Abstract
Predicted increases in atmospheric carbon dioxide (CO2) are widely anticipated to increase biomass accumulation by accelerating rates of photosynthesis in many plant taxa. Little, however, is known about how soil-borne plant antagonists might modify the effects of elevated CO2 (eCO2), with root-feeding insects being particularly understudied. Root damage by insects often reduces rates of photosynthesis by disrupting root function and imposing water deficits. These insects therefore have considerable potential for modifying plant responses to eCO2. We investigated how root damage by a soil-dwelling insect (Xylotrupes gideon australicus) modified the responses of Eucalyptus globulus to eCO2. eCO2 increased plant height when E. globulus were 14 weeks old and continued to do so at an accelerated rate compared to those grown at ambient CO2 (aCO2). Plants exposed to root-damaging insects showed a rapid decline in growth rates thereafter. In eCO2, shoot and root biomass increased by 46 and 35%, respectively, in insect-free plants but these effects were arrested when soil-dwelling insects were present so that plants were the same size as those grown at aCO2. Specific leaf mass increased by 29% under eCO2, but at eCO2 root damage caused it to decline by 16%, similar to values seen in plants at aCO2 without root damage. Leaf C:N ratio increased by >30% at eCO2 as a consequence of declining leaf N concentrations, but this change was also moderated by soil insects. Soil insects also reduced leaf water content by 9% at eCO2, which potentially arose through impaired water uptake by the roots. We hypothesise that this may have impaired photosynthetic activity to the extent that observed plant responses to eCO2 no longer occurred. In conclusion, soil-dwelling insects could modify plant responses to eCO2 predicted by climate change plant growth models.
Introduction
Background and Rationale
Predicted increases in atmospheric carbon dioxide (CO2) concentrations are typically expected to increase plant biomass of C3 plants by 10–20% and C4 plants by 0–10% [1]. Increased rates of photosynthesis in response to elevated CO2 (eCO2) underpin these increases in plant biomass, but this is only sustainable with improved nitrogen and water use efficiency in the plant [1], although other physiological processes are clearly important (e.g. [2]). Root function plays an important role in nitrogen and water use efficiency [3], and root growth usually increases relative to shoot growth for most plant species under elevated eCO2 conditions [4]–[6]. Combined with greater water use efficiency through reduced stomatal conductance, this investment in root growth and changes in root architecture potentially allows plants to sustain higher levels of photosynthesis at eCO2 and ultimately accumulate more biomass [7], [8].
While a number of studies address how insect herbivores moderate plant growth responses to eCO2, with several reviews [9]–[11] now published, these largely overlook soil-borne pests of plant roots [12], [13]. There is virtually no information about how soil-dwelling insects are affected by eCO2 [14] and even less about how this might impact on plant growth responses to eCO2. For example, only three studies have examined the effects of eCO2 on root herbivores [15]–[17]. Soil-dwelling insects have the capacity to damage roots either through direct herbivory or physical abrasion to the roots as they move around the rhizosphere [18]. Soil-dwelling insects can be particularly damaging to plant physiology since even minor root damage can: (i) decrease nutrient and water uptake, (ii) cause disproportionate resource losses by severing roots, (iii) divert assimilates belowground for root re-growth and (iv) impair photosynthesis by imposing water deficits [18], [19]. This last point may be critical for plant growth responses under eCO2 since increased rates of photosynthesis underpin enhanced growth. Root damaging insects might therefore have greater capacity to reduce, negate or even reverse the effects of eCO2 than aboveground herbivores. This hypothesis is supported by two meta-analyses which reported contrasting effects of above- and belowground herbivores on photosynthesis rates; the former often accelerated photosynthesis rates, potentially to compensate for loss of photosynthetic tissue [20], whereas soil insect herbivores significantly reduced it [19].
Eucalypts and Soil-borne Antagonists
Hovenden & Williams [21] report that 11 species of Eucalyptus have been studied in the context of eCO2 before 2010, and at least eight of these show strong positive responses in terms of growth. Eucalyptus therefore represented a model system to test whether the effects of eCO2 would be modified when roots were challenged by soil insect herbivores because of this consistently positive response to eCO2. Moreover, eucalypts dominate the 164 million ha of forest in Australia [22], and are now the most widely planted hardwood species in the world [23].
Soil-borne pests and pathogens of Eucalyptus roots include numerous microbial diseases [24] and nematodes [25], but also a number of soil insect herbivores [26]. These include termites [27], [28], moth larvae [29] and scarab beetles [26], which have the capacity to cause significant losses in nursery production. Some soil-dwelling insects feed on both living roots and decaying organic matter, but even in the latter case they can cause physical damage of roots through their activity in the rhizosphere [18]. Moreover, with the global spread and movement of Eucalyptus it is likely that new and exotic soil-dwelling insects may be accidentally introduced to Eucalyptus [22].
Study System
This study was based on predicted atmospheric CO2 concentrations for 2050 onwards [30] and used Eucalyptus globulus Labill. (Myrtaceae), which is both a dominant eucalypt species in South Eastern Australia and globally the most widely planted hardwood species in temperate regions [31]. To impose insect damage to E. globulus roots we used the soil-dwelling larvae of the generalist feeder Xylotrupes gideon australicus L. (Coleoptera: Scarabaeidae). Xylotrupes spp. are sporadic pests of forestry and horticulture [32], with soil-dwelling larval stages feeding on decaying organic matter [33] and roots [34]. While this species has not been reported in eucalypt plantations, we used this scarab beetle as a model substitute that has the capacity to impose root damage via herbivory and mechanical abrasion. Since secondary metabolites are not readily inducible by herbivory in eucalypts [35], [36], root damage arising from either activity should be functionally similar.
Aims and Hypotheses
This study aimed to determine the effects of aCO2 and eCO2 (400 and 600 µmol mol−1, respectively) on the growth, biomass accumulation, leaf morphology and primary chemistry of developing E. globulus saplings and determine whether brief (14d) exposure to root damage by soil insects moderated these effects. We hypothesised that eCO2 would promote plant height, biomass accumulation, increase specific leaf mass and decrease nitrogen concentrations in plant tissue, but each of these effects would be arrested when roots are challenged by root herbivores.
Materials and Methods
Growth Conditions and Experimental Design
Six glasshouse chambers, three maintained at ambient CO2 (aCO2) concentrations of 400 µmol mol−1 and the other three at eCO2 concentrations of 600 µmol mol−1, were used in this study. Glasshouse chambers (3 m×5 m×3 m; width×length×height) with UV transparent plexiglass (6 mm thick) walls and roof were used and naturally lit throughout the experiment. During the experiment, air temperature within each chamber was maintained according to a diurnal cycle, peaking at 25°C and falling to 20°C (±4°C). Humidity was controlled at 60% (±5%). CO2 levels were controlled via a monitoring and control system, PlantVisorPRO (Carel Industries, Padova, Italy). Briefly, CO2 levels within each chamber were monitored by a CO2 probe (GMP222, Vaisala, Vantaa, Finland), with CO2 (food grade, AirLiquide, Australia) injected from pressurized cylinders through solenoid valves. Before entering a chamber, CO2 was passed through a Purafils column to eliminate possible ethylene contamination. Eucalyptus globulus plants were grown from seed (CSIRO Australian Tree Seed Centre, Seedlot number 18673) in commercial potting mixture (Plugger Custom, Debco Pty Ltd., NSW, Australia). Once established, 96 viable and similar sized plants were transferred into square pots (90 mm×90 mm×180 mm, width×length×height) filled with c. 750 g of air dried soil sieved <4 mm. The soil was loamy-sand with low (0.7%) organic matter (see [37] for full soil properties). Plants were then randomly assigned to each of the six climate chambers (16 in each). Plants were watered daily (c. 300 mL) to maintain soil water content at around 10% (verified with two rod soil moisture probe, Hydrosense, Campbell Scientific, Australia) and supplemented monthly with liquid fertilizer (1.6 g L−1 Aquasol, N:P:K 23∶4:18). Xylotrupes gideon australicus larvae were maintained in culture at 20°C ±5°C in a mixture of pine bark mulch (Richgro, Jandakot, WA, Australia) and soil (as above) until required.
Experimental Procedure
Once plants were 13 weeks old, plant height was recorded at weekly intervals until the end of the experiment. After a further week, 48 of the X. gideon larvae (first instar) which had been starved for 48 hr were weighed and individually applied to half of the plants (assigned at random) in each of the chambers. Detailed information about likely densities of soil-dwelling insects in eucalypt systems is lacking, but our previous research indicated grass-feeding scarab densities in eucalypt plantations would approximate this [38]. Larvae were placed in an excavated hole at the corner of the pot, which was then backfilled with soil. After two weeks, larvae were removed from the pots by gentle excavation of the soil and re-weighed. Similar soil excavation was performed on plants without larvae to replicate any effects of this disturbance. Plants were left for a further week before carefully removing from the soil, whereupon they were weighed and separated into stems, leaves and roots. All detached root material in the pots was also collected and included in the root mass evaluation to establish the extent of root herbivory as opposed to mechanical damage caused by larval movement. To calculate specific leaf mass, a single leaf from the middle of the plant was weighed, measured for leaf area, snap frozen in liquid nitrogen, freeze dried and re-weighed. All remaining plant tissue was snap frozen in liquid nitrogen and stored at −20°C. All plant material was subsequently freeze dried, weighed and milled to analyse carbon (C) and nitrogen (N) concentrations using a LECO TruSpec® CHN analyser.
Statistical Analysis
All plant responses were analysed with two-way analysis of variance (ANOVA) tests, with chamber replicate (three chambers at each CO2 regime) included as a block term to avoid issues of pseudo-replication of CO2 treatment. CO2 and insect presence, and an interaction of the two, were the two fixed effects. Differences between individual treatment combinations were determined with least square difference tests. In the case of plant height, separate ANOVAs for each time point were conducted since repeated measures ANOVA was inappropriate due to insects only being present during three of five points that height was measured (i.e. these were not fully repeated events). Final mass of insects was analysed with a one-way ANOVA with CO2 as the fixed effect, chamber included as the block term and initial mass included as a covariate. Unless otherwise stated in Table 1 all analysis was conducted on untransformed data using Genstat (version 15, VSN International, UK). Transformations were chosen to give residual diagnostic plots which best fitted a normal distribution and showed least heteroscedasticity.
Table 1. Results of ANOVA tests of plant responses to aCO2 and eCO2 and root damaging insects (RD) relating to Figs. 1–4.
| Plant response | Figure | Factors | |||||
| CO2 | RD | CO2 × RD | |||||
| F1,4 | P | F1,88 | P | F1,88 | P | ||
| Plant height –13 weeks | 1 | 1.53 | 0.284 | 0.76 | 0.3861 | 0.09 | 0.762 |
| Plant height –14 weeks | 7.48 | 0.050 | 0.89 | 0.3481 | 0.06 | 0.814 | |
| Plant height –15 weeks | 5.54 | 0.078 | 2.76 | 0.100 | 0.43 | 0.541 | |
| Plant height –16 weeks | 10.06 | 0.034 | 18.30 | <0.001 | 5.70 | 0.019 | |
| Plant height –17 weeks | 13.25 | 0.022 | 22.52 | <0.001 | 2.67 | 0.106 | |
| Plant mass(total) | 2 | 83.20 | <0.001 | 12.83 | <0.001 | 0.94 | 0.335 |
| Shoot mass | 107.45 | <0.001 | 12.63 | <0.001 | 1.00 | 0.321 | |
| Root mass | 28.87 | 0.006 | 6.83 | 0.011 | 0.07 | 0.789 | |
| Shoot : root | 3.19 | 0.148 | 1.28 | 0.261 | 1.81 | 0.182 | |
| Specific leafmass | 3A | 12.56 | 0.024 | 6.28 | 0.014 | 2.68 | 0.105 |
| Leaf watercontent | 3B | 0.10 | 0.762 | 5.69 | 0.019 | 0.38 | 0.541 |
| Leaf C:N | 4 | 14.27 | 0.019 | 0.01 | 0.942 | 5.24 | 0.024 |
Significant effects (P<0.05) indicated in bold.
Measurements taken on plants assigned for root herbivore treatment prior to inoculation with insects. Statistical tests indicating no priori difference between plants assigned for inoculation.
Results
All insects were recovered alive and roots showed considerable herbivory and root detachment. Including detached root tissue, total root mass was c. 15% lower in pots containing larvae indicating that root tissue had been consumed by insects. While absolute root consumption and removal could not be determined exactly on the basis of differences between infested and control (i.e. insect-free) plants, this differences was similar under aCO2 and eCO2 (910 and 1130 mg in dry mass, respectively) and suggested root consumption was similar. The final body mass of beetle larvae was largely unaffected by eCO2 (F1,3 = 0.57, P = 0.613; data not shown).
Plant Growth and Biomass Accumulation
Plant height was significantly greater for plants grown at eCO2 than those at aCO2 by the time plants were 14 weeks old, which was also the case at 16 and 17 weeks (Fig. 1; Table 1). Application of insects had no impact for the first week, but caused a sharp decline in growth after 14 days which persisted after their removal (Fig. 1; Table 1).
Figure 1. Plant height as affected by root damage and CO2.
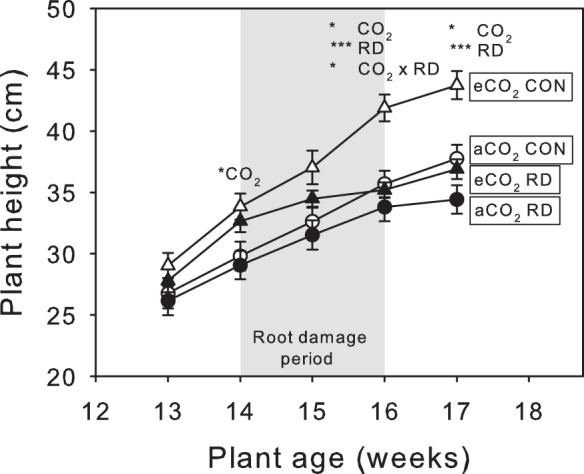
Height immediately preceding, during (shaded grey) and after root damage (RD) on plants under aCO2 (circles) and eCO2 (triangles). Open symbols are control plants (CON), closed symbols are plants with insect root damage (RD). Mean values ± S.E. shown, N = 24. Statistical significance of treatments indicated *(P<0.05), **(P<0.01) and *** (P<0.001).
eCO2 promoted growth of both shoots and roots, resulting in bigger plants overall (Fig. 2; Table 1). On plants without insects, eCO2 caused a 46% and 35% increase in root and shoot biomass, respectively, though there was no statistically significant change in the shoot:root ratio (Table 1). Exposure to insects had the opposite effect to eCO2, reducing both root and shoot mass. This arrested the positive effects of CO2 and left plants with insects under eCO2 effectively the same size as those grown at aCO2 without root damage (Fig. 1). Under aCO2, application of insects resulted in a in a 14.7% reduction in shoot biomass which increased to a 19.4% reduction under eCO2. Root loss due to insect damage was 18.42% and 15.7% under aCO2 and eCO2, respectively.
Figure 2. Plant biomass (dry) as affected by root damage and CO2.
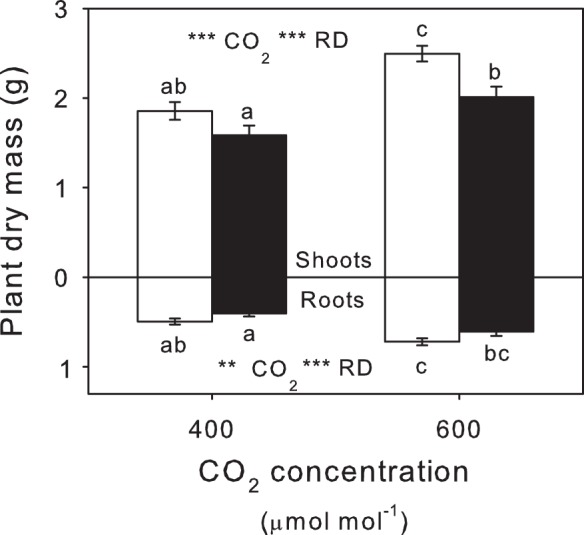
Shoot and root mass of plants at aCO2 and eCO2 without (open bars) and with (closed bars) root damage (RD). Mean values ± S.E. shown, N = 24. Statistical significance of treatments indicated **(P<0.01) and *** (P<0.001) with lowercase superscript letters indicating significant differences (P<0.05) between treatments.
Leaf Traits
Specific leaf mass was positively affected by eCO2 (Fig. 3A; Table 1), whereas root damage caused this to decline (Table 1). Moreover, plants at eCO2 with root herbivores had specific leaf mass values similar to those at aCO2 (Fig. 3A). While the interaction between CO2 and insect presence was not statistically significant at a 95% confidence interval (P = 0.105; Table 1), insects appeared to be having a more negative impact on specific leaf mass at eCO2. Root damage reduced leaf water concentrations overall (Table 1), but this difference largely occurred under eCO2 (Fig. 3B).
Figure 3. Leaf traits as affected by CO2 and root damage.
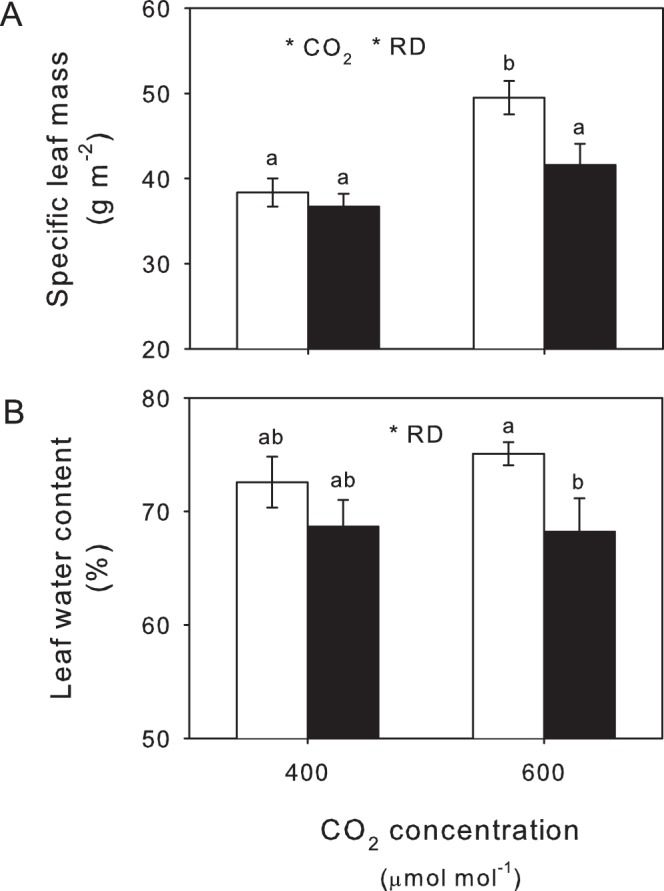
Effects of aCO2 and eCO2 on (A) specific leaf mass and (B) leaf water content (%) with (closed bars) and without (open bars) root damage (RD). Mean values ± S.E. shown, N = 24. Statistical significance of treatments indicated *(P<0.05) with lowercase superscript letters indicating significant differences (P<0.05) between treatments.
Primary Chemistry
Leaf C:N ratio rose in plants grown under eCO2 (Fig. 4; Table 1), driven largely by a decline in leaf N concentrations under eCO2 (Table 2). The significant interaction between eCO2 and root damage (Table 2) reflected the opposing effects of root damage on leaf N concentrations, causing a small reduction and increase at aCO2 and eCO2, respectively. Root damage had no impact on root C and N concentrations and similarly these remained largely unchanged by eCO2 (Table 2).
Figure 4. Leaf C:N ratios. Effects of aCO2 and eCO2 on leaf C:N from plants without (open bars)and with root damage (RD).
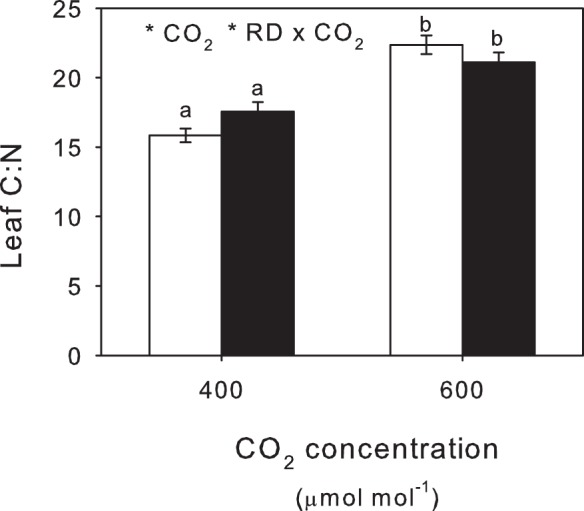
Mean values ± S.E. shown, N = 24. Statistical significance of treatments indicated *(P<0.05) with lowercase superscript letters indicating significant differences (P<0.05) between treatments.
Table 2. Carbon and nitrogen concentrations of shoots and roots of plants grown at aCO2 (400 µmol mol−1) and eCO2 (600 µmol mol−1) with and without root damaging insects (RD).
| CO2 concentration(µmol mol−1) | Herbivory | Leaf N (mg g−1) | Leaf C1 (mg g−1) | Root N (mg g−1) | Root C (mg g−1) |
| 400 | Absent | a 30.7±6.3 | 477.0±97.3 | 12.8±2.6 | 470.7±96.1 |
| Present | a 28.7±5.9 | 479.1±97.8 | 12.6±2.6 | 473.6±96.7 | |
| 600 | Absent | b 22.0±4.5 | 480.0±98.0 | 12.4±2.5 | 467.9±95.5 |
| Present | b 23.4±4.8 | 480.6±98.1 | 13.3±2.7 | 470.3±96.0 | |
| CO2 | F1,4 = 12.02 | F1,4 = 0.15 | F1,4 = 0.30 | F1,4 = 0.18 | |
| P = 0.026 | P = 0.716 | P = 0.611 | P = 0.697 | ||
| RD | F1,88 = 0.15 | F1,88 = 0.07 | F1,88 = 0.19 | F1,88 = 1.37 | |
| P = 0.701 | P = 0.790 | P = 0.667 | P = 0.244 | ||
| CO2 × RD | F1,88 = 4.82 | F1,88 = 0.11 | F1,88 = 2.32 | F1,88 = 0.01 | |
| P = 0.031 | P = 0.746 | P = 0.132 | P = 0.907 | ||
Mean values ± S.E shown, N = 24. Significant effects indicated in bold. Lowercase superscript letters indicates significant differences (P<0.05) between treatments.
Arcsine square root transformation applied.
Discussion
This study set out to establish whether root damage by soil-dwelling insects modified the response of E. globulus seedlings to eCO2. The findings suggest that this is the case, with root damage substantially reducing biomass accumulation by E. globulus under eCO2 and effectively reversing effects of eCO2 on specific leaf mass.
Plant Growth and Leaf Traits
In agreement with the meta-analysis by Zvereva & Kozlov [19], we found that insect herbivory or damage substantially reduced aboveground biomass by 19.4% and 14.7% at aCO2 and eCO2, respectively; both are similar values to their global prediction of 16.3%. The fact that enhanced plant growth was not achieved at eCO2 in the presence of root damaging insects suggests that plants were likely unable to accelerate or maintain rates of photosynthesis to capitalise on eCO2 conditions. The link between increased eucalypt growth under eCO2 and higher rates of photosynthesis is well established [39], so it is possible that root damage by soil insects imposed water deficits (consistent with the reported lower foliar water %) which limited photosynthetic activity.
Attendant changes in leaf traits also support this hypothesis, with insects reducing specific leaf mass which we reported as being increased under eCO2, in common with at least seven other eucalypt species [39]–[45]. Specific leaf mass, and implicitly leaf thickness, commonly increase under eCO2 which also renders leaves less palatable for leaf herbivores and reduces their performance [9], including eucalypts [44], [45]. Root herbivores frequently affect aboveground herbivores through plant-mediated mechanisms under aCO2 [46], so this raises the potential for root damaging insects to alter predicted effects of eCO2 on foliar herbivores. In this particular system, this could leave E. globulus more susceptible to defoliators under eCO2 when roots were under attack.
Leaf Chemistry
This study also found an increase in leaf C:N under eCO2 which is widely reported for many plant species [10], [47], and is normally attributed to a dilution effect as plants increase allocation to non-structural carbohydrates. In addition, higher leaf C:N can arise as plants increase N use efficiency and reduce allocation to Rubisco under eCO2 [1]. In common with E. saligna and E. sideroylon [39], we found that eCO2 reduced leaf N concentrations, which is consistent with plants allocating less N to Rubisco. The only other study, to our knowledge, to examine the effects of eCO2 on E. globulus also found changes in primary chemistry [48], though these were more modest, possibly due to different experimental conditions.
The effects of root damage on leaf chemistry were more complex, with a significant interactive effect of CO2 and insect presence. This arose because insects affected leaf N in opposing ways depending on CO2; marginally reducing leaf N concentrations under aCO2, but increasing it relative to control plants under eCO2 (with corresponding increases and decreases in leaf C:N, respectively). We hypothesise that root damage by insects could impair root uptake of N, resulting in a decrease in leaf N at aCO2 (for example, root herbivory has been reported to reduce N uptake by up to 30%; e.g. [49]). At eCO2, however, root damage could have reduced nitrogen use efficiency to the extent that plants could not re-allocate N (i.e. reduce foliar levels) to the same extent as in plants without root damaging insects.
Eucalypts and Soil-borne Antagonists in Future Climates
Most attention concerning soil-borne antagonists of eucalypts focus on plant pathogens [24], but several soil insect herbivores clearly attack eucalypt roots [26]. These are less conspicuous and currently pose less of a threat than aboveground herbivores (the latter are reviewed by [22], [50]). However, Wilcken et al. [28] reported that up to 70% of nursery eucalypt seedlings were killed by root herbivores, clearly demonstrating their destructive potential. Moreover, root-feeding insect herbivores are often highly invasive, with exotic species becoming significant pests of forest systems, as is the case in North America [51], so new pests could become apparent [22]. In particular, the results of the present study indicate that beneficial effects of eCO2 on eucalypt performance would be negated by root damaging insects in nursery aged plants.
In the current study, applying root damaging insects and eCO2 in a controlled manner necessitated glasshouse experiments. Glasshouse studies do not always reflect plant responses seen under field conditions [3], so our conclusions have to be viewed in this context. Having said this, early results from field based whole tree chambers [37] suggest E. globulus sapling growth responses to eCO2 in the field are consistent with the findings reported here (D. Ellsworth, pers. comm.) and elsewhere ( [21] and references therein). Another constraint was the use of pots to conduct this experiment, which sometimes affect growth responses [52] and may have slightly increased root damage by constraining herbivores. We minimised these effects by using a free draining soil (which minimises the chances of hypoxic conditions recommended in [52]). Also, root herbivores generally show restricted movement and usually remain associated with the root system when resources are adequate [53] so this probably was not a major issue for the brief period of root damage we applied.
Conclusions
This study has illustrated the potential for soil-dwelling insect herbivores to arrest or reverse the effects of eCO2 on plant physiology and biomass accumulation. Our results suggest that root damage by these insects (arising through herbivory and mechanical attrition) impaired water uptake which may have curtailed photosynthesis activity and limited the plant’s capacity for biomass accumulation at eCO2. The recent revelation that the majority of root herbivores reduce plant photosynthesis rates (by an average of 12%), whereas defoliators do the opposite [19], suggests that belowground herbivores might have more scope for modifying plant responses to eCO2 than aboveground herbivores. The present study provides some empirical basis for developing and testing hypotheses about how root damage by soil-dwelling insects may moderate plant responses to eCO2.
Acknowledgments
We are grateful to Adam Frew for assisting with experimental procedures, Dr Kaushal Tewari for the chemical analysis, Renee Smith and Prof. David Tissue for supplying plant material and Dr Tara Murray for experimental advice.
Funding Statement
This research was funded with a personal start-up research allowance from the University of Western Sydney. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Newman JA, Abnand M, Henry HAL, Hunt S, Gedalof Z (2011) Climate Change Biology. WallingfordOxfordshireUK: CABI. 304 p. [Google Scholar]
- 2. Jacob J, Greitner C, Drake BG (1995) Acclimation of photosynthesis in relation to Rubisco and non-structural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi grown at elevated CO2 in the field. Plant Cell Environ 18: 875–884. [Google Scholar]
- 3.Gregory PJ (2006) Plant roots – growth, activity and interaction with soils. OxfordUK: Blackwell Publishing. 328 p. [Google Scholar]
- 4. Rogers HH, Prior SA, Runion GB, Mitchell RJ (1996) Root to shoot ratio of crops as influenced by CO2 . Plant Soil 187: 229–248. [Google Scholar]
- 5. Rogers HH, Runion GB, Krupa SV (1994) Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ Pollut 83: 155–189. [DOI] [PubMed] [Google Scholar]
- 6. Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Global Change Biol 5: 807–837. [Google Scholar]
- 7. Jongen M, Jones MB, Hebeisen T, Blum H, Hendrey G (1995) The effects of elevated CO2 concentrations on the root growth of Lolium perenne and Trifolium repens grown in a FACE system. Global Change Biol 1: 361–371. [Google Scholar]
- 8. Prior SA, Rogers HH, Runion GB, Kimball BA, Mauney JR, et al. (1995) Free air carbon dioxide enrichment of cotton root morphological characteristics. J Environ Qual 24: 678–683. [Google Scholar]
- 9. Robinson EA, Ryan GD, Newman JA (2012) A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol 194: 321–336. [DOI] [PubMed] [Google Scholar]
- 10. Stiling P, Cornelissen T (2007) How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Global Change Biol 13: 1823–1842. [Google Scholar]
- 11. Zavala JA, Nabity PD, DeLucia EH (2013) An emerging understanding of mechanisms governing insect herbivory under elevated CO2 . Annu Rev Entomol 58: 79–97. [DOI] [PubMed] [Google Scholar]
- 12. Gregory PJ, Johnson SN, Newton AC, Ingram JSI (2009) Integrating pests and pathogens into the climate change/food security debate. J Exp Bot 60: 2827–2838. [DOI] [PubMed] [Google Scholar]
- 13. Newton AC, Johnson SN, Gregory PJ (2011) Implications of climate change for diseases, crop yields and food security. Euphytica 179: 3–18. [Google Scholar]
- 14.Staley JT, Johnson SN (2008) Climate change impacts on root herbivores. In: Johnson SN, Murray PJ, editors. Root Feeders – an ecosystem perspective. Wallingford, UK: CABI. 192–213.
- 15. Salt DT, Fenwick P, Whittaker JB (1996) Interspecific herbivore interactions in a high CO2 environment: root and shoot aphids feeding on Cardamine . Oikos 77: 326–330. [Google Scholar]
- 16. Johnson SN, McNicol JW (2010) Elevated CO2 and aboveground–belowground herbivory by the clover root weevil. Oecologia 162: 209–216. [DOI] [PubMed] [Google Scholar]
- 17. Johnson SN, Barton AT, Clark KE, Gregory PJ, McMenemy LS, et al. (2011) Elevated atmospheric carbon dioxide impairs the performance of root-feeding vine weevils by modifying root growth and secondary metabolites. Global Change Biol 17: 688–695. [Google Scholar]
- 18.Johnson SN, Murray PJ, editors (2008) Root Feeders – an ecosystem perspective. 1st ed. WallingfordUK: CABI. 256 p. [Google Scholar]
- 19. Zvereva EL, Kozlov MV (2012) Sources of variation in plant responses to belowground insect herbivory: a meta-analysis. Oecologia 169: 441–452. [DOI] [PubMed] [Google Scholar]
- 20. Nykänen H, Koricheva J (2004) Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos 104: 247–268. [Google Scholar]
- 21. Hovenden MJ, Williams AL (2010) The impacts of rising CO2 concentrations on Australian terrestrial species and ecosystems. Austral Ecol 35: 665–684. [Google Scholar]
- 22. Paine TD, Steinbauer MJ, Lawson SA (2011) Native and exotic pests of Eucalyptus: a worldwide perspective. Annu Rev Entomol 56: 181–201. [DOI] [PubMed] [Google Scholar]
- 23. Turnbull JW (1999) Eucalypt plantations. New Forests 17: 37–52. [Google Scholar]
- 24.Keane PJ (2000) Diseases and pathogens of eucalypts. Melbourne, Australia: CSIRO Publishing.
- 25. Ruehle JL (1973) Nematodes and forest trees – types of damage to tree roots. Annu Rev Phytopathol 11: 99–118. [Google Scholar]
- 26.Wylie FR, Speight MR (2011) Insect Pests in Tropical Forestry. WallingfordUK: CABI. 365 p. [Google Scholar]
- 27. Cowie RH, Logan JWM, Wood TG (1989) Termite (Isoptera) damage and control in tropical forestry with special reference to Africa and Indo-Malaysia – a review. B Entomol Res 79: 173–184. [Google Scholar]
- 28. Wilcken CF, Raetano CG, Forti LC (2002) Termite pests in Eucalyptus forests of Brazil. Sociobiology 40: 179–190. [Google Scholar]
- 29. Kile GA, Hardy RJ, Turnbull CRA (1979) Association between Abantiades latipennis (Lepidoptera, Family Hepialidae) and Eucalyptus obliqua and Eucalyptus regnans in Tasmania. J Aust Entomol Soc 18: 7–17. [Google Scholar]
- 30.IPCC (2007) Impacts, adaptations and vulnerability. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press. 391–431.
- 31.Eldridge KG, Davidson J, Harwood C, van Wyk G (1993) Eucalypt domestication and breeding. OxfordUK: Clarendon. 308 p. [Google Scholar]
- 32.Hangay G, Zborowski P (2010) A guide to the beetles of Australia. CollingwoodVictoriaAustralia: CSIRO Publishing. 248 p. [Google Scholar]
- 33. Bedford GO (1975) Observations on the biology of Xylotrupes gideon (Coleoptera: Scarabaeidae: Dynastinae) in Melanesia. J Aust Entomol Soc 14: 213–216. [Google Scholar]
- 34.Menzel C (2002) The lychee crop in Asia and the Pacific. Bankok, Thailand: FAO.
- 35. Henery ML, Wallis IR, Stone C, Foley WJ (2008) Methyl jasmonate does not induce changes in Eucalyptus grandis leaves that alter the effect of constitutive defences on larvae of a specialist herbivore. Oecologia 156: 847–859. [DOI] [PubMed] [Google Scholar]
- 36. Rapley LP, Allen GR, Potts BM, Davies NW (2007) Constitutive or induced defences – how does Eucalyptus globulus defend itself from larval feeding? Chemoecology 17: 235–243. [Google Scholar]
- 37. Barton CVM, Ellsworth DS, Medlyn BE, Duursma RA, Tissue DT, et al. (2010) Whole-tree chambers for elevated atmospheric CO2 experimentation and tree scale flux measurements in south-eastern Australia: The Hawkesbury Forest Experiment. Agr Forest Meteorol 150: 941–951. [Google Scholar]
- 38. Frew A, Nielsen UN, Riegler M, Johnson SN (2013) Do eucalypt plantation management practices create understory reservoirs of scarab beetle pests in the soil? Forest Ecol Manag 306: 275–280. [Google Scholar]
- 39. Ghannoum O, Phillips NG, Sears MA, Logan BA, Lewis JD, et al. (2010) Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric CO2 and temperature. Plant Cell Environ 33: 1671–1681. [DOI] [PubMed] [Google Scholar]
- 40. Roden JS, Ball MC (1996) Growth and photosynthesis of two eucalypt species during high temperature stress under ambient and elevated CO2 . Global Change Biol 2: 115–128. [Google Scholar]
- 41. Gleadow RM, Foley WJ, Woodrow IE (1998) Enhanced CO2 alters the relationship between photosynthesis and defence in cyanogenic Eucalyptus cladocalyx F. Muell. Plant Cell Environ 21: 12–22. [Google Scholar]
- 42. Roden JS, Egerton JJG, Ball MC (1999) Effect of elevated CO2 on photosynthesis and growth of snow gum (Eucalyptus pauciflora) seedlings during winter and spring. Aust J Plant Physiol 26: 37–46. [Google Scholar]
- 43. Crous KY, Zaragoza-Castells J, Loew M, Ellsworth DS, Tissue DT, et al. (2011) Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: impacts of elevated atmospheric CO2 and summer drought. Global Change Biol 17: 1560–1576. [Google Scholar]
- 44. Murray TJ, Ellsworth DS, Tissue DT, Riegler M (2013) Interactive direct and plant-mediated effects of elevated atmospheric CO2 and temperature on a eucalypt-feeding insect herbivore. Global Change Biol 19: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 45. Murray TJ, Tissue DT, Ellsworth DS, Riegler M (2013) Interactive effects of pre-industrial, current and future CO2 and temperature on an insect herbivore of Eucalyptus . Oecologia 171: 1025–1035. [DOI] [PubMed] [Google Scholar]
- 46. Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW, et al. (2012) Aboveground–belowground herbivore interactions: a meta-analysis. Ecology 93: 2208–2215. [DOI] [PubMed] [Google Scholar]
- 47. Luo YQ, Hui DF, Zhang DQ (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 87: 53–63. [DOI] [PubMed] [Google Scholar]
- 48. McKiernan AB, O’Reilly-Wapstra JM, Price C, Davies NW, Potts BM, et al. (2012) Stability of plant defensive traits among populations in two Eucalyptus species under elevated carbon dioxide. J Chem Ecol 38: 204–212. [DOI] [PubMed] [Google Scholar]
- 49. Newingham BA, Callaway RM, BassiriRad H (2007) Allocating nitrogen away from a herbivore: a novel compensatory response to root herbivory. Oecologia 153: 913–920. [DOI] [PubMed] [Google Scholar]
- 50. Ohmart CP, Edwards PB (1991) Insect herbivory on Eucalyptus . Annu Rev Entomol 36: 637–657. [Google Scholar]
- 51.Coyle DR, Mattson MJ, Raffa KF (2008) Invasive root-feeding insects in natural forest ecosystems of North America. In: Johnson SN, Murray PJ, editors. Root Feeders – an ecosystem perspective. Wallingford, UK: CABI. 134–149.
- 52. Passioura JB (2006) The perils of pot experiments. Funct Plant Biol 33: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 53.Barnett K, Johnson SN (2013) Living in the soil matrix: abiotic factors affecting root herbivores. Adv Insect Physiol 45: in press.


