the most. Such additional information may help ADAPs to better manage their resources and best serve the needs of their target populations.
Supporting Information
Appendix. Table S1. Mechanisms at individual clinics in NA-ACCORD to assist patients in accessing prescription drugs in 2008 (N = 22). Table S2. Sensitivity analyses for association between state ADAP features at time of eligibility and outcomes.
(DOCX)
Acknowledgments
Cohorts and representatives of the NA-ACCORD
AIDS Link to the IntraVenous Experience: Gregory D. Kirk.
Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson, Ronald J. Bosch, and Ann C. Collier.
Fenway Health HIV Cohort: Stephen Boswell, Chris Grasso, and Ken Mayer.
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Richard Harrigan, Julio Montaner, and Angela Cescon.
HIV Outpatient Study: John T. Brooks and Kate Buchacz.
HIV Research Network: Kelly A. Gebo.
Johns Hopkins HIV Clinical Cohort: Richard D. Moore.
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez.
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg.
Kaiser Permanente Northern California: Michael J. Silverberg.
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne.
Multicenter Hemophilia Cohort Study–II: James J. Goedert.
Multicenter AIDS Cohort Study: Lisa P. Jacobson.
Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein.
Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann Burchell, and Anita R. Rachlis.
Retrovirus Research Center, Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor.
Southern Alberta Clinic Cohort: M. John Gill.
Studies of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin.
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig.
University of North Carolina, Chapel Hill, HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik.
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane.
Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin.
Vanderbilt-Meharry Centers for AIDS Research Cohort: Timothy R. Sterling, David Haas, Sally Bebawy, and Megan Turner.
Women's Interagency HIV Study: Stephen J. Gange and Kathryn Anastos.
NA-ACCORD Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Keri N. Althoff, Mari M. Kitahata, Rosemary G. McKaig, Amy C. Justice, and Aimee M. Freeman.
NA-ACCORD Administrative Core: Richard D. Moore, Aimee M. Freeman, Carol Lent and Aaron Platt.
NA-ACCORD Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Eric Webster, Liz Morton, and Brenda Simon.
NA-ACCORD Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Alison G. Abraham, Bryan Lau, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Shari Modur, David B. Hanna, Peter Rebeiro, Cherise Wong and Adell Mendes.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH).
Funding Statement
This work was supported by grants F31-DA30254, U01-AI069918, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI42590, U01-HD32632, UL1-RR024131, UL1-RR024131, M01-RR-00052, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, M01-RR025747, P30-AI27757, P30-AI27767, P30-AI27763, P30-AI50410, P30-AI54999, P30-AI036219, R01-DA04334, R01-DA12568, R01-DA11602, R01-AA16893, R24-AI067039, AHQ290-01-0012, N02-CP55504, K01-AI071725, K01-AI071754, K01-AI093197, K23-AI610320, K24-DA00432, and K24-AI1065298 from the National Institutes of Health; contract CDC200-2006-18797 from the CDC; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036, KRS-86251, and 169621 from the Canadian Institutes of Health Research; the Canadian HIV Trials Network, project 24; and the government of British Columbia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Available: http://www.whitehouse.gov/administration/eop/onap/nhas. Accessed 15 Jul 2010.
- 2. Centers for Disease Control and Prevention (2011) Vital signs: HIV prevention through care and treatment–United States. MMWR Morb Mortal Wkly Rep 60 (47) 1618–23. [PubMed] [Google Scholar]
- 3. Palella FJ, Baker RK, Buchacz K, Chmiel JS, Tedaldi EM, et al. (2011) Increased mortality among publicly insured participants in the HIV Outpatient Study despite HAART treatment. AIDS 25 (15) 1865–76. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, et al. (1999) Variations in the care of HIV-infected adults in the United States: Results from the HIV Cost and Services Utilization Study. JAMA 281 (24) 2305–15. [DOI] [PubMed] [Google Scholar]
- 5. Yehia BR, Fleishman JA, Hicks PL, Ridore M, Moore RD, et al. (2010) Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr 53 (3) 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chander G, Himelhoch S, Fleishman JA, Hellinger J, Gaist P, et al. (2009) HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care 21 (5) 655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, et al. (2013) Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 56 (8) 1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore RD, Keruly JC, Bartlett JG (2012) Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis 55 (9) 1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, et al. (2011) Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 203 (4) 442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hellinger FJ, Fleishman JA (2011) Location, race, and hospital care for AIDS patients: An analysis of 10 states. Inquiry 38 (3) 319–30. [DOI] [PubMed] [Google Scholar]
- 11. Fleishman JA, Hellinger FH (2003) Recent trends in HIV-related inpatient admissions 1996–2000: a 7-state study. J Acquir Immune Defic Syndr 34 (1) 102–10. [DOI] [PubMed] [Google Scholar]
- 12. Hanna DB, Selik RM, Tang T, Gange SJ (2012) Disparities among US states in HIV-related mortality in persons with HIV infection, 2001–2007. AIDS 26 (1) 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, et al. (2011) Clinical and behavioral characteristics of adults receiving medical care for HIV infection — Medical Monitoring Project, United States, 2007. MMWR Surveill Summ 60 (11) 1–20. [PubMed] [Google Scholar]
- 14.National Alliance of State and Territorial AIDS Directors. National ADAP Monitoring Project Annual Report. Available: http://www.nastad.org/Docs/020035_2011%20NASTAD%20National%20ADAP%20Monitoring%20Project%20Annual%20Report.pdf. Accessed 8 Dec 2011.
- 15.National Alliance of State and Territorial AIDS Directors. National ADAP Monitoring Project Annual Report. Available: http://www.nastad.org/Docs/020035_2011%20NASTAD%20National%20ADAP%20Monitoring%20Project%20Annual%20Report.pdf. Accessed 8 Dec 2011.
- 16. Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338 (13) 853–60. [DOI] [PubMed] [Google Scholar]
- 17. Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, et al. (2009) Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 360 (18) 1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Alliance of State and Territorial AIDS Directors. National ADAP Monitoring Project Annual Report, Module One. Available: http://www.nastad.org/Docs/081741_Module%20One%20-%20National%20ADAP%20Monitoring%20Project%20Annual%20Report%20-%20January%202012.pdf. Accessed 9 May 2012.
- 19. Pinkerton SD, Kibicho J, Galletly CL (2013) Is the US AIDS drug assistance program cost-effective? AIDS Behav 17 (1) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johri M, David Paltiel A, Goldie SJ, Freedberg KA (2002) State AIDS Drug Assistance Programs: Equity and efficiency in an era of rapidly changing treatment standards. Med Care 40 (5) 429–41. [DOI] [PubMed] [Google Scholar]
- 21. Linas BP, Zheng H, Losina E, Rockwell A, Walensky RP, et al. (2006) Optimizing resource allocation in United States AIDS drug assistance programs. Clin Infect Dis 43 (10) 1357–64. [DOI] [PubMed] [Google Scholar]
- 22. Linas BP, Losina E, Rockwell A, Walensky RP, Cranston K, et al. (2009) Improving outcomes in state AIDS drug assistance programs. J Acquir Immune Defic Syndr 51 (5) 513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bassett IV, Farel C, Szmuilowicz ED, Walensky RP (2008) HIV/AIDS: AIDS Drug Assistance Programs in the era of routine HIV testing. Clin Infect Dis 47 (5) 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGowan CC, Weinstein DD, Samenow CP, Stinnette SE, Barkanic G, et al. (2011) Drug use and receipt of highly active antiretroviral therapy among HIV-infected persons in two U.S. clinic cohorts. PLoS One 6 (4) e18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westergaard RP, Hess T, Astemborski J, Mehta SH, Kirk GD (2013) Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS ePub 13 Jun 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, et al. (2007) Cohort profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 36 (2) 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panel on Clinical Practices for Treatment of HIV infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL02052001009.pdf. Accessed 15 Aug 2011.
- 28. Relucio K, Holodniy M (2002) HIV-1 RNA and viral load. Clin Lab Med 22 (3) 593–610. [DOI] [PubMed] [Google Scholar]
- 29. Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, et al. (2013) Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 56 (8) 1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau B, Gange SJ, Moore RD (2007) Interval and clinical cohort studies: epidemiological issues. AIDS Res Hum Retroviruses 23 (6) 769–76. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau. Population estimates. Available: http://www.census.gov/popest/estimates.html. Accessed 31 Jan 2010.
- 32.Henry J. Kaiser Family Foundation. statehealthfacts.org: HIV/AIDS. Available: http://www.statehealthfacts.org/comparecat.jsp?cat=11&rgn=6&rgn=1. Accessed 1 Feb 2013.
- 33.U.S. Census Bureau. Current Population Survey (CPS). Available: http://www.census.gov/cps/data/. Accessed 31 Jan 2010.
- 34. Ho D, Imai K, King G, Stuart E (2007) Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Pol Analysis 15: 199–236. [Google Scholar]
- 35.Crump RK, Hotz VJ, Imbens GW, Mitnik O. Moving the goalposts: Addressing limited overlap in estimation of average treatment effects by changing the estimand (working paper, Univ. California-Berkeley). Available: http://elsa.berkeley.edu/users/webfac/mcfadden/e242_s05/imbens.pdf. Accessed 26 Mar 2012.
- 36.Reif S, Whetten K, Wilson E. HIV/AIDS epidemic in the South reaches crisis proportions in last decade. Available: http://southernaids.files.wordpress.com/2011/10/research-report-final-revised10-26-121.pdf. Accessed 12 Dec 2011.
- 37. Walensky RP, Paltiel AD, Freedberg KA (2002) AIDS Drug Assistance Programs: Highlighting inequities in human immunodeficiency virus-infection health care in the United States. Clin Infect Dis 35 (5) 606–10. [DOI] [PubMed] [Google Scholar]
- 38.Human Rights Watch. Southern Exposure: Human Rights and HIV in the Southern United States. Available: http://www.hrw.org/node/94476. Accessed 17 Apr 2012.
- 39. Martin EG, Keenan PS (2011) Sticky dollars: Inertia in the evolution of federal allocations for HIV care through the Ryan White HIV/AIDS Program. Publius 4 (1) 101–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, et al. (2006) Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 163 (3) 262–70. [DOI] [PubMed] [Google Scholar]
- 41. Blackstock OJ, Wang KH, Fiellin DA (2011) State variation in AIDS drug assistance program prescription drug coverage for modifiable cardiovascular risk factors. J Gen Intern Med 26 (12) 1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yi T, Cocohoba J, Cohen M, Anastos K, DeHovitz JA, et al. (2011) The impact of the AIDS Drug Assistance Program (ADAP) on use of highly active antiretroviral and antihypertensive therapy among HIV-infected women. J Acquir Immune Defic Syndr 56 (3) 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin EG, Barry CL (2011) The adoption of mental health drugs on state AIDS drug assistance program formularies. Am J Public Health 101 (6) 1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheever LW, Kresina TF, Cajina A, Lubran R (2011) A model federal collaborative to increase patient access to buprenorphine treatment in HIV primary care. J Acquir Immune Defic Syndr 56 Suppl 1: S3–6. [DOI] [PubMed] [Google Scholar]
- 45. Godwin NC, Willig JH, Nevin CR, Lin HY, Allison J, et al. (2011) Underutilization of the AIDS Drug Assistance Program: associated factors and policy implications. Health Serv Res 46 (3) 982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, et al. (2011) Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS 25 (8) 1113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley JS, Kates J. Updating the Ryan White HIV/AIDS Program for a New Era: Key Issues and Questions for the Future. Available: http://www.kff.org/hivaids/upload/8431.pdf. Accessed 1 Apr 2013.
- 48. Althoff KN, Buchacz K, Hall IH, Zhang J, Hanna DB, et al. (2012) U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 157 (5) 325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL002111.pdf. Accessed 22 Dec 2009.
- 50.Committee to Review Data Systems for Monitoring HIV Care, Board on Population Health and Public Health Practice (2012) Monitoring HIV Care in the United States: Indicators and Data Systems. Washington: Natl Academies Press. [PubMed] [Google Scholar]
- 51. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ (2011) The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 52 (6) 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention (2011) Results of the Expanded HIV Testing Initiative–25 jurisdictions, United States, 2007–2010. MMWR Morb Mortal Wkly Rep 60 (24) 805–10. [PubMed] [Google Scholar]
- 53.U.S. Department of Health and Human Services. Overview of the uninsured in the United States: A summary of the 2011 Current Population Survey. Available: http://aspe.hhs.gov/health/reports/2011/CPSHealthIns2011/ib.pdf. Accessed 18 Apr 2012.
- 54. Martin EG, Schackman BR (2012) What does US health reform mean for HIV clinical care? J Acquir Immune Defic Syndr 60 (1) 72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McManus KA, Engelhard CL, Dillingham R (2013) Current challenges to the United States' AIDS Drug Assistance Program and possible implications of the Affordable Care Act. AIDS Res Treat 2013: 350169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martin EG, Meehan T, Schackman BR (2013) AIDS Drug Assistance Programs: Managers confront uncertainty and need to adapt as the Affordable Care Act kicks in. Health Aff (Millwood) 32 (6) 1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix. Table S1. Mechanisms at individual clinics in NA-ACCORD to assist patients in accessing prescription drugs in 2008 (N = 22). Table S2. Sensitivity analyses for association between state ADAP features at time of eligibility and outcomes.
(DOCX)