Abstract
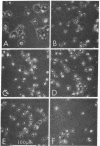
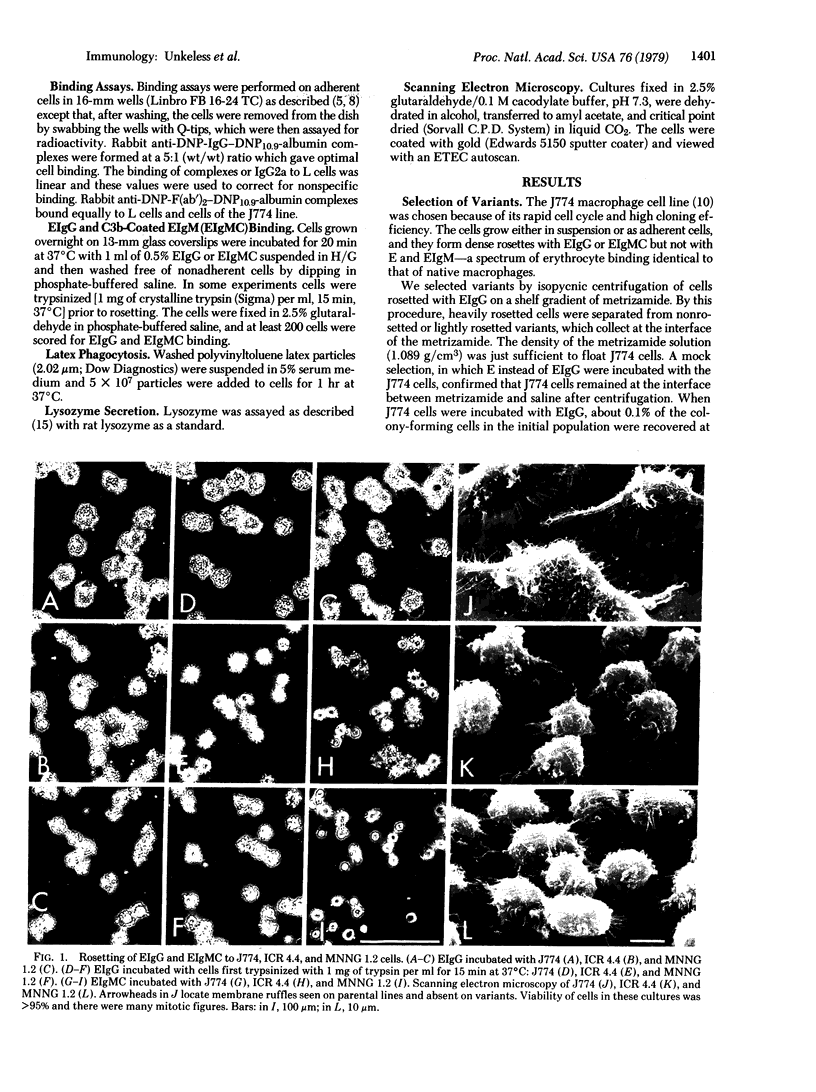
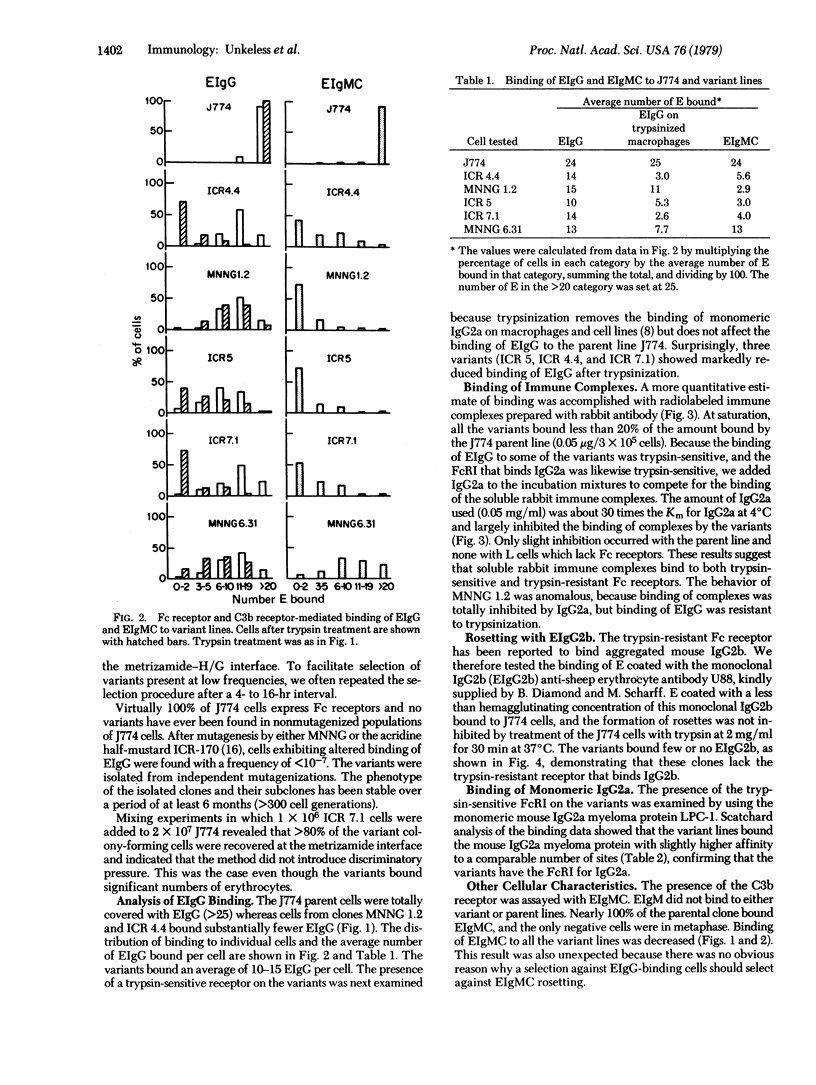
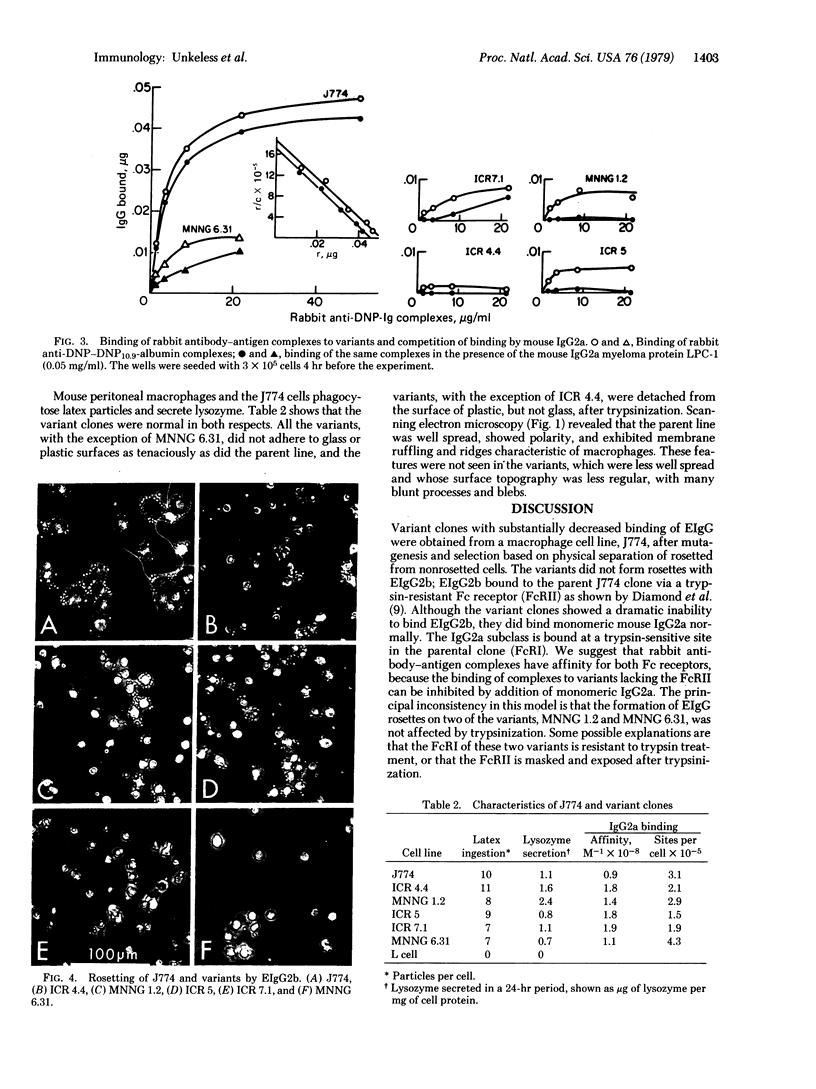
Variants of the J774 mouse macrophage cell line that lack immunologically important membrane receptors were isolated. After mutagenesis, variants were selected in a metrizamide gradient that separated cells heavily rosetted with sheep erythrocytes (E) coated with rabbit anti-E IgG (EIgG) from poorly rosetted cells. Stable variants that exhibited altered binding were found with a frequency of <10-7, and five clones were studied in detail. The variants failed to bind E opsonized with a monoclonal mouse IgG2b anti-E antibody but bound monomeric IgG2a normally when compared to the parental J774 line (Ka 4°C=≈1×108 M-1; ≈2×105 sites per cell). This demonstrates the independence of the receptor for mouse IgG2b complexes (FcRII) from the trypsin-sensitive receptor for mouse IgG2a monomer (FcRI). The variants bound an average of 10-15 EIgG per cell, compared to >20 per cell for J774. After trypsinization, three variants bound only three to five EIgG per cell; the J774 line was not affected by this treatment. Monomeric IgG2a could inhibit the binding of soluble rabbit IgG—antigen complexes to the variants but not to the parent line. Finally, E coated with IgM and complement (EIgMC) were bound poorly by all the variants, relative to the J774 parent. These results show that rabbit IgG complexes are bound by both FcRI and FcRII on mouse macrophages. The impairment of EIgMC rosetting in the variants suggests that the C3b receptor and FcRII are related.
Keywords: somatic cell genetics, complement receptor, antigen-antibody complexes, mouse IgG subclasses
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgois A., Abney E. R., Parkhouse R. M. Structure of mouse Fc receptor. Eur J Immunol. 1977 Oct;7(10):691–695. doi: 10.1002/eji.1830071008. [DOI] [PubMed] [Google Scholar]
- Creech H. J., Preston R. K., Peck R. M., O'Connell A. P. Antitumor and mutagenic properties of a variety of heterocyclic nitrogen and sulfur mustards. J Med Chem. 1972 Jul;15(7):739–746. doi: 10.1021/jm00277a011. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Siegal F. P., Good R. A. Isolation and characterization of a human mononuclear cell Fc receptor. Immunochemistry. 1978 Jun;15(6):365–370. doi: 10.1016/0161-5890(78)90132-3. [DOI] [PubMed] [Google Scholar]
- Diamond B., Bloom B. R., Scharff M. D. The Fc receptors of primary and cultured phagocytic cells studied with homogeneous antibodies. J Immunol. 1978 Oct;121(4):1329–1333. [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger B. S., Koren H. S. The nature of the effector cell in antibody-dependent, cell-mediated cytolysis (ADCC): the cytotoxic activity of murine tumor cells and peritoneal macrophages. Clin Immunol Immunopathol. 1976 Mar;5(2):272–281. doi: 10.1016/0090-1229(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Haskill J. S., Fett J. W. Possible evidence for antibody-dependent macrophage-mediated cytotoxicity directed against murine adenocarcinoma cells in vivo. J Immunol. 1976 Nov;117(5 PT2):1992–1998. [PubMed] [Google Scholar]
- Heusser C. H., Anderson C. L., Grey H. M. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977 May 1;145(5):1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Genetic dissection of the control of normal differentiation in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5554–5558. doi: 10.1073/pnas.74.12.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loube S. R., McNabb T. C., Dorrington K. J. Isolation of an Fcgamma-binding protein from the cell membrane of a macrophage-like cell line (P388D1) after detergent solubilization. J Immunol. 1978 Mar;120(3):709–715. [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Seglen P. O. The use of Metrizamide as a gradient medium for isopycnic separation of rat liver cells. FEBS Lett. 1974 Aug 1;43(3):252–256. doi: 10.1016/0014-5793(74)80654-x. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Bloom B. R. Isolation of variants in phagocytosis of a macrophage-like continuous cell line. J Exp Med. 1977 Jan 1;145(1):175–186. doi: 10.1084/jem.145.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. Studies on the immunoglobulins which stimulate the ingestion of glutaraldehyde-treated red cells attached to macrophages. J Immunol. 1967 Dec;99(6):1115–1120. [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Segal D. M., Titus J. A. The subclass specificity for the binding of murine myeloma proteins to macrophage and lymphocyte cell lines and to normal spleen cells. J Immunol. 1978 Apr;120(4):1395–1403. [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. The synthesis and properties of T25 blycoprotein in Thy-1-negative mutant lymphoma cells. Cell. 1978 May;14(1):21–32. doi: 10.1016/0092-8674(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. The presence of two Fc receptors on mouse macrophages: evidence from a variant cell line and differential trypsin sensitivity. J Exp Med. 1977 Apr 1;145(4):931–945. doi: 10.1084/jem.145.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Separate Fc-receptors for immunoglogulins IgG2a and IgG2b on an established cell line of mouse macrophages. J Immunol. 1976 Apr;116(4):911–914. [PubMed] [Google Scholar]
- Weitzman S., Nathenson S. G., Scharff M. D. Abnormalities in the glycosylation of immunoglobulin heavy chain and an h-2 transplantation antigen in a mouse myeloma mutant. Cell. 1977 Apr;10(4):679–687. doi: 10.1016/0092-8674(77)90101-5. [DOI] [PubMed] [Google Scholar]