Abstract
Candida albicans is the most common human fungal pathogen and has a high propensity to develop biofilms that are resistant to traditional antifungal agents. In this study, we investigated the effect of tetrandrine (TET) on growth, biofilm formation and yeast-to-hypha transition of C. albicans. We characterized the inhibitory effect of TET on hyphal growth and addressed its possible mechanism of action. Treatment of TET at a low concentration without affecting fungal growth inhibited hyphal growth in both liquid and solid Spider media. Real-time RT-PCR revealed that TET down-regulated the expression of hypha-specific genes ECE1, ALS3 and HWP1, and abrogated the induction of EFG1 and RAS1, regulators of hyphal growth. Addition of cAMP restored the normal phenotype of the SC5314 strain. These results indicate that TET may inhibit hyphal growth through the Ras1p-cAMP-PKA pathway. In vivo, at a range of concentrations from 4 mg/L to 32 mg/L, TET prolonged the survival of C. albicans-infected Caenorhabditis elegans significantly. This study provides useful information for the development of new strategies to reduce the incidence of C. albicans biofilm-associated infections.
Introduction
Candida albicans is the most common fungal pathogen and may cause life-threatening invasive infections, especially in immunocompromised individuals [1], [2]. Antifungal agents available are limited in clinic, and drug resistance has become a significant threat [3], [4]. C. albicans has a high propensity to develop biofilms on the surfaces of almost any medical devices, such as stents, shunts, prostheses, implants, endotracheal tubes, pacemakers and various types of catheters [5], resulting in biofilm-associated infections [6]–[8]. More specifically, it is the fourth leading cause of vascular catheter-related infections and the third leading cause of urinary catheter-related infections [9]–[12]. Among vascular catheter-related infections, those due to Candida spp. are associated with the highest rate of mortality [9], [13], [14]. The C. albicans biofilms are structured microbial communities with C. albicans cells embedded in a matrix of extracellular polymeric substances produced by the cells [15]–[18]. Comparing to planktonic cells, C. albicans cells in biofilms display severe resistance to a wide variety of clinical antifungal agents, including amphotericin B and fluconazole [19]–[22]. There is an urgent need to develop new antifungal agents against C. albicans biofilms.
Tetrandrine (TET) (Fig. 1) is a bis-benzylisoquinoline alkaloid compound originating from several natural plant sources, including Stephania tetrandra [23], [24]. This alkaloid displays low toxicity [25] and has been used in China for the treatment of angina, hypertension, silicosis and arthritis [26]–[30]. Besides, TET could reduce acute radiation injury [31], [32] and exhibited anti-inflammatory [32]–[34] and anti-tumor [35], [36], [37], [38] activities. In more details, TET was reported to block voltage-gated Ca2+ channels in mammalian cells [38], inhibit NF-κB activation in the alveolar macrophage [33], induce apoptosis and growth arrest in human leukemic HL-60 cells and lung carcinoma cells [36], [37], serve as a MDR (multidrug drug resistance) modulator for the treatment of P-glycoprotein-mediated MDR cancers [35]. Interestingly, it exhibited synergistic effect with ketoconazole against drug resistant C. albicans [39] and synergism with econazole against Trichophyton mentagrophytes [40]. Nevertheless, its activity against C. albicans biofilms has not yet been investigated.
Figure 1. Chemical structure of TET.
In this study, we evaluated the activity of TET against C. albicans biofilms, and revealed that the anti-biofilm activity of TET was associated with Ras/cAMP pathway.
Results
TET inhibits the formation of C. albicans biofilms in vitro
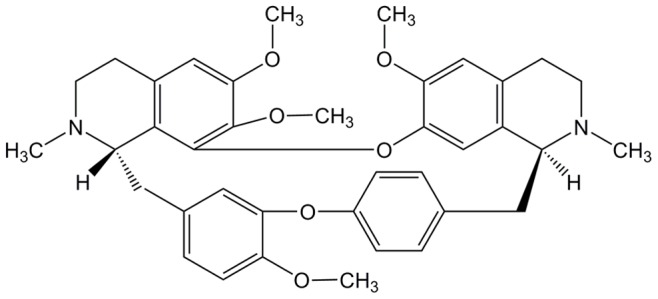
The effect of TET on C. albicans biofilm formation was evaluated by XTT reduction assay (Fig. 2). It was found that addition of TET to C. albicans cells after 90-min adhesion inhibited biofilm formation in a dose-dependent manner (Fig. 2A). More specifically, 16 mg/L TET inhibited biofilm formation significantly (P<0.05), and this anti-biofilm effect increased with increasing TET concentrations. In the 64 mg/L TET group, the biofilm formation was less than 5% as compared with the control group without TET treatment. Notably, under the condition that the biofilms were mature after 24 h incubation at 37°C, TET also inhibited biofilms in a dose-dependent manner (Fig. 2C). 32 mg/L TET inhibited mature biofilms significantly (P<0.05). The anti-biofilm effect increased with increasing TET concentrations. In the 64 mg/L TET group, the maintenance of biofilm architecture was about 15% as compared with the control group without TET treatment. Collectively, TET showed a significant anti-biofilm effect on both developing biofilms and mature biofilms.
Figure 2. TET inhibits C. albicans SC5314 biofilm formation in vitro.
(A) Effects of different concentrations of TET on biofilm formation. (B) Effects of different concentrations of Amphotericin B on biofilm formation. AmB: amphotericin B. (C) Effects of different concentrations of TET on the maintenance of mature biofilms. Biofilm formation was evaluated by XTT reduction assay, and the results were presented as the percentage compared to the control biofilms formed without TET treatment. Biofilm formation results represent the mean ± standard deviation for five independent experiments. * P<0.05 compared to the control biofilms, ** P<0.01 compared to the control biofilms. (D) Effects of different concentrations of TET on biofilms formed on silicone pads. Standard deviations are depicted and based on 6 silicone pad measurements. ** P<0.01. (E) Screen for TET-treated biofilms formed on silicone pads. The wells are shown for a: normal biofilm. b: cells were treated with 4 mg/L TET. c: 8 mg/L TET. d: 16 mg/L TET. e: 32 mg/L TET. f: uninoculated control. (F) Effects of different concentrations of TET on biofilm formation presented by using CLSM. a: Control. b: 8 mg/L of TET. c: 16 mg/L TET. d: 32 mg/L TET. (G) Effects of different concentrations of TET on biofilm formation presented by using SEM. The inset in the 500 ×, 2000× panels show the area that was magnificated.
The result of biofilm biomass determination confirmed the dose-dependent anti-biofilm effect of TET, and 32 mg/L TET inhibited biofilm formation significantly (P<0.01; Fig. 2D). Accordingly, the anti-biofilm effect of TET could be observed visually (Fig. 2E). Compared with the TET-free control biofilms on silicone pads (Fig. 2Ea), biofilms in the 4, 8 mg/L TET group (Fig. 2Eb, c) were thinner and incomplete. With increasing TET concentrations, the effect of TET on biofilm formation became more obvious. In the 32 mg/L TET group (Fig. 2Ee), the silicone pad was maintained clean, indicating that the biofilm formation was disrupted completely.
The anti-biofilm effect of TET was further confirmed by confocal laser scanning microscopy (CLSM, Fig. 2F) and scanning electron microscopy (SEM, Fig. 2G). Compared with the normal thick biofilm with true hyphae criss-crossing (Fig. 2Fa, 2Ga-c), C. albicans biofilm formation was disrupted by TET in a dose-dependent manner. 8 mg/L of TET (Fig. 2Fb, 2Gd-f) led to the reduction in cell density and defect in filamentation. With increasing the TET concentration to 16 mg/L and 32 mg/L (Fig. 2Fc-d, 2Gg-l), cell density was further reduced and the defect in filamentation became more obvious.
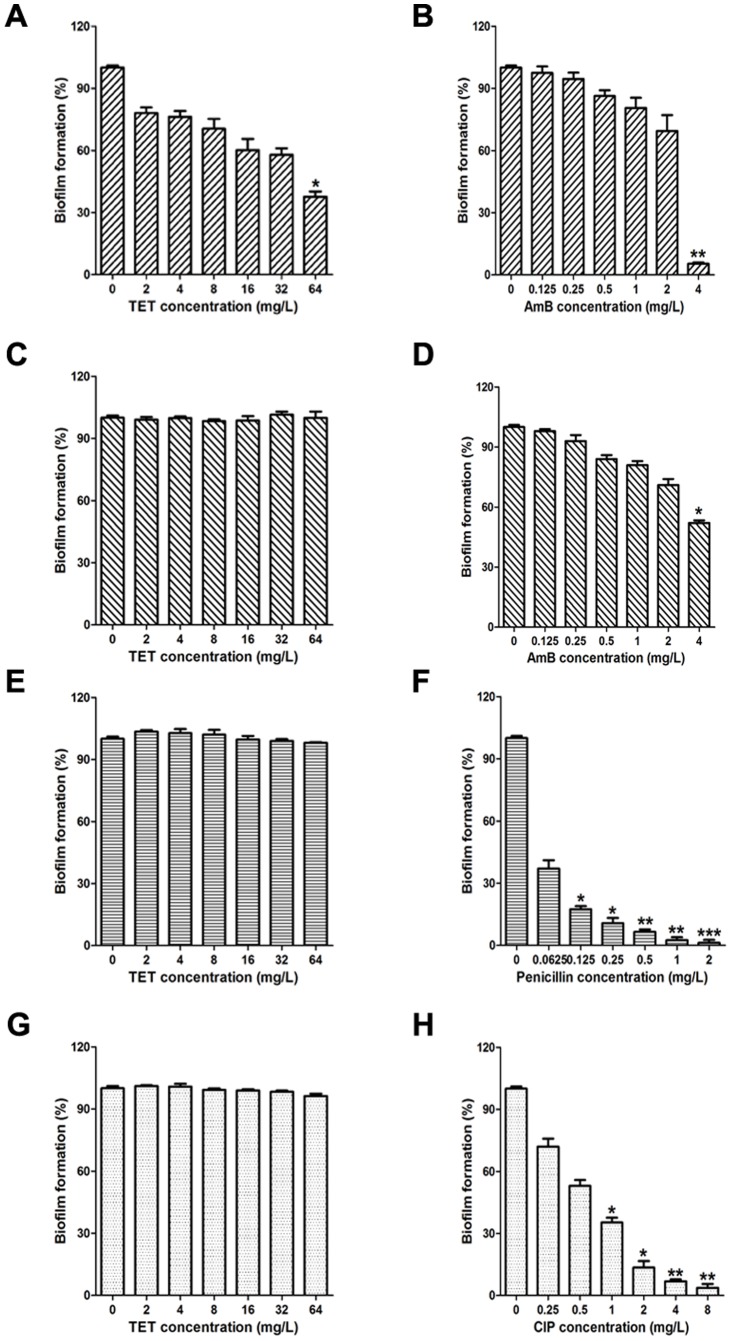
We further evaluated the activity of TET against biofilms of other fungi and bacteria (Fig. 3). Cryptococcus neoformans strain H99, Aspergillus fumigatus strain T308073458, Staphylococcus aureus strain Newman and Pseudomonas aeruginosa strain PA14 were used in this study. TET exhibited weak anti-biofilm effect against C. neoformans: it inhibited the biofilms significantly only when the drug concentration was as high as 64 mg/L (Fig. 3A). No anti-biofilm effect was observed of TET against A. fumigatus, S. aureus and P. aeruginosa, even under the condition that the concentration was high as 64 mg/L (Fig. 3C, E, G). Collectively, the strong anti-biofilm effect of TET was selective against C. albicans.
Figure 3. Effects of TET on fungal and bacterial biofilm formation in vitro.
(A) TET against C. neoformans H99; (B) Amphotericin B against C. neoformans H99, AmB: amphotericin B; (C) TET against A. fumigatus T308073458; (D) Amphotericin B against A. fumigatus T308073458, AmB: amphotericin B; (E) TET against S. aureus; (F) Penicillin against S. aureus; (G) TET against P. aeruginosa; (H) Ciprofloxacin against P. aeruginosa, CIP: ciprofloxacin. * P<0.05 compared to the treatment-free control biofilm, ** P<0.01 compared to the treatment-free control biofilm, *** P<0.001 compared to the treatment-free control biofilm.
TET decreases cellular surface hydrophobicity (CSH) of C. albicans biofilm
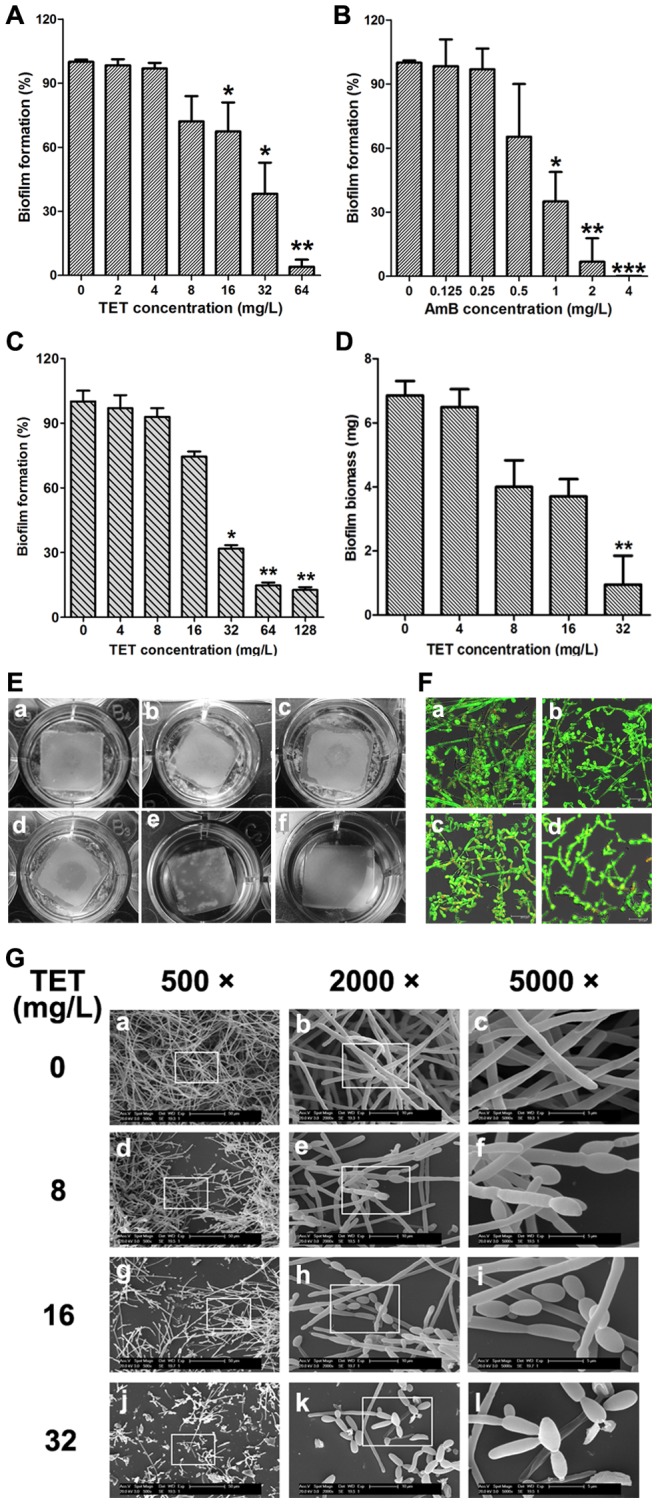
Knowing that there is a positive correlation between CSH and adhesion of C. albicans [41]-[43], we examined the effect of TET on CSH. The normal CSH of C. albicans was shown as 0.73 in this work. Our results showed that 4 mg/L TET significantly decreased CSH to 0.56 (P<0.01; Fig. 4). In addition, TET decreased CSH of C. albicans biofilm in a dose-dependent manner, and it decreased to 0.04 in the 32 mg/L TET group (Fig. 4).
Figure 4. Effects of different concentrations of TET on CSH of C. albicans SC5314.
CSH was estimated by using the water-hydrocarbon two-phase assay. Standard deviations are depicted and based on three independent experiments. ** P<0.01, *** P<0.001.
TET retards growth of C. albicans
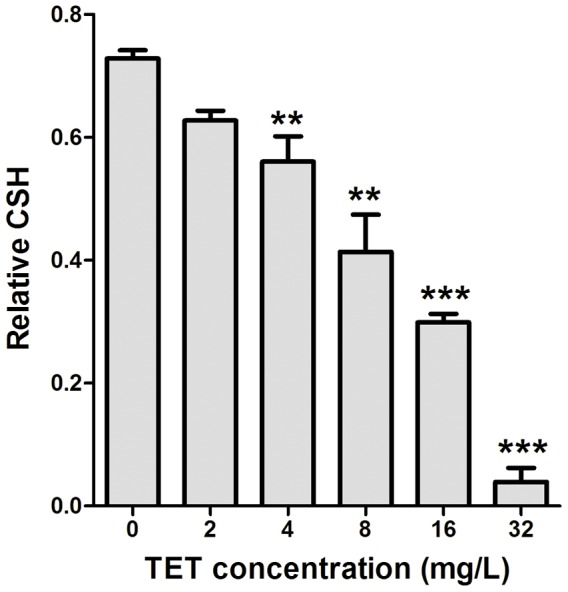
The effect of TET on growth of C. albicans was further investigated. Time-growth curves indicated that 8 and 16 mg/L TET could not affect the growth of C. albicans significantly, where the cell density reached 1×108 cells/ml after 12-h culture, which was similar to that in the control group without TET treatment (Fig. 5). At 32 mg/L, TET slowed down the growth of C. albicans, and the cell density were 3.7×107 cells/ml after 12 h culture (Fig. 5).
Figure 5. Time-growth curves of different concentrations of TET on C. albicans strain SC5314.
We also carried out a standard antifungal susceptibility test to investigate the activity of TET on growth of C. albicans. Besides the normally used C. albicans strain SC5314, another fluconazole-susceptible strains Y0109 and two fluconazole-resistant strains, 0304103 and 01010, were used in this experiment. MIC50 was determined as the lowest concentration of the drug that inhibited fungal growth by 50%. TET exhibited weak antifungal activity: the MIC50s against both the fluconazole-susceptible strains and the fluconazole-resistant strains were 32 mg/L (Table 1), and the MIC80s of TET against all the four strains tested were 64 mg/L.
Table 1. The MIC50 of TET and fluconazole against C. albicans strains.
| Strains | MIC50 (mg/L) | |
| TET | Fluconazole | |
| Fluconazole-susceptible strains | ||
| SC5314 | 32 | 0.125 |
| Y0109 | 32 | 0.25 |
| Fluconazole-resistant strains | ||
| 304103 | 32 | >64 |
| 1010 | 32 | >64 |
TET inhibits hyphal formation of C. albicans
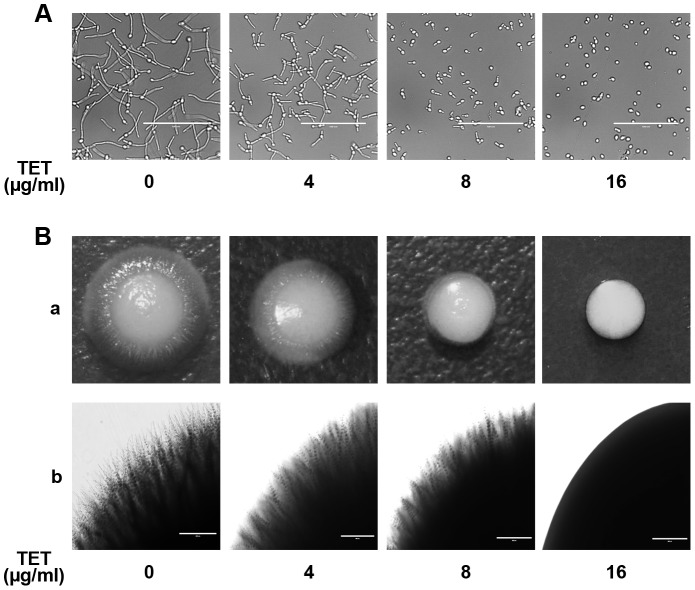
To study the effect of TET on yeast-to-hypha morphological transition of C. albicans, C. albicans cells were grown in liquid Spider medium known to induce morphological transition. In TET free Spider medium, C. albicans cells formed true hyphae (Fig. 6A). At 4 mg/L, TET inhibited the yeast-to-hypha morphological transition to some extent, and the inhibition occurred in a dose-dependent manner. The addition of 16 mg/L TET totally disrupted the hyphal formation (Fig. 6A). In accordance, the inhibition effect of TET on hyphal formation was also observed on solid Spider medium (Fig. 6B). More specifically, at 4 mg/L, TET inhibited the developing of radial colonies to some extent (Radial colonies usually indicate mycelial cells inside the colonies while smooth colonies indicate budding yeast cells inside[44]), and in 16 mg/L TET group, only smooth-edged colonies were observed (Fig. 6B). Collectively, TET inhibited the yeast-to-hypha morphological transition in a dose-dependent manner in Spider medium.
Figure 6. Effects of different concentrations of TET on hyphal formation in Spider medium.
(A) Log phase cells were incubated in liquid Spider medium at 37°C. Cells were photographed after 4 h of incubation in Spider medium. Observed with a inverted phase contrast microscope (AMG® EVOS xl) with a×40 objective. (B) Approximately 10 cells were plated on Spider solid medium. Incubation time and temperature were 5 d at 37°C.
Exposure to TET alters C. albicans gene expression
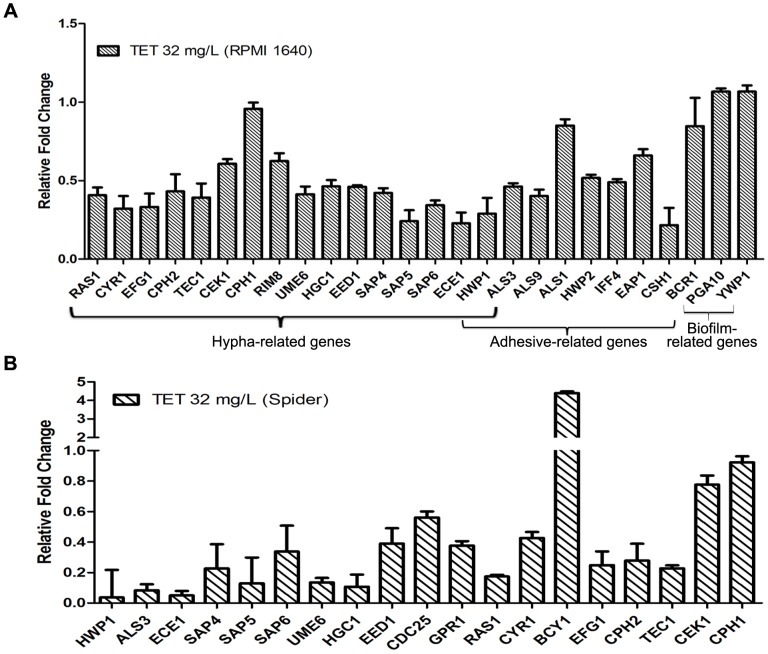
To understand the anti-biofilm mechanism of TET, we further investigated the expression changes of the known adhesion-related, hypha-related and biofilm-related genes after TET treatment using real-time RT-PCR. In RPMI 1640 medium at 37°C, the hypha-specific genes such as ECE1, HWP1, ALS3, SAP4, SAP5, SAP6, UME6, EED1 and HGC1 were down-regulated after 32 mg/L TET treatment (Fig. 7A). Some regulation genes, including RAS1, CYR1, EFG1, CPH2, and TEC1 were also down-regulated. Moreover, adhesion-specific genes CSH1, IFF4 and ALS9 were down-regulated by 0.22, 0.49 and 0.40 fold respectively. Nevertheless, ALS1, EAP1 and HWP2, three critical adhesion-related genes were not affected significantly after 32 mg/L TET treatment. Taken together, the real-time RT-PCR results indicated that TET treatment down-regulated the expression of some hypha-specific genes and some genes known to regulate the yeast-to-hypha transition in RPMI 1640 medium.
Figure 7. Gene expression changes of some important biofilm formation related genes.
The C. albicans strain tested was SC5314. The concentration of TET was 32 mg/L. All genes were examined by real-time RT-PCR with gene-specific primers. Gene expression was indicated as a fold change relative to that of the control group treated with DMSO. (A) in RPMI 1640 medium. (B) in Spider medium. Data are shown as mean ± SD from three experiments.
Similar results were obtained in Spider at 37°C (Fig. 7B). Hypha-specific genes HWP1, ALS3 and ECE1 were down-regulated with the relative fold being 0.036, 0.083 and 0.050 respectively. Besides, SAP4, SAP5, SAP6, UME6, HGC1 and EED1 were also down-regulated. Some regulation genes including RAS1, CYR1, EFG1, CPH2 and TEC1 were down-regulated as well.
Exogenous cAMP reverts the morphogenesis defect caused by TET
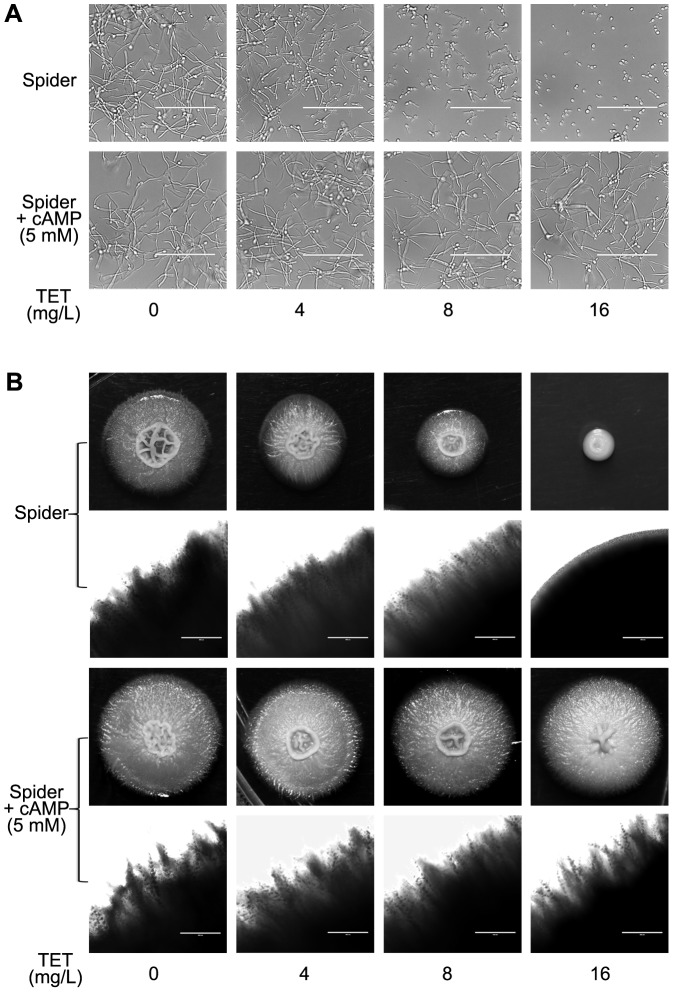
Since a series of important genes in Ras/cAMP pathway, including RAS1, CYR1, EFG1, CPH2, TEC1, BCY1, ECE1, ALS3, HWP1 and HGC1 [45], [46] were down-regulated after TET treatment, cAMP levels were measured in C. albicans cells. A significant decrease of cAMP level was observed in the 32 mg/L TET-treated cells (P<0.01; Fig. 8). Interestingly, exogenous cAMP reverted the morphogenesis defect caused by TET (Fig. 9). More specifically, with the addition of 5 mM cAMP in TET treated cultures, true hyphae were observed both in liquid and on solid Spider media (Fig. 9).
Figure 8. Determination of intracellular cAMP level.
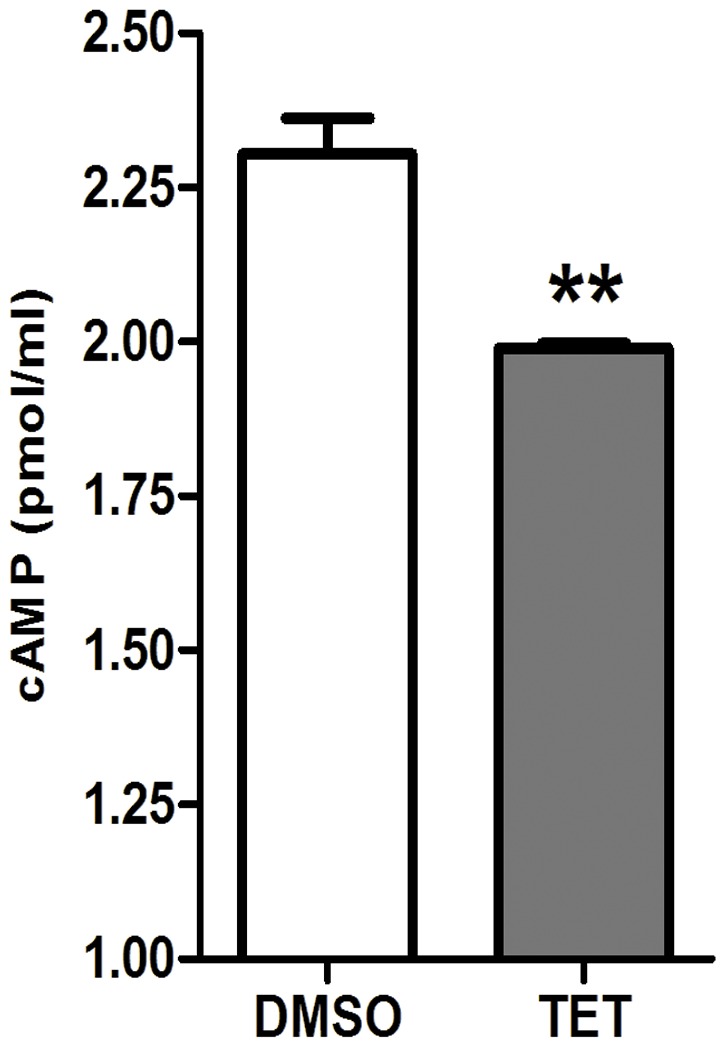
Exponentially growing C. albicans SC5314 cells incubated at 37°C in Spider medium in the presence of 32 mg/L TET and harvested at the 60 min time point. The cAMP content was measured using the cAMP Enzyme Immunoassay Kit according to the manufacturer’s instructions. ** P<0.01.
Figure 9. Addition of exogenous cAMP reverts the morphological transition defect of C. albicans SC5314 caused by TET.
Exponentially growing C. albicans SC5314 cells were transferred to Spider medium. (A) Cells were incubated in liquid Spider medium supplemented without or with cAMP (final concentration 5 mM). The cells were incubated at 37°C for 4 h. Magnification 40 ×. (B) Hyphal formation on solid Spider medium plate with the same concentrations of cAMP and TET as in liquid Spider medium.
TET exhibits antifungal activity in vivo in a Caenorhabditis elegans infection model
Since TET inhibited yeast-to-hypha morphological transition, the most widely acknowledged pathogenic trait of C. albicans, we further investigated the in vivo antifungal activity of TET using a C. elegans–C. albicans infection model. At a range of concentrations, from 4 mg/L to 32 mg/L, TET significantly protected C. elegans from C. albicans infection (P<0.0001; Fig. 10A). More specifically, we found that under the conditions used in this study more than half of the worms died within the first 48 h after infection with C. albicans strain SC5314 (Fig. 10A), and every dead worm had visible hyphae piercing the cuticle (Fig. 10Ba). At 144 h after infection, less than 20% of the worms were alive (Fig. 10A). In contrast, with 4 mg/L to 32 mg/L TET treatment, > 60% of the worms were alive until the end of 144 h of our observation period (Fig. 10A), and little even no hyphae were observed on the living worms (Fig. 10Bb-e).
Figure 10. TET prolongs the survival of C. elegans glp-4; sek-1 nematodes infected by C. albicans SC5314.
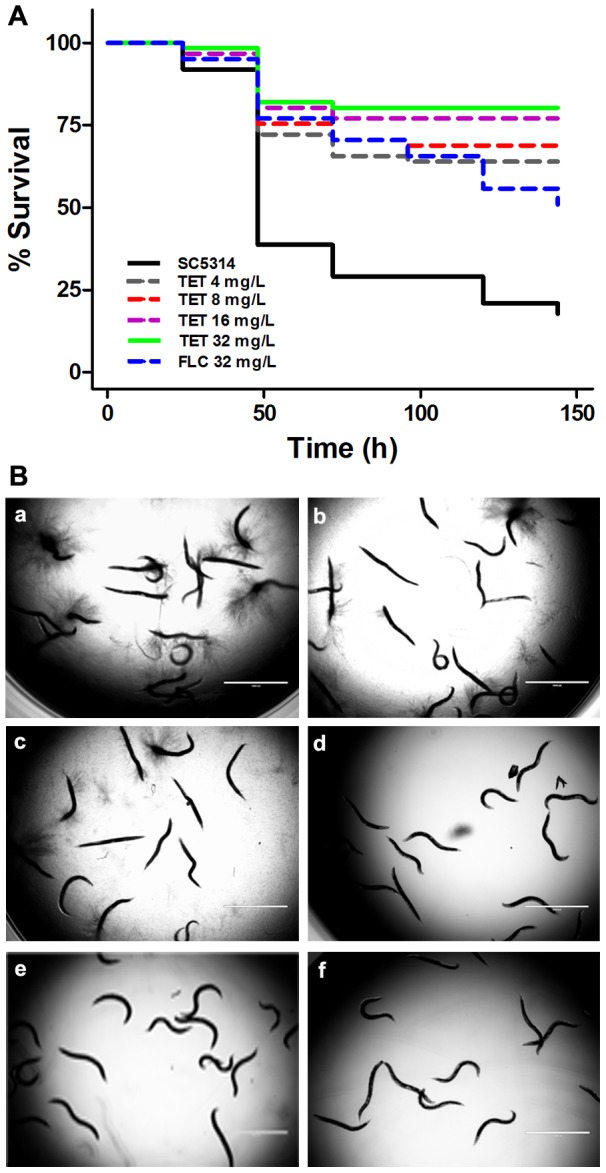
(A) Nematodes were infected with C. albicans for 4 h and then moved to pathogen-free liquid medium in the presence of TET (4 mg/L, 8 mg/L, 16 mg/L, 32 mg/L, P<0.0001), FLC (32 mg/L, P<0.0001) or DMSO. Dead worms were counted and removed daily. (B) After exposure to strain C. albicans SC5314, C. elegans nematodes were piped into 12-well plates that contain TET or DMSO. TET exhibited antifungal activity. a: Treatment free control group (DMSO added); b: TET 4 mg/L; c: TET 8 mg/L; d: TET 16 mg/L; e: TET 32 mg/L; f: FLC 32 mg/L.
We further tested the toxicity of TET using uninfected adult C. elegans worms. Final concentrations at 32 mg/L, 64 mg/L and 128 mg/L were used respectively. At all the concentrations tested, no toxicity of TET was observed, and the worms all looked healthy. There was no difference between the TET treatment groups and the drug-free group (Fig. 11).
Figure 11. TET shows no toxicity on uninfected C. elegans glp-4; sek-1 nematodes.
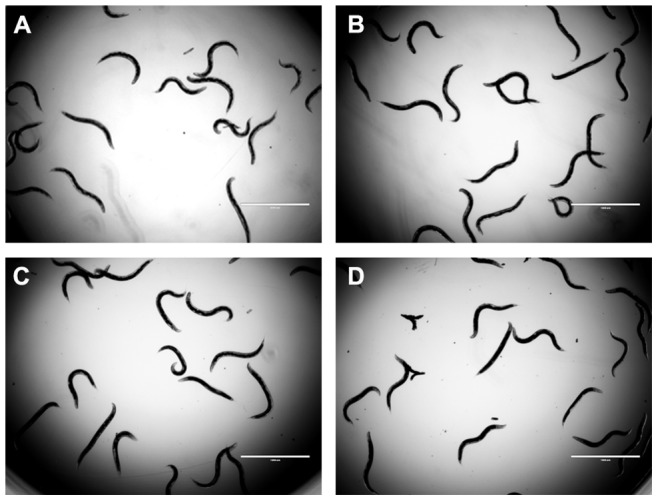
C. elegans glp-4; sek-1 nematodes were pipetted into 12-well plates that contained different concentrations of TET, incubated at 25°C, and observed daily. On day 2, the worms were photographed. (A) 32 mg/L TET; (B) 64 mg/L TET; (C) 128 mg/L TET; (D) Treatment free group with the solvent DMSO added.
Discussion
C. albicans biofilms constitute a threat to successful antifungal treatment [47]. In this study, we revealed the anti-biofilm activity of TET against C. albicans. TET exhibited significant effect against both biofilm formation and maintenance of mature biofilms in vitro. Interestingly, the compound exhibited satisfactory anti-biofilm activity selectively against C. albicans. TET showed only weak anti-biofilm activity against C. neoformans and no effect against A. fumigatus, S. aureus and P. aeruginosa, at least at the concentrations we tested. We further investigated the mechanism of TET against C. albicans biofilms and found that TET could decrease CSH, retard the growth of C. albicans at high concentrations, and suppress the yeast-to-hypha morphological transition. The results of real-time RT-PCR indicated that some important filamentation genes were differentially expressed after exposure to TET. We further revealed that the effect of TET was related to Ras/cAMP pathway, and exogenous cAMP could restore the morphological transition of C. albicans under the condition of TET exposure. Finally, we revealed the antifungal activity of TET in vivo using the C. elegans-C. albicans infection model.
To our knowledge, this is the first report indicating that TET had a significant anti-biofilm effect against C. albicans. Notably, TET could not only inhibit the formation of biofilms but also destroy the maintenance of mature biofilms. More specifically, we revealed that the MIC50 of TET against C. albicans SC5314 was 32 mg/L. 32 mg/L (1×MIC) of TET inhibited the development of more than 60% biofilms, and destroyed the maintenance of about 60% mature biofilms. The effect of TET was good compared with various other antifungal agents. Vila et al [48] found that at the concentration of 16×MIC, fluconazole could not inhibit the development of biofilms, and that amphotericin B at the concentration of 1×MIC could inhibit the development of biofilms but could not affect the maintenance of mature biofilms. Collectively, TET possesses satisfactory anti-biofilm activity.
Our data indicate that TET could inhibit biofilm formation through decreasing adhesion and morphological transition, rather than inhibiting the growth of C. albicans. Adhesion to biomaterial surfaces, growth of cells to form an anchoring layer, and morphological transition to form a complex three-dimensional structure are known to be three stages for biofilm formation [49]-[53]. Our data indicate that 16 mg/L (1/2×MIC50) TET significantly inhibited biofilm formation, severely decreased CSH (indicating adhesion ability [43]), and obviously inhibited the yeast-to-hypha morphological transition in both liquid and solid Spider medium, while 16 mg/L TET had no significant influence on the growth of C. albicans. Thus, the anti-biofilm effect of TET seems attributable to its anti-adhesion and anti-morphological-transition activities.
Our real-time RT-PCR data indicate that some important hypha and adhesion-related genes, including ECE1, HGC1, RAS1, CYR1, EFG1, CPH2, TEC1, HWP1 and ALS3 were down-regulated after 32 mg/L TET treatment. ECE1 is a hypha-specific gene and its expression correlates with the extent of hyphal cell elongation [54]. HGC1 encodes a cyclin partner and functions in maintaining hyphal growth [55]. Ras1p is a GTPase that plays roles in inducing hyphal formation by activating both Ras/cAMP pathway and MAPK [56], [57] pathway. Cyr1 integrates environmental signals from a range of sources and is essential for hyphal formation [58]. Efg1 [59], Cph2 [60] and Tec1 [61] are transcription factors that positively regulate the expression of hypha-specific genes. More specifically, Efg1 is a transcription factor of the Ras/cAMP pathway, which plays important roles in regulating the expression of some hypha-specific genes, including ECE1, HWP1 and ALS3 [62]. Accordingly, ECE1, HWP1 and ALS3 were all down-regulated after TET treatment. Besides the hypha-specific character, HWP1 is also a unique adhesion gene expressing on the hyphal surface. Biofilms lacking HWP1 gene were prone to detach from the abiotic substrate [63], [64]. ALS3 is an ALS family gene that plays an essential role in the adherence stage of C. albicans [65], [66]. The down-regulation of these genes may contribute to the hyphal formation and adhesion defect of C. albicans after TET treatment.
Interestingly, many down-regulated genes after TET treatment, including HWP1, ALS3, ECE1 and HGC1, were genes regulated by Ras/cAMP pathway [45], [46]. Thus we speculated that the anti-biofilm effect of TET might be related to the down-regulation of Ras/cAMP pathway. To verify this hypothesis, we determined the cAMP level and revealed a significant decrease in cAMP level after TET treatment. Moreover, exogenous cAMP restored the hyphal formation in the TET treatment groups. These results indicate that TET may inhibit the filamentous growth by down-regulating the Ras/cAMP pathway.
Using the C. elegans infection model, we revealed the antifungal activity of TET in vivo. Since 4 mg/L of TET significantly protected C. elegans from C. albicans infection in vivo but the agent at this concentration could not inhibit the growth of C. albicans in vitro, we may attribute the antifungal activity of TET in vivo to the inhibitory effect of TET on pathogenic traits. Consistently, TET inhibited yeast-to-hyphae morphological transition, which is the most widely acknowledged pathogenic trait of C. albicans [67]. In addition, we tried to use mouse infection model to verify the antifungal activity of TET in vivo. The mouse infection model was established by infusion of C. albicans via the tail vein. In treatment groups, TET was administered intragastrically at 2 mg/kg, 8 mg/kg or 12 mg/kg for 4 d, and 12 mice was used in each group. TET at 8 mg/kg exhibited protective effect against C. albicans infection, but no significance was obtained (P > 0.05). Moreover, in 12 mg/kg TET group, mice were thiner and died faster than the control group. Further anatomic study found no food in the stomachs of the mice in the 8 mg/kg and 12 mg/kg TET groups. We used healthy C. elegans worms to study the toxicity of TET, and the results indicated that at the final concentration as high as 128 mg/L TET did not exhibit toxicity, which is in accordance with the safe clinical use of TET for silicosis treatment in China since the 1960s[68]. Moreover, it was reported that even when TET was administrated intramuscularly at the dosage of 240 mg, three times daily, it is not toxic to humans[68], [69]. Collectively, TET exhibits low toxicity. Thus, we assumed that the fast death of mice in the above 12 mg/kg TET group was caused by anorexia due to TET intragastrical administration rather than cytotoxicity. Collectively, TET exhibits potential antifungal activity in vivo, and further studies should be carried out to improve pharmaceutical preparations and minimize the side effects on mammals.
In conclusion, TET exhibits anti-biofilm effect selectively against C. albicans, and the anti-biofilm mechanism may be related to the Ras/cAMP pathway. TET may serve as a tool drug to dissect the difference between C. albicans biofilm and other microbial (C. neoformans, A. fumigatus, S. aureus and P. aeruginosa) biofilms, and further translational study is required to determine whether the anti-biofilm effect of TET is applicable in clinical settings.
Materials and Methods
Strains, culture and agents
C. albicans strains and C. neoformans strain H99 were routinely grown in YPD (1% yeast extract, 2% peptone and 2% dextrose) liquid medium at 30°C in a shaking incubator [70]. For A. fumigatus strain T308073458, conidia were harvested from 3-day-old cultures on Sabouraud dextrose agar plates by flooding the surface of the plates with PBS containing 0.025% (vol/vol) Tween-20 and shaking gently [70]. S. aureus was routinely grown in TSB liquid medium at 37°C in a shaking incubator [71]. P. aeruginosa was incubated in LB liquid medium at 37°C in a shaking incubator. TET, amphotericin B, ciprofloxacin and penicillin were purchased from Sigma-Aldrich, and fluconazole was purchased from Pfizer inc.
In vitro biofilm formation assays
To investigate the activities of TET against fungal biofilms, the assays were performed on TET (Sigma, cat. no. T2695) according to the methods that Pierce et al described [70] with slight modifications. Briefly, biofilm formation assays were performed in 96-well tissue culture plates (Corning, cat. no. 3599) by seeding 100 µl 1.0×106 cells/ml C. albicans cell suspension in RPMI 1640 medium, 200 µl 1.0×107 cells/ml C. neoformans cell suspension in DMEM medium (Cellgro, cat. no. 10-013-CV), or 200 µl 1.0×105 cells/ml A. fumigatus cell suspension in RPMI 1640 medium (Gibco, Bethesda, MD) respectively, and incubating them statically at 37°C. After 90-min (C. albicans) or 4 h (A. fumigatus and C. neoformans) adhesion, the media were aspirated, non-adherent cells were removed, and fresh medium was added. The plates were further incubated at 37°C for 24∼48 h until formation of mature biofilms. A semiquantitative measure of the formed biofilms was calculated using an XTT reduction assay [72]. To detect the effect of TET and the positive control drug Amphotericin B on the formation of biofilms, different concentrations of the drugs were added to the fresh medium after 90-min/4 h adhesion, incubated at 37°C for 24∼48 h. To detect the effect of the drugs on mature biofilms, the drugs were added after 24-h incubation with the formed mature biofilms at 37°C, and the plates were incubated at 37°C for further 24 h.
To investigate the activities of TET against bacterial biofilms, the assays were conducted as previously described by Malena Sandberg et al [71] with slight modifications. Briefly, exponentially growing bacteria were diluted with fresh TSB (for S. aureus)/ LB (for P. aeruginosa) medium to an OD600 of 0.02, then 200 µl cell suspensions were added to flat-bottomed 96-well microplates. Drugs and bacterial suspensions were added simultaneously to the wells of microplates and incubated at 37°C for 18 h. Crystal violet staining of the biofilms was conducted as previously described by Adyary Fallarero et al [73]. Briefly, the planktonic suspension was removed from the wells following incubation and the wells were washed with PBS followed by the addition of crystal violet solution (2.3% w/v) and incubated at room temperature for 5 min. The stain was removed and wells were washed twice with PBS. The stained biofilms were then diluted in 96% by volume ethanol and plates were incubated at room temperature for 1 h. The absorbance at 595 nm was measured. Penicillin was used as positive control drug against S. aureus, and ciprofloxacin was used as positive control drug against P. aeruginosa.
Measurement of biofilm biomass
Biofilm biomass was measured as described in Nobile et al [64] with slight modifications. Sterile silicone squares (1.5×1.5 cm, cut from Cardiovascular Instrument silicone sheets [PR72034-06N, Bentec Medical Inc, United States]) were pretreated with bovine serum (Sigma) overnight and washed with PBS before inoculation. Exponentially growing C. albicans cells were diluted to an OD600 of 0.2 with Spider medium, and the suspension was added to a sterile 12-well plate with one prepared silicone square in each well. The inoculated plate was incubated at 37°C for 90 min with gentle agitation (150 rpm) until adhesion occurred. To remove non-adherent cells, the squares were washed with 2 ml PBS, and then moved to a fresh 12-well plate containing 2 ml fresh Spider medium. For TET treatment groups, TET was added to the fresh Spider medium. The plate was incubated at 37°C for an additional 60 h at 75 rpm agitation to allow biofilm formation. For dry mass measurements, each biofilm was removed from the substrate by vortexing the silicone square in PBS and then filtering the cell suspension on preweighted filter paper. The filtrate and filter were dried at 75°C overnight and then weighted. The total biomass of each biofilm was calculated by subtracting the weight of the filter paper. The mean dry biomass was calculated from six independent samples. Statistical significance was determined by analysis of variance (ANOVA). Comparison between TET treatment groups and non-treatment group was performed by Student t test. A P value of less than 0.05 was considered statistically significant.
Confocal laser scanning microscopy (CLSM) assay
CLSM was performed as described previously [74] to determine the inhibitory effect of TET on biofilm formation. Briefly, plastic disks were inoculated with C. albicans statically at 37°C for 90 min to allow adhesion. After removing non-adherent cells, the disk was incubated with fresh RPMI 1640 medium at 37°C for 24 h to allow biofilm formation. For TET treatment groups, TET was added to the fresh RPMI 1640 medium after 90-min adhesion. The disk was then transferred to a new 6-well plate and incubated at 37°C for 45 min in 4 ml PBS containing fluorescent stain FUN-1 (10 µM) (Molecular Probes, Eugene, OR) and concanavalin A-Alexa Fluor 488 conjugate (ConA; 25 mg/L; Molecular Probes). FUN-1 (excitation wavelength 543 nm; emission wavelength 560 nm; long-pass filter) is converted to an orange-red cylindrical intravascular structure by metabolically active cells, while ConA (excitation wavelength 488 nm; emission wavelength 505 nm; long-pass filter) binds to glucose and mannose residues of cell wall polysaccharides and emits green fluorescence. After incubation with the dye, the disk was flipped and C. albicans cells were observed under a Leica TCS SP2 CLSM.
Scanning electron microscopy (SEM) assay
SEM was performed to investigate the ultrastructure of biofilms [75]. Sterile glass disks coated with poly-L-lysine hydrobromide (Sigma, cat. no. P6282) were used to develop C. albicans biofilms. The disks were inoculated with C. albicans SC5314 statically at 37°C for 90 min to allow adhesion. After removing non-adherent cells, the disks were incubated with fresh RPMI 1640 medium at 37°C for 24 h. For TET treatment groups, TET was added with the fresh RPMI 1640 medium after 90-min adhesion. Biofilms were washed and placed in a fixative consisting of 2% (v/v) glutaraldehyde in 0.15 M sodium cacodylate buffer (pH 7.2) for 2h. The samples were rinsed twice in cacodylate buffer, garnish with 1% osmic acid for 2 h, dehydrated in an ascending ethanol series, treated with hexamethyldisilazane (Polyscience Europe GmbH, Eppelheim, Germany) and dried overnight. The specimens were coated with gold and observed through a Philips XL-30 scanning electron microscope (Philips, The Netherlands) in high vacuum mode [75].
Cellular surface hydrophobicity (CSH) assay
C. albicans CSH was measured by water–hydrocarbon two-phase assay as described previously [76]. In brief, the formed C. albicans biofilms were removed from the flask surface with a sterile scraper (Corning, P.R. Mexico) to prepare a cell suspension (OD600 = 1.0 in YPD medium). 1.2 ml suspension was pipetted into a clean glass tube and overlaid with 0.3 ml of octane. The mixture was vortexed for 3 min. After the separation of the two phases, OD600 of the aqueous phase was determined. OD600 for the group without the octane overlay was used as the control. Three repeats were performed for each group. Relative hydrophobicity was obtained as [(OD600 of the control minus OD600 after octane overlay)/OD600 of the control] ×100.
Time-growth curve assay
Overnight culture of C. albicans was diluted with YPD medium to an OD600 of 0.01 (about 1.5×105 cells/ml) and divided into 4 bottles. Different concentrations of TET were added to the C. albicans suspension. The samples were cultured at 30°C under constant shaking (200 rpm), and cells were counted at the designated time points after culture (0, 3, 6, 9 and 12 h). Three independent experiments were performed [77].
Antifungal susceptibility test
The MIC values was evaluated for TET in 96-well microtiter plates (Greiner, Germany) as described previously [77], using a broth microdilution protocol modified from the Clinical and Laboratory Standards Institute M27-A3 methods [78], [79]. MIC50 was determined as the lowest concentration of the drug that inhibited growth by 50%. MIC80 was determined as the lowest concentration of the drug that inhibited growth by 80%.
Real-time RT-PCR assay
Real-time RT-PCR was used to investigate gene expression changes [80]. Briefly, C. albicans SC5314 cells (1.0×106 cells/ml) were added to 80 ml RPMI 1640 medium in 150 mm×25 mm cell culture dishes. The dishes were incubated statically for 90 min to allow initial adhesion, after which the medium was removed and replaced with 80 ml fresh RPMI 1640 medium containing 32 mg/L TET or DMSO as the control. The dishes were then incubated statically at 37°C for further 1 h. C. albicans cells were then collected and used for the subsequent RNA extraction. Triplicate independent experiments were conducted on each sample. Total RNA was isolated using Fungal RNAout kit (TIANDS, China). First-strand cDNAs were synthesized using a cDNA synthesis kit (TaKaRa Biotechnology, Dalian, China). Real-time PCR was performed as described previously [80]. Primers are shown in Table S1. Triplicate quantitative real-time PCRs were performed on each sample with SYBR Green II (TaKaRa Biotechnology, Dalian, P.R. China) using ABI 7500 Real-Time PCR system (Applied Biosystems Co, California, USA).
Determination of cAMP level
Determination of intracellular cAMP level was performed as described previously [81]. C. albicans samples were collected as described above for real-time RT-PCR. The C. albicans cells were washed once with sterile water and once with MES buffer (10 mM MES [morpholineethanesulfonic acid] containing 0.1 mM EDTA; pH 6). Cells were re-suspended with MES buffer to an OD600 of 8, and 500 µl aliquots were taken from the suspension. Samples were transferred to 1.5-ml microcentrifuge tubes containing 0.5 g glass beads and 500 µl 10% trichloroacetic acid, briefly vortexed, and frozen immediately in liquid nitrogen. After centrifugation, trichloroacetic acid was extracted four times with water-saturated ether. The cAMP content was measured using the cAMP Enzyme Immunoassay Kit (Sigma, cat.no.CA200) according to the manufacturer’s instructions.
Antifungal effect evaluation using a C. elegans infection model
C. elegans-C. albicans infection model was used to evaluate the antifunagl effect of TET. C. elegans was infected by C. albicans as described previously [82], [83]. Briefly, C. elegans glp-4; sek-1 adult nematodes were added to the center of C. albicans SC5314 lawns on BHI kanamycin (45 mg/L) agar plates and incubated at 25°C for 4 h to allow infections. Worms were washed four times with sterile M9. Sixty worms were then pipetted into each well of six-well tissue culture plates (Corning, USA) containing 2 ml of liquid medium (80% M9, 20% BHI) and kanamycin (45 mg/L). For TET treatment groups, TET was added with a series of concentrations, including 4 mg/L, 8 mg/L, 16 mg/L and 32 mg/L. 32 mg/L FLC treatment group was set as the positive control, and the DMSO solvent group was set as the negative control. Worms were scored daily and dead worms were removed from the assay. Survival was examined by using the Kaplan-Meier method and differences were determined by using the log-rank test (STATA 6; STATA, College Station, TX). A P value of<0.05 was considered statistically significant.
Toxicity evaluation using C. elegans worms
To evaluate the toxicity of TET, C. elegans glp-4; sek-1 adult nematodes were prepared and the assay was performed as described previously [84]. Briefly, the nematodes were moved from Escherichia coli OP50 to pathogen-free liquid medium containing 32, 64, and 128 mg/L TET or the solvent DMSO at the same volume. The worms were incubated at 25°C for 2 d and observed daily.
Supporting Information
Primers used for real-time RT-PCR in this study.
(DOC)
Acknowledgments
We thank Dr. William A Fonzi (Department of Microbiology and Immunology, Georgetown University, Washington DC, USA) for providing C. albicans strain SC5314, Dr. Jun Gu (Changhai Hospital, Shanghai, China) for providing C. albicans strains Y0109, 0304103 and 01010, Dr. Ying Wang (Changhai Hospital, Shanghai, China) for providing A. fumigatus strain T308073458, Dr. Wangqing Liao (Changzheng Hospital, Shanghai, China) for providing C. neuformans strain H99, Dr. David C. Hooper (Division of Infectious Disease, Massachusetts General Hospital, USA) for providing S. aureus strain Newman, and Dr. Frederick M. Ausubel (Department of Molecular Biology, Massachusetts General Hospital, USA) for providing P. aeruginosa strain PA14 and C. elegans glp-4; sek-1 strain.
Funding Statement
This work was supported by the National Key Basic Research Program of China (2013CB531602), the National Natural Science Foundation of China (81273558, 81072678 and 90913008), the National Science and Technology Major Project of the Ministry of Science and Technology of China (2011ZX09102-002-01), and Shanghai Science and Technology Major Project (10431902200). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nkechi A, Dionissios N, Michael P, Herwig-Ulf MK, Quan SP, et al. (2012) The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 73: 293–300. [DOI] [PubMed] [Google Scholar]
- 2. Ferreira AV, Prado CG, Carvalho RR, Dias KS, Dias AL (2013) Candida albicans and Non-C. albicans Candida Species: Comparison of Biofilm Production and Metabolic Activity in Biofilms, and Putative Virulence Properties of Isolates from Hospital Environments and Infections. Mycopathologia 175: 265–272. [DOI] [PubMed] [Google Scholar]
- 3. Alexander BD, Perfect JR (1997) Antifungal resistance trends towards the year 2000. Implications for therapy and new approaches. Drugs 54: 657–678. [DOI] [PubMed] [Google Scholar]
- 4. Nett JE, Sanchez H, Cain MT, Ross KM, Andes DR (2011) Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot Cell 10: 1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, et al. (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183: 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramage G, Saville SP, Thomas DP, López-Ribot JL (2005) Candida biofilms: an update. Eukaryot Cell 4: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J (2009) Characterization of mucosal Candida albicans biofilms. PLoS One 4: e7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasan F, Xess I, Wang X, Jain N, Fries BC (2009) Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect 11: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagué CMB, Jarvis WR (1993) Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis 167: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 10. Maki DG, Tambyah PA (2001) Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 7: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen MH, Peacock JE Jr, Tanner DC, Morris AJ, Nguyen ML, et al. (1995) Therapeutic approaches in patients with candidemia: evaluation in a multicenter, prospective, observational study. Arch Intern Med 155: 2429. [PubMed] [Google Scholar]
- 12. Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, et al. (2003) Guidelines for treatment of candidiasis. Clin Infect Dis 38: 161–189. [DOI] [PubMed] [Google Scholar]
- 13. Crnich CJ, Maki DG (2002) The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin Infect Dis 34: 1232–1242. [DOI] [PubMed] [Google Scholar]
- 14. Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP (1988) Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med 148: 2642. [DOI] [PubMed] [Google Scholar]
- 15. Costerton J, Stewart PS, Greenberg E (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 16. Davey ME, O'Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64: 847–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15: 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33: 1387–1392. [DOI] [PubMed] [Google Scholar]
- 19. Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, et al. (2001) Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80: 903–908. [DOI] [PubMed] [Google Scholar]
- 20. Ramage G, Vandewalle K, Wickes BL, Lopez-Ribot JL (2001) Characteristics of biofilm formation by Candida albicans . Rev Iberoam Micol 18: 163–170. [PubMed] [Google Scholar]
- 21. Tobudic S, Lassnigg A, Kratzer C, Graninger W, Presterl E (2010) Antifungal activity of amphotericin B, caspofungin and posaconazole on Candida albicans biofilms in intermediate and mature development phases. Mycoses 53: 208–214. [DOI] [PubMed] [Google Scholar]
- 22. Hawser SP, Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39: 2128–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Lv Y, Cui Z, Bei G, Qin G, et al. (2013) Tetrandrine ameliorates cognitive impairment via inhibiting astrocyte-derived S100B activation in a rat model of chronic cerebral hypoperfusion. Neurol Res 36: 614–621. [DOI] [PubMed] [Google Scholar]
- 24. Gralla EJ, Coleman GL, Jonas AM (1974) Toxicology studies with d-tetrandrine (NSC-77037), a plant alkaloid with vascular and lymphotoxic effects in dogs and monkeys. Cancer Chemother Rep 3 5: 79–85. [PubMed] [Google Scholar]
- 25. Tainlin L, Tingyi H, Changqi Z, Peipei Y, Qiong Z (1982) Studies of the chronic toxicity of tetrandrine in dogs: an inhibitor of silicosis. Ecotoxicol Environ Saf 6: 528–534. [DOI] [PubMed] [Google Scholar]
- 26. Gao Y, Chang M, Mao H, Chao H, Chen D (1965) The clinical observation of tetrandrine in the treatment of 270 cases of hypertensive patients and hypertensive crisis. Chin J Int Med 13: 504–508. [Google Scholar]
- 27. Yao W-X, Jiang M-X (2002) Effects of tetrandrine on cardiovascular electrophysiologic properties. Acta Pharmacologica Sinica 23: 1069–1074. [PubMed] [Google Scholar]
- 28. Kim HS, Zhang YH, Oh KW, Ahn HY (1997) Vasodilating and hypotensive effects of fangchinoline and tetrandrine on the rat aorta and the stroke-prone spontaneously hypertensive rat. J Ethnopharmacol 58: 117–123. [DOI] [PubMed] [Google Scholar]
- 29. Yu X, Zou C, Lin M (1983) Observation of the effect of tetrandrine on experimental silicosis of rats. Ecotoxicol Environ Saf 7: 306–312. [DOI] [PubMed] [Google Scholar]
- 30. Li Q (1981) The therapeutic effect of tetrandrine on silicosis (author's transl). Zhonghua Jie He He Hu Xi Za Zhi 4: 321–323. [PubMed] [Google Scholar]
- 31. Chen Y, Chen JC, Tseng SH (2009) Effects of tetrandrine plus radiation on neuroblastoma cells. Anticancer Res 29: 3163–3171. [PubMed] [Google Scholar]
- 32. Chen YJ, Dai YS, Chen BF, Chang A, Chen HC, et al. (1999) The effect of tetrandrine and extracts of Centella asiatica on acute radiation dermatitis in rats. Biol Pharm Bull 22: 703–706. [DOI] [PubMed] [Google Scholar]
- 33. Chen F, Sun S, Kuhn DC, Lu Y, Gaydos LJ, et al. (1997) Tetrandrine inhibits signal-induced NF-κB activation in rat alveolar macrophages. Biochem Biophys Res Commun 231: 99–102. [DOI] [PubMed] [Google Scholar]
- 34. Shen YC, Chou CJ, Chiou WF, Chen CF (2001) Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae: comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions. Mol pharmacol 60: 1083–1090. [PubMed] [Google Scholar]
- 35. Fu L, Zhang Y, Liang Y, Yang X, Pan Q (2002) The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF-7/adr cells. Eur J cancer 38: 418. [DOI] [PubMed] [Google Scholar]
- 36. Lee JH, Kang GH, Kim KC, Kim KM, Park DI, et al. (2002) Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol 21: 1239. [PubMed] [Google Scholar]
- 37. Dong Y, Yang MMP, Kwan CY (1997) In vitro inhibition of proliferation of HL-60 cells by tetrandrine and coriolus versicolor peptide derived from Chinese medicinal herbs. Life Sci 60: PL135–PL140. [DOI] [PubMed] [Google Scholar]
- 38. Wang G, Lemos JR, Iadecola C (2004) Herbal alkaloid tetrandrine: from an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci 25: 120–123. [DOI] [PubMed] [Google Scholar]
- 39. Zhang H, Wang K, Zhang G, Ho HI, Gao A (2010) Synergistic anti-candidal activity of tetrandrine on ketoconazole: an experimental study. Planta Med 76: 53. [DOI] [PubMed] [Google Scholar]
- 40. Zhang D, Zhang H, Li H, Li S, Shi J (2010) An in vivo study on tetrandrine as a synergist to econazole against Trichophyton mentagrophytes . Chinese Journal of Zoonoses 3: 010. [Google Scholar]
- 41. Luo G, Samaranayake LP (2002) Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans . APMIS 110: 601–610. [DOI] [PubMed] [Google Scholar]
- 42. Pompilio A, Piccolomini R, Picciani C, D'Antonio D, Savini V, et al. (2008) Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol Lett 287: 41–47. [DOI] [PubMed] [Google Scholar]
- 43. Samaranayake YH, Wu PC, Samaranayake LP, So M (1995) Relationship between the cell surface hydrophobicity and adherence of Candida krusei and Candida albicans to epithelial and denture acrylic surfaces. APMIS 103: 707–713. [PubMed] [Google Scholar]
- 44. Miwa T, Takagi Y, Shinozaki M, Yun CW, Schell WA, et al. (2004) Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans . Eukaryot Cell 3: 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braun BR, Johnson AD (2000) TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans . Genetics 155: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hogan DA, Sundstrom P (2009) The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans . Future Microbiol 4: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 47. Ramage G, Saville SP, Wickes BL, López-Ribot JL (2002) Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68: 5459–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vila TV, Ishida K, de Souza W, Prousis K, Calogeropoulou T, et al. (2013) Effect of alkylphospholipids on Candida albicans biofilm formation and maturation. J Antimicrob Chemother 68: 113–125. [DOI] [PubMed] [Google Scholar]
- 49. Nobile CJ, Mitchell AP (2006) Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 8: 1382–1391. [DOI] [PubMed] [Google Scholar]
- 50. Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL (2005) Candida biofilms: an update. Eukaryot Cell 4: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seneviratne CJ, Jin L, Samaranayake LP (2008) Biofilm lifestyle of Candida: a mini review. Oral Dis 14: 582–590. [DOI] [PubMed] [Google Scholar]
- 52. Li DD, Wang Y, Dai BD, Li XX, Zhao LX, et al. (2012) ECM17-Dependent Methionine/Cysteine Biosynthesis Contributes to Biofilm Formation in Candida albicans . Fungal Genet Biol 51: 50–59. [DOI] [PubMed] [Google Scholar]
- 53. Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J (2009) Our current understanding of fungal biofilms. Crit Rev Microbiol 35: 340–355. [DOI] [PubMed] [Google Scholar]
- 54. Birse CE, Irwin MY, Fonzi WA, Sypherd PS (1993) Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans . Infect Immun 61: 3648–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng X, Wang Y (2004) Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J 23: 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feng Q, Summers E, Guo B, Fink G (1999) Ras signaling is required for serum-induced hyphal differentiation in Candida albicans . J Bacteriol 181: 6339–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, et al. (2001) Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans . Mol microbiol 42: 673–687. [DOI] [PubMed] [Google Scholar]
- 58. Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, et al. (2001) Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans . Mol Biol Cell 12: 3631–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stoldt VR, Sonneborn A, Leuker CE, Ernst JF (1997) Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J 16: 1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lane S, Zhou S, Pan T, Dai Q, Liu H (2001) The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Mol Cell Biol 21: 6418–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lane S, Birse C, Zhou S, Matson R, Liu H (2001) DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans . J Biol Chem 276: 48988–48996. [DOI] [PubMed] [Google Scholar]
- 62. Hogan DA, Sundstrom P (2009) The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans . Future Microbiol 4: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 63. Chaffin WL (2008) Candida albicans cell wall proteins. Microbiol Mol Biol Rev 72: 495–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, et al. (2006) Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo . PLoS Pathog 2: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sundstrom P (2002) Adhesion in Candida spp. Cell Microbiol 4: 461–469. [DOI] [PubMed] [Google Scholar]
- 66. Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara JP (2008) Adherence mechanisms in human pathogenic fungi. Med Mycol 46: 749–772. [DOI] [PubMed] [Google Scholar]
- 67. Sudbery PE (2011) Growth of Candida albicans hyphae. Nat Rev Microbiol 9: 737–748. [DOI] [PubMed] [Google Scholar]
- 68. Xu WL, Shen HL, Ao ZF, Chen BA, Xia W, et al. (2006) Combination of tetrandrine as a potential-reversing agent with daunorubicin, etoposide and cytarabine for the treatment of refractory and relapsed acute myelogenous leukemia. Leuk Res 30: 407–413. [DOI] [PubMed] [Google Scholar]
- 69. Fang J, Fang Y (1996) Tetrandine: pharmacology and clinical usefulness. Chin Pharm J 31: 454–457. [Google Scholar]
- 70. Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, et al. (2008) A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3: 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sandberg M, Maattanen A, Peltonen J, Vuorela PM, Fallarero A (2008) Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: an approach to screening of natural antimicrobial compounds. Int J Antimicrob Agents 32: 233–240. [DOI] [PubMed] [Google Scholar]
- 72. Klotz SA, Drutz DJ, Zajic JE (1985) Factors governing adherence of Candida species to plastic surfaces. Infect Immun 50: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fallarero A, Skogman M, Kujala J, Rajaratnam M, Moreira VM, et al. (2013) (+)-Dehydroabietic Acid, an Abietane-Type Diterpene, Inhibits Staphylococcus aureus Biofilms in Vitro . Int J Mol Sci 14: 12054–12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cao Y, Dai B, Wang Y, Huang S, Xu Y, et al. (2008) In vitro activity of baicalein against Candida albicans biofilms. Int J Antimicrob Agents 32: 73–77. [DOI] [PubMed] [Google Scholar]
- 75. Braga PC, Culici M, Alfieri M, Dal Sasso M (2008) Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int J Antimicrob Agents 31: 472–477. [DOI] [PubMed] [Google Scholar]
- 76. Klotz S, Drutz D, Zajic J (1985) Factors governing adherence of Candida species to plastic surfaces. Infect Immun 50: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Quan H, Cao YY, Xu Z, Zhao JX, Gao PH, et al. (2006) Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother 50: 1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arikan S, Gur D, Akova M (1997) Comparison of Etest, microdilution and colorimetric dilution with reference broth macrodilution method for antifungal susceptibility testing of clinically significant Candida species isolated from immunocompromised patients. Mycoses 40: 291–296. [DOI] [PubMed] [Google Scholar]
- 79. Martinez-Martinez L, Rodriguez G, Pascual A, Suarez AI, Perea EJ (1996) In-vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii . J Antimicrob Chemother 38: 1107–1108. [DOI] [PubMed] [Google Scholar]
- 80. Liang R, Cao Y, Fan K, Xu Y, Gao P, et al. (2009) 2-Amino-nonyl-6-methoxyl-tetralin muriate inhibits sterol C-14 reductase in the ergosterol biosynthetic pathway. Acta Pharmacol Sin 30: 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miwa T, Takagi Y, Shinozaki M, Yun CW, Schell WA, et al. (2004) Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans . Eukaryot Cell 3: 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E (2009) Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell 8: 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Okoli I, Coleman JJ, Tempakakis E, An WF, Holson E, et al. (2009) Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS One 4: e7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, et al. (2007) Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog 3: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for real-time RT-PCR in this study.
(DOC)