Summary
While the presence of hyperlipidaemia in glycogen storage disease (GSD) type Ia and Ib is generally accepted, few investigators have adequately assessed lipid profiles of GSD III in children, in whom the presence of hyperlipidaemia may be most prominent. We analysed the lipid profiles in 44 GSD III patients from 6 months to 30 years of age. Hypertriglyceridaemia and hypercholesterolaemia were common in children younger than 3 years of age. Hypertriglyceridaemia correlated negatively with age, and may reflect increased severity of hypoglycaemia in this younger population. The presence of hyperlipidaemia during childhood in these patients identifies another GSD population that could be at risk for early cardiovascular disease (CVD). Consequently, the outcome of clinical trials investigating the vascular effect of hyperlipidaemia in GSD applies to types other than GSD I.
Introduction
Type III glycogen storage disease (GSD III; OMIM 232400) results from a defect in glycogen debranching enzyme (GDE) that interrupts glycogenolysis. In the first step of glycogenolysis, glycogen phosphorylase and GDE remove glucose from the outer branches of glycogen to form glucose 1-phosphate. Impairment of GDE leads to the accumulation of an abnormal form of glycogen, limit dextrin, in liver, heart and skeletal muscle. As the biochemical abnormalities in GSD III are present in these affected organs, creatine kinase (CK), aspartate transaminase (AST) and alanine transaminase (ALT) are used as markers of control in the management of this disorder (Wolfsdorf and Weinstein 2003).
In the United States, 80% of patients with GSD III have liver, heart and skeletal muscle involvement (type IIIa), while most of the remaining patients have only liver involvement (type IIIb). The predominant manifestation of GSD III in infancy and childhood is hypoglycaemia with fasting. As patients age, however, muscle damage occurs (type IIIa), and most patients have severe physical limitations by the fourth decade of life (Wolfsdorf and Weinstein 2003).
Unlike the other hepatic GSDs, researchers debate the presence of hyperlipidaemia in GSD III. Geberhiwot et al (2007) described the lipid profiles in GSD I, III and IX. He found evidence of pro-atherogenic lipid profiles in GSD Ia characterized by hypercholesterolaemia, hypertriglyceridaemia and reduced HDL. However, he found no significant abnormalities in the lipid profiles of a cohort of six patients with GSD III aged 14–to 54 years. Likewise, Hershkovitz et al (1999) reported no abnormality of plasma lipid and lipoprotein profile in 11 GSD III patients aged 17–54 years. Despite this evidence, the small number of patients studied does not yet justify the conclusion that GSD III patients have no risk for hyperlipidaemia.
Given that the majority of studies reviewing hyperlipidaemia in GSD III do not include patients younger than 14 years of age, in whom hypoglycaemia is most severe, we analysed the largest collection of GSD III patient data available. We ascertained the prevalence of hyperlipidaemia in GSD III at all ages. We postulated that hyperlipidaemia correlates with hypoglycaemia, which is most severe in early childhood.
Methods
Design
The study was based on a secondary analysis of patient data recorded through the collaborative International Study on GSD III (ISGSD III) database. This database was designed to collect outcome data of natural history and clinical interventions to develop clinical guidelines and therapeutic strategies for GSD III. Clinical and laboratory data from participants are entered annually into this longitudinal database. We included results for total cholesterol, triglycerides and ALT. These data were analysed within five age groups that were defined before the analysis: 0–3, 3–6, 6–12, 12–18, and greater than 18 years.
Participants
The diagnosis of GSD III was established either by histological examination of liver biopsy specimens or by enzymatic methods and, more recently, through mutation analysis of DNA obtained from a blood or saliva sample. IRB approval was obtained for the collection of data in the ISGSD III database, and informed consent with assent of minors was obtained from all participants.
A total of 44 patients (32 GSD IIIa and 12 GSD IIIb) identified from hospital records of 16 metabolic centres in eight countries had a complete set of variables with 204 observations. A quality review check by looking at date of birth and initials ensured that no participant was duplicated. Data from patient records were compiled by either the treating physician or one of the investigators. In order to assess changes with time, at least two observation times were needed for inclusion of a patient in the analysis. There were 32 individuals with multiple time points for triglycerides and 30 with multiple time points for cholesterol.
Statistical methods
The key inferences were based on personal linear regression slopes to see whether the quantitative outcomes (serum triglycerides and serum cholesterol) were changing over time. Since these may be outlier-prone, we used the Wilcoxon signed-rank test, which is stable against such outliers, to compare with a null value of zero slope in the target population. Secondarily, we correlated the fitted slopes for triglycerides with ALT to see whether the changes tended to be concordant (positive association) versus discordant (negative association) by the Spearman rank correlation test. All reported p-values are two-sided.
For descriptive purposes, we also provide means and standard deviations for outcomes by age period. To avoid bias, each subject was weighted equally, regardless of the number of observations in the reporting period. The participant’s individual mean for the age period was used as the outcome. This method was also applied to estimate the prevalence of hypertriglyceridaemia and hypercholesterolaemia, which are 0=no or 1=yes at each participant’s observation point in the age group.
Results
Triglycerides
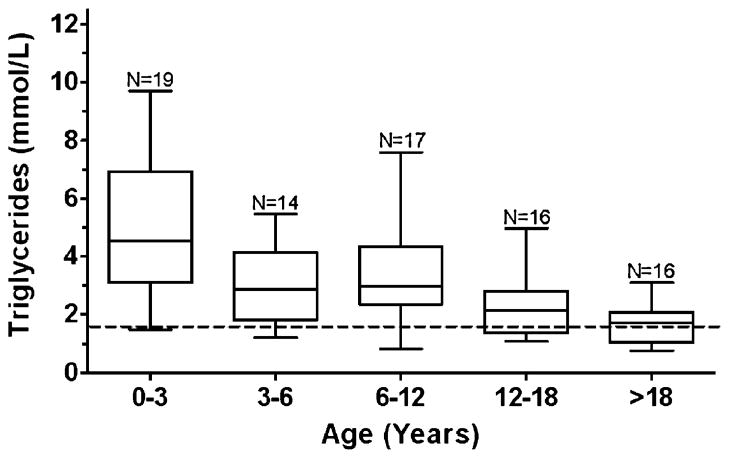
Prevalence of hypertriglyceridaemia (defined as >1.69 mmol/L) was estimated to be 67% (standard error=6%; Table 1). Elevated triglyceride concentrations, however, were most commonly found in younger children (Fig. 1), with a significant decrease in triglycerides with increasing age (p=0.026). Approximately 75% of the subjects had a negative slope and the median slope was −0.11 mmol/L per year.
Table 1.
Prevalence of hypercholesterolaemia and hypertriglyceridaemia by age
| Age in years | Number of patients Chol/TG | Prevalence of hypercholesterolaemia (SE) | Prevalence of hypertriglyceridaemia (SE) |
|---|---|---|---|
| 0–3.0 | 19/19 | 58% (10%) | 89% (6%) |
| 3.0–6.0 | 15/14 | 33% (12%) | 86% (8%) |
| 6.0–12.0 | 18/17 | 51% (11%) | 87% (7%) |
| 12.0–18.0 | 15/16 | 22% (9%) | 63% (12%) |
| >18.0 | 16/16 | 22% (9%) | 44% (11%) |
This table demonstrates the prevalence of both hypercholesterolaemia and hypertriglyceridaemia within pre-determined age brackets. The prevalence across all age groups for hypercholesterolaemia was 31% and for hypertriglyceridaemia was 67%
Fig. 1.
Triglyceride box-and-whisker plot. Boxes represent the 25th to 75th percentiles, and the median is represented in each box. The range of the observations is depicted by the whiskers. The dotted line represents the cut-off for hypertriglyceridemia defined as >1.69 mmol/L (150 mg/dl). One observation – the mean of multiple values – per patient was plotted within each age bracket. N=the number of observations within each age bracket
Cholesterol
Prevalence of hypercholesterolaemia (defined as total cholesterol >5.17 mmol/L) was estimated to be 31% (standard error=6%; Table 1). Unlike triglycerides, however, the elevated cholesterol concentrations did not demonstrate an age predilection (Fig. 2), nor did absolute cholesterol concentrations significantly associate with age (p=0.59) with a median slope of −0.02 mmol/L per year.
Fig. 2.
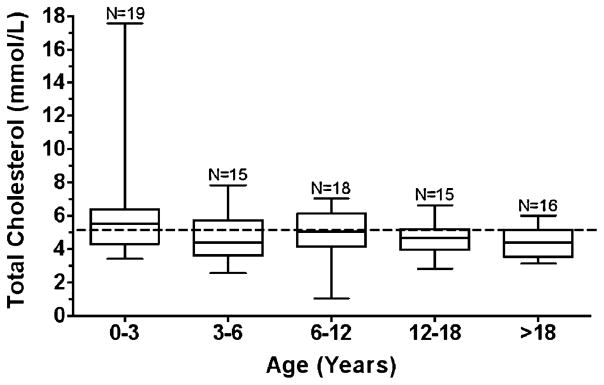
Total cholesterol box-and-whisker plot. Boxes represent the 25th to 75th percentiles, and the median is represented in each box. The range of the observations is depicted by the whiskers. The dotted line represents the cut-off for hypercholesterolemia defined as >5.17 mmol/L (200 mg/dl). One observation – the mean of multiple values - per patient was plotted within each age bracket. N=the number of observations within each age bracket
Markers of control
Spearman correlation analysis of the individual slopes with age revealed no significant association between changes in ALT and changes in triglycerides (r=−0.05, p=0.85).
Discussion
Hyperlipidaemia in the hepatic GSDs develops from a combination of two mechanisms. The first, specific to GSD I, results from shunting of glycogen breakdown products through the glycolytic pathway, which increases formation of acetyl-CoA and synthesis of fatty acids and cholesterol in the liver (Bandsma et al 2008). The second mechanism, which occurs in GSD VI and IX, results from increased β-oxidation of fats in the setting of hypoglycaemia that, in turn, increases fatty acid flux from adipose tissue to the liver as an alternative source of fuel. This second mechanism is hypothesized to result in the observed hyperlipidaemia in GSD III.
Although the circumstances responsible for hyperlipidemia in other types of GSD are present in GSD III, hypertriglyceridaemia has not previously been characterized in children with this form of GSD. The inability to mobilize glycogen results in frequent hypoglycaemia in affected children. This frequency of hypoglycaemia in GSD III patients, however, decreases with age, which may explain why previous investigations did not reveal lipid abnormalities in this population.
With improving dietary management, patients with GSD are living well into adulthood. Patients with GSD I and III commonly reach ages where the potential contribution of hyperlipidaemia to the development of atherosclerosis becomes important. However, even though GSD I patients manifest a pro-atherogenic lipid profile characterized by hypercholesterolaemia, hypertriglyceridaemia, and reduced HDL, investigations have not demonstrated an increased frequency of cardiovascular disease (CVD) in this population (Ubels et al 2002). On the basis of early studies (Muhlhausen et al 2005; Nguyen et al 2006; Wittenstein et al 2002) that failed to establish a correlation between GSD patients with hyperlipidaemia and risk of early CVD, the current consensus guideline for management of GSD I does not mandate treatment with lipid-lowering agents unless patients are at risk for pancreatitis. Researchers have attempted to explain the apparent protective features of GSD I by proposing mechanisms such as increased cholesterol efflux, increased antioxidant potential, and decreased platelet aggregation (Muhlhausen et al 2005; Nguyen et al 2006; Wittenstein et al 2002). However, these hypotheses have been developed on the basis of very few patients.
Unlike in patients with GSD I, hypertriglyceridaemia in GSD III does not reach levels high enough to cause pancreatitis. Indeed, pancreatitis has not been described in GSD III, and treatment for elevated lipids may be less likely to be commenced. Nevertheless, the potential risk of elevated triglycerides has yet to be determined, especially regarding risk for vessel dysfunction and CVD. If vascular dysfunction does occur, aggressive treatment is warranted. Future studies in the GSD III population are therefore needed to determine whether the elevated lipids in GSD III are pro-atherogenic and, if so, whether pharmacological therapy will decrease the risk of CVD.
Limitations of this study arise from the lack of information regarding dietary therapy in this population. In addition, only total cholesterol and triglycerides were included in the database, while HDL and LDL were omitted, resulting in incomplete assessment of cardiovascular risk. Finally, while this study represents the largest assessment of hyperlipidaemia in GSD III, the numbers are still relatively small, and an assessment of lipids in the subtypes of GSD III could not be performed. Despite these limitations, hyperlipidaemia clearly occurs in this population, and a more thorough evaluation of cardiovascular risk factors is needed as part of new prospective studies assessing CVD.
In conclusion, hyperlipidaemia occurs in children with GSD III. The duration of this metabolic derangement throughout childhood may place adolescent and adult patients at risk for CVD. Investigations to assess this risk are warranted.
Acknowledgments
The authors acknowledge and thank the following physicians for contributing to the International Study on GSD III (ISGSD III) database: E. B. Haagsma (Universitair Medisch Centrum, Groningen), J. Zeman (Charles University, Prague), P. Lee (University College London) and M. Berry (Montreal Children’s Hospital, Quebec). Support for this project was provided by the Association for Glycogen Storage Disease, University of Florida Glycogen Storage Disease Research Fund, the National Center for Research Resources General Clinical Research Center grant M01 RR 00082, National Institutes of Health, and NIH Mentored Career Award K23 RR 017560 (D.A.W.).
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- CK
creatinine kinase
- CVD
cardiovascular disease
- GDE
glycogen debranching enzyme
- GSD I
glycogen storage disease type I
- GSD III
glycogen storage disease type III
- ISGSD III
International Study on GSD III
Footnotes
Communicating editor: Robert Steiner
Competing interests: None declared
References to electronic databases: Glycogen storage disease type III : OMIM 232400.
Contributor Information
A. V. Bernier, Division of Pediatric Endocrinology and Glycogen Storage Disease Program, Department of Pediatrics, University of Florida, Gainesville, FL, USA
C. P. Sentner, Department of Metabolic Diseases, Beatrix Children’s Clinic and University of Groningen, Groningen, The Netherlands
C. E. Correia, Division of Pediatric Endocrinology and Glycogen Storage Disease Program, Department of Pediatrics, University of Florida, Gainesville, FL, USA
D. W. Theriaque, General Clinical Research Center, University of Florida, Gainesville, FL, USA
J. J. Shuster, General Clinical Research Center, University of Florida, Gainesville, FL, USA. Division of Biostatistics, Department of Epidemiology and Health Policy Research, University of Florida, Gainesville, FL, USA
G. P. A. Smit, Department of Metabolic Diseases, Beatrix Children’s Clinic and University of Groningen, Groningen, The Netherlands
D. A. Weinstein, Email: weinsda@peds.ufl.edu, Division of Pediatric Endocrinology and Glycogen Storage Disease Program, Department of Pediatrics, University of Florida, Gainesville, FL, USA. Division of Pediatric Endocrinology, University of Florida, PO Box 100296, Gainesville, FL 32610-0296, USA
References
- Bandsma RH, Prinsen BH, van der Velden MD, et al. Increased de novo lipogenesis and delayed conversion of large VLDL into IDL particles contribute to hyperlipidemia in glycogen storage disease type 1a. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e31816c9013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Geberhiwot T, Alger S, McKiernan P, et al. Serum lipid and lipoprotein profile of patients with glycogen storage disease types I, III and IX. J Inherit Metab Dis. 2007;30(3):406. doi: 10.1007/s10545-007-0485-2. [DOI] [PubMed] [Google Scholar]
- Hershkovitz E, Donald A, Mullen M, et al. Blood lipids and endothelial function in glycogen storage disease type III. J Inherit Metab Dis. 1999;22(8):891–898. doi: 10.1023/A:1005687323096. [DOI] [PubMed] [Google Scholar]
- Muhlhausen C, Schneppenheim R, Budde U. Decreased plasma concentration of von Willebrand factor antigen (vWF:Ag) in patients with glycogen storage disease type 1a. J Inherit Metab Dis. 2005;28:945–950. doi: 10.1007/s10545-005-0184-9. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Pan CJ, Weinstein DA. Increased scavenger receptor class B type 1-mediated cellular cholesterol efflux and antioxidant capacity in the sera of glycogen storage disease type 1a patients. Mol Genet Metab. 2006;89(3):233–238. doi: 10.1016/j.ymgme.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Ubels FL, Rake JP, Slaets JP. Is glycogen storage disease 1a associated with atherosclerosis? Eur J Pediatr. 2002;161:s62–s64. doi: 10.1007/s00431-002-1006-9. [DOI] [PubMed] [Google Scholar]
- Wittenstein B, Klein M, Finckh B, Ullrich K, Kohlschutter A. Plasma antioxidants in pediatric patients with glycogen storage disease, diabetes mellitus, and hypercholesterolemia. Free Radic Biol Med. 2002;33(1):103–110. doi: 10.1016/S0891-5849(02)00863-8. [DOI] [PubMed] [Google Scholar]
- Wolfsdorf JI, Weinstein DA. Glycogen storage diseases. Rev Endocr Metab Disord. 2003;4(1):95–102. doi: 10.1023/A:1021831621210. [DOI] [PubMed] [Google Scholar]