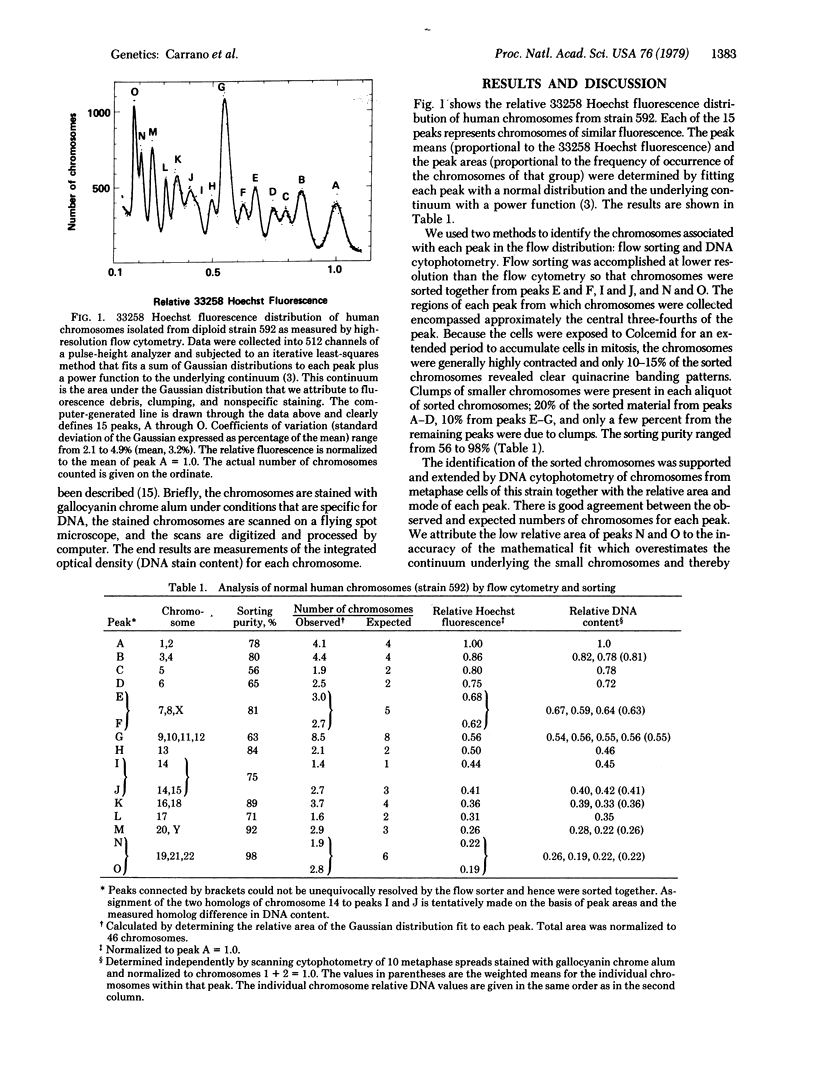
Abstract
The 24 human chromosome types of normal diploid fibroblast cell strain were classified into 15 groups by high-resolution flow cytometry on the basis of 33258 Hoechst fluorescence. Chromosomes associated with each group were flow sorted onto microscope slides and identified by quinacrine banding analysis. DNA cytophotometry of metaphase chromosomes from the same cell strain supported and extended this identification. Four of the groups purified were due to chromosomes of a single type--namely, chromosomes 5, 6, 13, and 17. Eight additional groups were also separated and found to contain the following chromosomes: 1 and 2; 3 and 4; 7, 8, and X; 9--12; 14 and 15; 16 and 18; 20 and Y; and 19, 21, and 22. The average purity for the 12 sorted fractions was 78%.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrano A. V., Gray J. W., Moore D. H., 2nd, Minkler J. L., Mayall B. H., van Dilla M. A., Mendelsohn M. L. Purification of the chromosomes of the Indian muntjac by flow sorting. J Histochem Cytochem. 1976 Jan;24(1):348–354. doi: 10.1177/24.1.1254929. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Drets M. E. Mechanisms of chromosome banding. IX. Are variations in DNA base composition adequate to account for quinacrine, Hoechst 33258 and daunomycin banding? Chromosoma. 1976 Jul 8;56(3):199–211. doi: 10.1007/BF00293185. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Pinkel D. High resolution dual laser flow cytometry. J Histochem Cytochem. 1978 Aug;26(8):622–627. doi: 10.1177/26.8.357646. [DOI] [PubMed] [Google Scholar]
- Fournier R. E., Ruddle F. H. Stable association of the human transgenome and host murine chromosomes demonstrated with trispecific microcell hybrids. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3937–3941. doi: 10.1073/pnas.74.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden J. R., Mitchell A. R., Buckland R. A., Clayton R. P., Evans H. J. The location of four human satellite DNAs on human chromosomes. Exp Cell Res. 1975 Apr;92(1):148–158. doi: 10.1016/0014-4827(75)90648-5. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Carrano A. V., Moore D. H., 2nd, Steinmetz L. L., Minkler J., Mayall B. H., Mendelsohn M. L., Van Dilla M. A. High-speed quantitative karyotyping by flow microfluorometry. Clin Chem. 1975 Aug;21(9):1258–1262. [PubMed] [Google Scholar]
- Gray J. W., Carrano A. V., Steinmetz L. L., Van Dilla M. A., Moore D. H., 2nd, Mayall B. H., Mendelsohn M. L. Chromosome measurement and sorting by flow systems. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1231–1234. doi: 10.1073/pnas.72.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. W., Purdom I. F., Prosser J., Corneo G. The chromosomal localisation of human satellite DNA I. Chromosoma. 1974;49(2):161–171. doi: 10.1007/BF00348888. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Brodie S., Munroe S. H. Optical studies of complexes of quinacrine with DNA and chromatin: implications for the fluorescence of cytological chromosome preparations. Chromosoma. 1974;49(1):17–40. doi: 10.1007/BF00284985. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Wohlleb J. C. Optical studies of the interaction of 33258 Hoechst with DNA, chromatin, and metaphase chromosomes. Chromosoma. 1975 Nov 11;52(4):297–316. doi: 10.1007/BF00364015. [DOI] [PubMed] [Google Scholar]
- Leif R. C., Easter H. N., Jr, Warters R. L., Thomas R. A., Dunlap L. A., Austin M. F. Centrifugal cytology. I. A quantitative technique for the preparation of glutaraldehyde-fixed cells for the light and scanning electron microscope. J Histochem Cytochem. 1971 Apr;19(4):203–215. doi: 10.1177/19.4.203. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Athwal R. S. Genetic analysis by chromosome-mediated gene transfer. In Vitro. 1976 Nov;12(11):777–786. doi: 10.1007/BF02835452. [DOI] [PubMed] [Google Scholar]
- McKenzie W. H., Lubs H. A. Human Q and C chromosomal variations: distribution and incidence. Cytogenet Cell Genet. 1975;14(2):97–115. doi: 10.1159/000130330. [DOI] [PubMed] [Google Scholar]
- Padgett T. G., Stubbledield E., Varmus H. E. Chicken macrochromosomes contain an endogenous provirus and microchromosomes contain sequences related to the transforming gene of ASV. Cell. 1977 Apr;10(4):649–657. doi: 10.1016/0092-8674(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Wullems G. J., van der Horst J., Bootsma D. Transfer of the human genes coding for thymidine kinase and galactokinase to Chinese hamster cells and human-Chinese hamster cell hybrids. Somatic Cell Genet. 1977 May;3(3):281–293. doi: 10.1007/BF01538746. [DOI] [PubMed] [Google Scholar]


