Abstract
While the molecular structures of angiotensin II (Ang II) type 1 (AT1) receptor blockers (ARBs) are very similar, they are also slightly different. Although each ARB has been shown to exhibit a unique mode of binding to AT1 receptor, different positions of the AT1 receptor have been analyzed and computational modeling has been performed using different crystal structures for the receptor as a template and different kinds of software. Therefore, we systematically analyzed the critical positions of the AT1 receptor, Tyr113, Tyr184, Lys199, His256 and Gln257 using a mutagenesis study, and subsequently performed computational modeling of the binding of ARBs to AT1 receptor using CXCR4 receptor as a new template and a single version of software. The interactions between Tyr113 in the AT1 receptor and the hydroxyl group of olmesartan, between Lys199 and carboxyl or tetrazole groups, and between His256 or Gln257 and the tetrazole group were studied. The common structure, a tetrazole group, of most ARBs similarly bind to Lys199, His256 and Gln257 of AT1 receptor. Lys199 in the AT1 receptor binds to the carboxyl group of EXP3174, candesartan and azilsartan, whereas oxygen in the amidecarbonyl group of valsartan may bind to Lys199. The benzimidazole portion of telmisartan may bind to a lipophilic pocket that includes Tyr113. On the other hand, the n-butyl group of irbesartan may bind to Tyr113. In conclusion, we confirmed that the slightly different structures of ARBs may be critical for binding to AT1 receptor and for the formation of unique modes of binding.
Introduction
Angiotensin II (Ang II) type 1 (AT1) receptor is a member of the G protein-coupled receptor (GPCR) superfamily and contains 359 amino acids [1]. It has a widespread tissue distribution and mediates most known cardiovascular functions including vasoconstriction, cardiovascular hypertrophy and hyperplasia [2]. AT1 receptor blockers (ARBs, including EXP3174, which is an active metabolite of losartan, candesartan, eprosartan, valsartan, telmisartan, olmesartan, irbesartan, and azilsartan) have been developed and are available for clinical use worldwide. Basic and clinical studies have shown that ARBs are useful for preventing the development of cardiovascular disease [2].
With the exception of eprosartan, ARBs that are widely used in clinics share a common molecular scaffold consisting of biphenyl-tetrazol and imidazole groups that have slightly different structures [3,4]. Recent clinical studies have demonstrated that not all ARBs have the same effects and some benefits conferred by ARBs may not be class (or common) effects, but rather molecule-specific (or differential) effects [4]. We and others previously indicated that each ARB has a unique mode of binding for AT1 receptor [5-16]. We have proposed that the molecule-specific effects may be due to small differences in the molecular structure of each ARB [3].
While the crystal structures of GPCRs obtained from the rhodopsin, opsin, and beta1- and beta2-adrenergic receptor systems have recently been described [17-24], the crystal structure of AT1 receptor has not been elucidated. Although we and others analyzed the mode of binding of ARBs to AT1 receptor [5-16], different approaches used showed different modes of binding of ARBs to AT1 receptor. These analyses considered different positions of the AT1 receptor using site-directed mutagenesis and performed computational modeling using different GPCR crystal structures as templates and also different softwares which could account for the different modes of binding of ARBs to AT1 receptor observed.
To resolve these problems, in this study we systematically analyzed the same critical positions of AT1 receptor, Tyr113, Tyr184, Lys199, His256 and Gln257, which may commonly bind to ARBs according to previous reports [5-16], using a mutagenesis study, and subsequently performed computational modeling of the binding mode between AT1 receptor and ARBs using human C-X-C chemokine receptor type 4 (CXCR4) receptor as a new template [25] and a single version of software. We confirmed here that the slightly different structures of ARBs are critical for unique modes of binding to AT1 receptor.
Materials and Methods
Materials
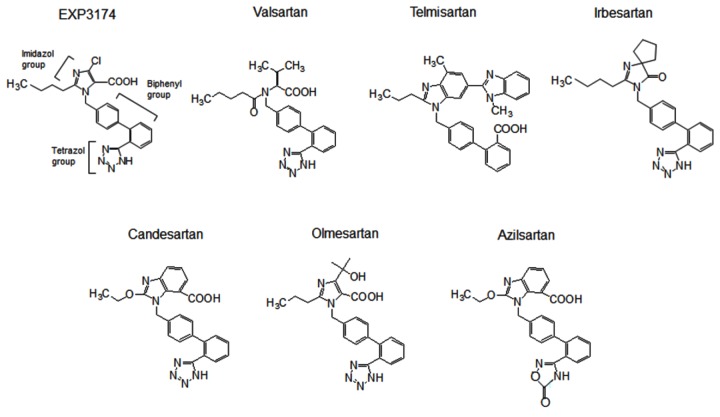
The following reagents were purchased or provided: EXP3174, candesartan, valsartan, telmisartan, irbesartan and azilsartan (Toronto Research Chemicals Inc., Ontario, Canada); olmesartan (Daiichi Sankyo Co., Tokyo, Japan); [Sar1, Ile8]Ang II (Sigma-Aldrich, MO, USA); and 125I-[Sar1, Ile8] Ang II (Amersham Biosciences, Buckinghamshire, UK). The molecular structures of the ARBs are shown in Figure 1.
Figure 1. Chemical structures of the angiotensin II type 1 receptor blockers EXP3174, which is an active metabolite of losartan, candesartan, valsartan, telmisartan, olmesartan, irbesartan and azilsartan.
Site-directed mutagenesis and expression of the AT1 receptor and membrane preparation
The synthetic rat AT1-wild-type (WT) receptor gene, cloned in the shuttle expression vector pMT-3, was used for expression and site-directed mutagenesis studies, as described previously [26].
Cell cultures, transfections, membrane preparation
COS1 cells (African green monkey kidney fibroblast-like cell line, #CRL-1650, American Type Culture Collection, VA, USA) were cultured. The cells were maintained in 10 % fetal bovine serum and penicillin- and streptomycin-supplemented Dulbecco’s modified Eagle’s essential medium (Invitrogen) in 5 % CO2 at 37°C. In the experiments, cells without cell-growth supplement were used. The WT and mutant AT1 receptors were transiently transfected into COS1 cells using Lipofectamine 2000 liposomal reagent (Roche Applied Science) according to the manufacturer’s instructions. Cell membranes were prepared by the nitrogen Parr bomb disruption method using a 0.25 M sucrose solution in the presence of protease inhibitors.
Competition binding study
The Kd values of receptor binding were determined by 125I-[Sar1, Ile8]Ang II-binding experiments under equilibrium conditions, as described previously [26]. Membranes expressing the AT1-WT or mutant receptors were incubated with 100 pM 125I-[Sar1, Ile8], [Sar1, Ile8]Ang II or ARBs for 1 h at 22 °C in a volume of 125 μl. The binding reaction was terminated by filtering the incubation mixture through Whatman GF/C glass filters, and the residues were extensively washed further with binding buffer. The bound ligand fraction was determined from the counts per minute (cpm) remaining on the membrane. Binding kinetics values were determined as previously described [26].
Molecular Modeling of the AT1 Receptor
Unless otherwise noted, all analyses were performed using Prime, version 3.0 (Schrödinger, LLC, New York, NY, 2011). The X-ray crystal structure of human CXCR4 (Protein Data Bank code 3OE0) was used as the template for the AT1 receptor model. The primary sequence of AT1 receptor was obtained from the Swiss-Prot Protein Database (AGTR1_HUMAN, P30556) and aligned with that of the CXCR4 structure by considering the sequence identity, secondary structure prediction results and typical consensus sequence of GPCRs (disulfide bond between transmembrane (TM) 3 and extracellular loop (ECL) 2, LAxAD in TM2, DRY in TM3, [F/Y]xxPxxxxxxxY in TM5 and (C)WxP in TM6). Using this alignment, we built a comparative homology model of the AT1 receptor from the X-ray structure of CXCR4. Ligand and the T4-Lysozyme region (1102-1161) in the CXCR4 structure were removed before modeling. The protonation state of His256 of the model was modified manually to the epsilon position. The final AT1 receptor model had 289 amino acids (15-303) and 31 % sequence identity with the template. The stereochemical quality of the model was assessed by PROCHECK analysis [27].
Induced fit docking (IFD) of olmesartan
The ligand 3D structure of olmesartan was generated by LigPrep version 2.5 (Smchrödinger, LLC, New York, NY, 2011). The ligand was docked into the AT1 receptor model using the Schrödinger Suite 2011 IFD protocol. The VDW scaling factors for both the ligand and the protein were set to 0.3 and Lys199 in TM5 was specified as the ligand binding site. All other parameters were at the default setting.
Thirteen models were obtained as a result of induced fit docking. The interaction fingerprints [28] were calculated for the models by using the Python script bundled in Schrödinger Suite 2011. Using this information, we chose the best model that satisfied polar interactions between the ligand and all four of the important residues (Tyr113, Lys199, His256, Gln257) in the AT1 receptor suggested by the mutation study. Energy minimization was performed with the model using MacroModel, version 9.9 (Schrödinger, LLC, New York, NY, 2011). The force field was set to OPLS2005 with the GB/SA water solvation model and all atoms of the protein backbone were fixed during minimization. The final AT1 receptor model with olmesartan was obtained by the Polak-Ribier Conjugate Gradient method with a convergence threshold of 0.005 for less than 5000 iterations. We used the AT1 receptor model as the docking template for the various ARBs as described below.
Docking of various ARBs to the AT1 receptor model
The 7 ARBs (candesartan, EXP3174, irbesartan, olmesartan, telmisartan, valsartan, azilsartan) were docked into the AT1 receptor model by using Glide, version 5.7 (Schrödinger, LLC, New York, NY, 2011) with the hydrogen bond constraint to Lys199. Extra precision mode was used for the docking studies, except the irbesartan. Standard precision mode was used for irbesartan since no poses were obtained with the XP mode. The docking poses that had the best glide score for each ARB were selected as the final model.
Results
Binding affinities of [Sar1, Ile8]Ang II and ARBs to AT1-WT and mutant receptors
First, we selected the candidate residues (Tyr113, Tyr184, Lys199, His256 and Gln257) for specific binding sites of ARBs based on the molecular models of the AT1 receptor complex described in previous reports [5-16]. As shown in Table S1, the binding affinities of [Sar1, Ile8]Ang II and ARBs to AT1-WT and mutant receptors were performed in the present study and our previous studies [8-10,12,16]. The ratio of Kd (mutant) to Kd (WT) [Kd (mutant)/Kd (WT)] were calculated.
First, the binding affinities of [Sar1, Ile8]Ang II and ARBs to AT1-WT were performed, and we confirmed that the data of binding affinities in the present study and our previous studies [8-10,12,16] were similar. The affinities of [Sar1, Ile8]Ang II were almost the same in some mutants and were decreased in other mutants, but not to less than 1/5 the affinity for the AT1-WT receptor.
The affinity of EXP3174 was reduced by more than 10-fold in Y113A, K199A and Q257A mutant receptors compared to the AT1-WT receptor, suggesting that Tyr113, Lys199 and Gln257 in the AT1 receptor are involved in binding to EXP3174. Similar to EXP3174, valsartan, candesartan and azilsartan showed a more than 10-fold loss of affinity in Y113A, K199A and Q257A mutant receptors compared to the AT1-WT receptor, which indicated that Tyr113, Lys199 and Gln257 in the AT1 receptor are also involved in binding to the 4 ARBs which have a carboxyl group as a common chemical structure.
Telmisartan (13-fold reduction) and irbesartan (27-fold reduction) only exhibited a >10-fold reduction in binding affinity to the Y113A mutant receptor. These ARBs retained their binding affinities toward other mutant receptors (<10-fold reduction). Telmisartan and irbesartan do not contain a carboxyl group and instead have a benzimidazole portion and a cyclopentyl group, respectively.
Among the remaining ARBs, olmesartan, which contains a hydroxyl group in addition to a carboxyl group, showed a >10-fold reduction in binding affinity to the Y113F (23-fold reduction), K199A (60-fold reduction), K199Q (14-fold reduction), H256A (16-fold reduction), and Q257A (98-fold reduction) mutant receptors. Olmesartan may bind to Tyr113, Lys199, His256 and Gln257 in the AT1 receptor.
Binding affinities of telmisartan to other AT1 mutant receptors
Ohno et al. reported that all of the ARBs interacted with Phe171, Phe182, Tyr184, Lys199 and His256, and telmisartan possessed unique additional interaction sites that could bind to hydrophobic residues (Val116, Phe204, Phe208 and Trp253) [14]. Since they indicated that the binding mode of telmisartan is much different than those of other ARBs, we performed an additional site-directed mutagenesis study. At these positions in the AT1 receptor, we constructed AT1 mutant receptors, i.e., V116A, F182A, Y184A, F208A and W253A (Table S1). The affinities of [Sar1, Ile8]Ang II were almost the same in the mutants (<3-fold reduction). The positions Val116, Phe182, Tyr184, Phe208 and Trp253 are all not important for binding to telmisartan because telmisartan retained its binding affinity against the mutant receptors, i.e., V116A, F182A, Y184A, F208A and W253A (<2-fold reduction).
Molecular models of the interactions between ARBs and the AT1 receptor
Next, molecular modeling of the binding modes of AT1 receptor and ARBs was performed. Previous studies performed computational modeling using different crystal structures of the receptor as a template, mainly bovine rhodopsin [5-16]. We used the recently published structure of the CXCR4 receptor as a new template [25]. The position of ECL2 in the model of AT1 receptor with bovine rhodopsin as a template was much different from the position of Tyr184 with CXCR4 receptor (Figure 2A). The position of TM5 in the AT1 receptor was also different.
Figure 2. A. Position of the second extracellular loop (ECL2) in the model of AT1 receptor using bovine rhodopsin (orange) or CXCR4 (yellow) receptor as a template.
B. Molecular modeling of the interaction between olmesartan and the AT1 receptor. The AT1 receptor is shown as a ribbon and Tyr113, Lys199, His256, Gln257 and olmesartan are shown as stick models. Color notation in the helix is as follows: transmembrane (TM)1, orange; TM3, yellow; TM5, dark green; TM6, blue; and TM7, purple.
For modeling, IFD was performed using the AT1 receptor model and olmesartan among the 7 kinds of ARBs. Since there are a lot of different modes of binding between olmesartan and AT1 receptor, it is generally difficult to classify them. A couple of binding modes were selected among the different modes, since olmesartan binds to 4 sites in the AT1 receptor (Tyr113, Lys199, His256 and Gln257) as suggested from the mutation experiment. Among a couple of modes, the mode of binding that showed the highest IFD score was selected (Figure 2B). The interactions between Tyr113 in the AT1 receptor and the hydroxyl group of olmesartan (distance of 3.0 Å), between Lys199 and the carboxyl (2.7 Å) or tetrazole (3.3 Å) group, and between His256 (3.1 Å) or Gln257 (3.1 Å) and the tetrazole group were examined. These are reasonable distances for contributing to electrostatic and/or hydrogen bond interactions.
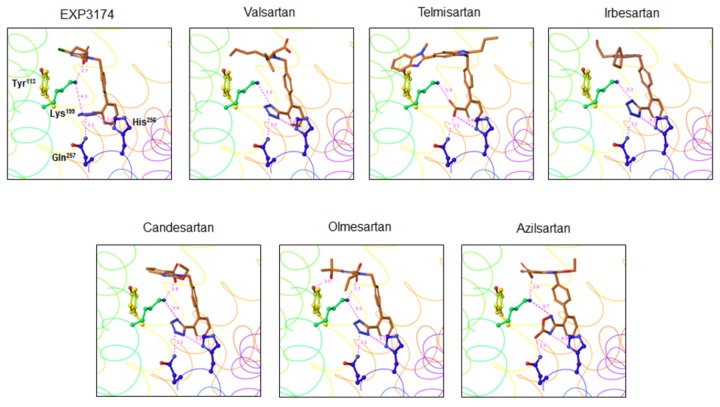
As shown in Figures 3 and 4, we also performed molecular modeling of the modes of binding between the remaining 6 ARBs and AT1 receptor based on the mode of binding between olmesartan and AT1 receptor (Figure 2B). A tetrazole group, which is a common chemical structure of ARBs, binds similarly to Lys199, His256 and Gln257 in the AT1 receptor. This tetrazole group is replaced by a carboxyl group and 5-oxo-1,2,4-oxadiazole in telmisartan and azilsartan, respectively. Lys199 in the AT1 receptor binds to the carboxyl groups in EXP3174, candesartan and azilsartan. Although valsartan also contains a carboxyl group, this carboxyl group did not interact with Lys199 and may bind to Ser105 in the AT1 receptor. Instead, oxygen of the amidecarbonyl group of valsartan may bind to Lys199.
Figure 3. Molecular modeling of the interactions between the 7 ARBs and the AT1 receptor.
Color notation in the helix is as follows: transmembrane (TM)1, orange; TM2, dark yellow; TM3, yellow; TM4, green; TM5, dark green; TM6, blue; and TM7, purple.
Figure 4. Molecular modeling of a close-up view of the interactions between the 7 ARBs and the AT1 receptor.
Color notation in the helix is as follows: transmembrane (TM)1, orange; TM3, yellow; TM5, dark green; TM6, blue; and TM7, purple.
Telmisartan and irbesartan do not contain a carboxyl group, and instead have a benzimidazole portion and a cyclopentyl group, respectively. The benzimidazole portion of telmisartan may bind to a lipophilic pocket that includes Tyr113 in the AT1 receptor. On the other hand, the n-butyl group of irbesartan may bind to Tyr113 according to the present modeling, although the cyclopentyl group of irbesartan should interact with the lipophilic pocket includes Tyr113 [29]. Instead, the cyclopentyl group of irbesartan may bind to Phe182, Leu195 and Lys199.
Discussion
In the present study, we confirmed that the slightly different structures of ARBs are important for unique modes of binding to AT1 receptor using mutagenesis-guided docking to AT1 receptor model based on CXCR4 receptor [25] as a new template and a single version of software. Although the tetrazole group (which is replaced by a carboxyl group in telmisartan and a 5-oxo-1,2,4-oxadiazole group in azilsartan) in ARBs commonly binds to Lys199, His256 and Gln257 in the AT1 receptor, distinctly different modifications of the biphenyl-tetrazolyl-imidazole scaffold lead to different modes of binding observed.
There are several reasons why we used CXCR4 receptor as a template. First, AT1 receptor and CXCR4 receptor show about 34 % homology, which is much higher than that between AT1 receptor and bovine rhodopsin (about 20 %), which has been used in most previous studies [5-16]. Second, in the GPCR evolution tree [30,31], the AT1 receptor is closer to the CXCR4 receptor, but far from rhodopsin. In this study, although we systematically analyzed critical positions of the AT1 receptor, i.e., Tyr113, Tyr184, Lys199, His256 and Gln257, the position of Tyr184 in ECL2 with bovine rhodopsin as a template was much different than the position of Tyr184 in the AT1 receptor using CXCR4 receptor as a template. The position of Tyr184 with CXCR4 receptor as a template is far from the ligand binding pocket of AT1 receptor. Although ECL2 is critical for receptor activation when Ang II binds to and changes the conformation of ECL2 [32,33], none of the ARBs can bind to Tyr184 in ECL2. In fact, none of the ARBs showed a loss of binding affinity in the Y184A mutant AT1 receptor in our study.
The main purpose of the present study was to confirm that the slightly different structures of ARBs may be important for binding to AT1 receptor and for the formation of unique modes of binding, although a tetrazole group in ARBs commonly binds to Lys199, His256 and Gln257 in the AT1 receptor. Our current understanding is that the hydroxyl and carboxyl groups of olmesartan interact with Tyr113 and Lys199, respectively. We previously indicated that the inverse agonist activity of olmesartan required two interactions that between the hydroxyl group of olmesartan and Tyr113 in the receptor and that between the carboxyl group and Lys199 and His256 in the receptor [8]. We confirmed that the hydroxyl and carboxyl groups in olmesartan were critical for binding. On the other hand, the carboxyl groups of EXP3174, candesartan and azilsartan bind to Lys199; these three ARBs do not contain a hydroxyl group and may not interact with Tyr113. In addition, valsartan also contains a carboxyl group, which does not bind to Lys199. Instead, oxygen of the amidecarbonyl group and the carboxyl group of valsartan may bind to Lys199 and Ser105, respectively, since we had reported that Ser105 is a candidate for binding to the carboxyl group of valsartan [9]. Thus, each ARB showed a slightly different mode of binding with regard to Tyr113 and Lys199 in the AT1 receptor.
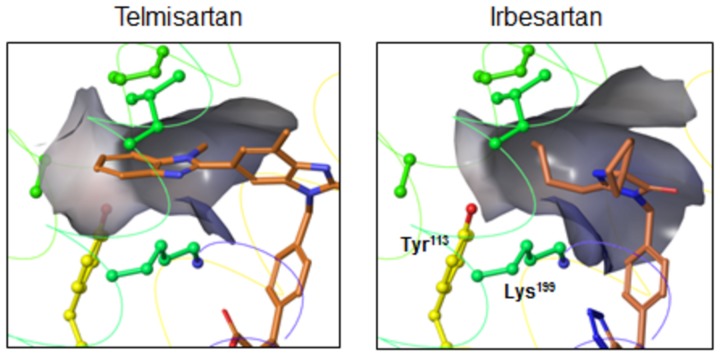
Since telmisartan and irbesartan do not contain an imidazole ring with a carboxyl group, these ARBs should be considered separately from the other ARBs which do contain a carboxyl group. According to results of modeling, the benzimidazole portion of telmisartan may bind to a lipophilic pocket that includes Tyr113 by the hydrophobic effect, such as van der Waals forces (Figure 5). Ohno et al. also reported that the benzimidazole portion of telmisartan may interact with the lipophilic pocket in a model of the AT1 receptor [14]. Our site-directed mutagenesis study indicated that Val116, Phe182, Tyr184, Phe208 and Trp253 in addition to Lys199, His256 and Gln257 are not important for binding to telmisartan. In contrast, the data suggest that the benzimidazole portion of telmisartan may be more important for binding to the lipophilic pocket of AT1 receptor. The present results showed that the n-butyl group of irbesartan may bind to Tyr113 (Figure 5). We previously reported that the phenyl group at Tyr113 in the AT1 receptor and the cyclopentyl group of irbesartan may exhibit hydrophobic interaction [12]. In fact, the cyclopentyl group should move toward Tyr113 to slightly move the protein side chains, but because the chains are fixed in the docking studies, steric interference may occur and thus the correct original position cannot be obtained in the present modeling.
Figure 5. Possible molecular modeling of the interactions between a lipophilic pocket (gray zone) in the AT1 receptor and telmisartan or irbesartan.
Color notation in the helix is as follows: transmembrane (TM)3, yellow; TM5, dark green; and TM6, blue.
In conclusion, we confirmed that the slightly different structures of ARBs may be critical for binding to AT1 receptor and for the formation of unique modes of binding.
Supporting Information
Binding affinities (Kd, nM) of [Sar1, Ile8]Ang II and ARBs to AT1 WT and mutants receptors.
(DOCX)
Acknowledgments
We thank S. Tomita for providing excellent technical assistance.
Funding Statement
KS and SM received funding from Daiichi-Sankyo Co. Ltd. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Miura S, Imaizumi S, Saku K (2013) Recent progress in molecular mechanisms of angiotensin II type 1 and 2 receptors. Curr Pharm Des 19: 2981-2987. doi: 10.2174/1381612811319170002. PubMed: 23176209. [DOI] [PubMed] [Google Scholar]
- 2. De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology XXIII. Angiotensin II receptors. Pharmacol Rev 52: 415-72 [PubMed] [Google Scholar]
- 3. Miura S, Karnik SS, Saku K (2011) Angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst 12: 1-7. doi: 10.1177/1470320310370852. PubMed: 20603272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imaizumi S, Miura S, Yahiro E, Uehara Y, Komuro I et al. (2013) Retrieved onpublished at whilst December year 1111. from Class- and molecule-specific differential effects of angiotensin II type 1 receptor blockers. Curr Pharm Des 19: 3002-3008. [DOI] [PubMed] [Google Scholar]
- 5. Noda K, Saad Y, Kinoshita A, Boyle TP, Graham RM et al. (1995) Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms. J Biol Chem 270: 2284-2289. doi: 10.1074/jbc.270.5.2284. PubMed: 7530721. [DOI] [PubMed] [Google Scholar]
- 6. Takezako T, Gogonea C, Saad Y, Noda K, Karnik SS (2004) "Network leaning" as a mechanism of insurmountable antagonism of the angiotensin II type 1 receptor by non-peptide antagonists. J Biol Chem 279: 15248-15257. doi: 10.1074/jbc.M312728200. PubMed: 14754891. [DOI] [PubMed] [Google Scholar]
- 7. Tuccinardi T, Calderone V, Rapposelli S, Martinelli A (2006) Proposal of a new binding orientation for non-peptide AT1 antagonists: homology modeling, docking and three-dimensional quantitative structure-activity relationship analysis. J Med Chem 49: 4305-4316. doi: 10.1021/jm060338p. PubMed: 16821790. [DOI] [PubMed] [Google Scholar]
- 8. Miura S, Fujino M, Hanzawa H, Kiya Y, Imaizumi S et al. (2006) Molecular mechanism underlying inverse agonist of angiotensin II type 1 receptor. J Biol Chem 281: 19228-19295. PubMed: 16690611. [DOI] [PubMed] [Google Scholar]
- 9. Miura S, Kiya Y, Kanazawa T, Imaizumi S, Fujino M et al. (2008) Differential bonding interactions of inverse agonists of angiotensin II type 1 receptor in stabilizing the inactive state. Mol Endocrinol 22: 139-146. PubMed: 17901125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y et al. (2008) Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep 9: 179-186. doi: 10.1038/sj.embor.7401157. PubMed: 18202720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhuiyan MA, Ishiguro M, Hossain M, Nakamura T, Ozaki M et al. (2009) Binding sites of valsartan, candesartan and losartan with angiotensin II receptor 1 subtype by molecular modeling. Life Sci 85: 136-140. doi: 10.1016/j.lfs.2009.05.001. PubMed: 19446572. [DOI] [PubMed] [Google Scholar]
- 12. Fujino M, Miura S, Kiya Y, Tominaga Y, Matsuo Y et al. (2010) A small difference in the molecular structure of AT1 receptor blockers induces AT1 receptor-dependent and -independent beneficial effects. Hypertens Res 33: 1044-1052. doi: 10.1038/hr.2010.135. PubMed: 20668453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sokkar P, Mohandass S, Ramachandran M (2011) Multiple templates-based homology modeling enhances structure quality of AT1 receptor: validation by molecular dynamics and antagonist docking. J Mol Model 17: 1565-1577. doi: 10.1007/s00894-010-0860-z. PubMed: 20924625. [DOI] [PubMed] [Google Scholar]
- 14. Ohno K, Amano Y, Kakuta H, Niimi T, Takakura S et al. (2011) Unique "delta lock" structure of telmisartan is involved in its strongest binding affinity to angiotensin II type 1 receptor. Biochem Biophys Res Commun 404: 434-437. doi: 10.1016/j.bbrc.2010.11.139. PubMed: 21130741. [DOI] [PubMed] [Google Scholar]
- 15. Miura S, Kiya Y, Hanzawa H, Nakao N, Fujino M et al. (2012) Small molecules with similar structures exhibit agonist, neutral antagonist or inverse agonist activity toward angiotensin II type 1 receptor. PLOS ONE 7: e37974. doi: 10.1371/journal.pone.0037974. PubMed: 22719858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miura S, Okabe A, Matsuo Y, Karnik SS, Saku K (2013) Unique binding behavior of the recently approved angiotensin II receptor blocker azilsartan compared to that of candesartan. Hypertens Res 36: 134-139. doi: 10.1038/hr.2012.147. PubMed: 23034464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H et al. (2000) Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289: 739-744. doi: 10.1126/science.289.5480.739. PubMed: 10926528. [DOI] [PubMed] [Google Scholar]
- 18. Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N et al. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455: 497-502. doi: 10.1038/nature07330. PubMed: 18818650. [DOI] [PubMed] [Google Scholar]
- 19. Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP (2008) Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454: 183-187. doi: 10.1038/nature07063. PubMed: 18563085. [DOI] [PubMed] [Google Scholar]
- 20. Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS et al. (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318: 1258-1265. doi: 10.1126/science.1150577. PubMed: 17962520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS et al. (2007) GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318: 1266-1273. doi: 10.1126/science.1150609. PubMed: 17962519. [DOI] [PubMed] [Google Scholar]
- 22. Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D et al. (2011) Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature 469: 236-240. doi: 10.1038/nature09665. PubMed: 21228876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P et al. (2011) Structure of a nanobody-stabilized active state of the beta (2) adrenoceptor. Nature 469: 175-180. doi: 10.1038/nature09648. PubMed: 21228869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC et al. (2011) The structural basis for agonist and partial agonist action on a beta (1)-adrenergic receptor. Nature 469: 241-244. doi: 10.1038/nature09746. PubMed: 21228877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu B, Chien EY, Mol CD, Fenalti G, Liu W et al. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330: 1066-1071. doi: 10.1126/science.1194396. PubMed: 20929726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miura S, Feng YH, Husain A, Karnik SS (1999) Role of aromaticity of agonist switches of angiotensin II in the activation of the AT1 receptor. J Biol Chem 274: 7103-7110. doi: 10.1074/jbc.274.11.7103. PubMed: 10066768. [DOI] [PubMed] [Google Scholar]
- 27. Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26: 283-291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- 28. Deng Z, Chuaqui C, Singh J (2004) Structural interaction fingerprint (SIFt): a novel method for analyzing three-dimensional protein-ligand binding interactions. J Med Chem 47: 337-344. doi: 10.1021/jm030331x. PubMed: 14711306. [DOI] [PubMed] [Google Scholar]
- 29. Fujino M, Miura S, Kiya Y, Tominaga Y, Matsuo Y et al. (2010) A small difference in the molecular structure of angiotensin II receptor blockers induces AT1 receptor-dependent and -independent beneficial effects. Hypertens Res 33: 1044-1052. doi: 10.1038/hr.2010.135. PubMed: 20668453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kufareva I, Rueda M, Katritch V, Stevens RC, Abagyan R et al. (2011) Status of GPCR modeling and docking as reflected by community-wide GPCR Dock 2010 assessment. Structure 19: 1108-1126. doi: 10.1016/j.str.2011.05.012. PubMed: 21827947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stevens RC, Cherezov V, Katritch V, Abagyan R, Kuhn P et al. (2013) The GPCR Network: a large-scale collaboration to determine human GPCR structure and function. Nat Rev Drug Discov 12: 25-34. PubMed: 23237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Unal H, Jagannathan R, Bhat MB, Karnik SS (2010) Ligand-specific conformation of extracellular loop-2 in the angiotensin II type 1 receptor. J Biol Chem 285: 16341-16350. doi: 10.1074/jbc.M109.094870. PubMed: 20299456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Unal H, Jagannathan R, Bhatnagar A, Tirupula K, Desnoyer R et al. (2013) Long range effect of mutations on specific conformational changes in the extracellular loop 2 of angiotensin II type 1 receptor. J Biol Chem 288: 540-551. doi: 10.1074/jbc.M112.392514. PubMed: 23139413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Binding affinities (Kd, nM) of [Sar1, Ile8]Ang II and ARBs to AT1 WT and mutants receptors.
(DOCX)