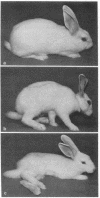
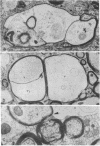
Abstract
Eosinophils contain a substance that is neurotoxic when injected intracerebrally or intrathecally into laboratory animals—an effect known as the “Gordon phenomenon.” We found neurotoxic activity in eosinophils from three patients with eosinophilic syndromes by injecting cell preparations into rabbits and guinea pigs. These animals developed a syndrome of muscular rigidity and ataxia, progressing to severe paralysis. No neurotoxic activity was found in preparations of polymorphonuclear or mononuclear leukocytes from normal donors. Examination of the brains of affected animals confirmed widespread loss of Purkinje cells, as described by earlier investigators. A new finding was severe spongy change occurring in the white matter of the cerebellum, brainstem, and spinal cord. Electron microscopic examination showed that vacuoles formed within the myelin sheaths of axons by separation of lamellae. Associated axonal degeneration was common and was also seen occasionally in peripheral nerves. Gray matter in the cerebral hemispheres and spinal cord was normal. This eosinophil-derived neurotoxin was partially purified by ultracentrifugation of sonicated eosinophils and fractionation of the supernate by gel filtration. Fractions with neurotoxic activity eluted at a position consistent with a molecular weight of approximately 15,000. The neurotoxic activity of this material withstood lyophilization and dialysis but was destroyed by heating to 90°C. Injection of eosinophil-derived neurotoxin into laboratory animals may provide a useful short-term experimental model for study of mechanisms of damage to myelinated nerve fibers. The clinical significance of the Gordon phenomenon has yet to be established.
Keywords: Gordon phenomenon, leukocytes, white matter, Purkinje cells
Full text
PDF
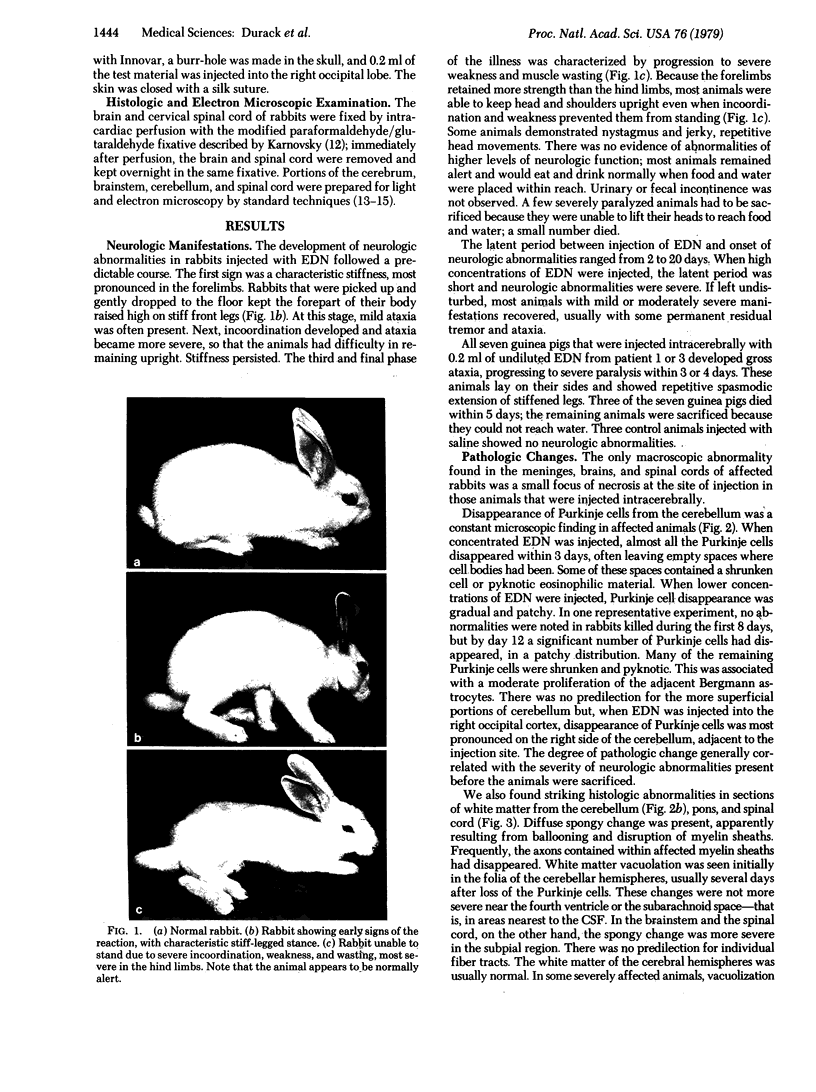

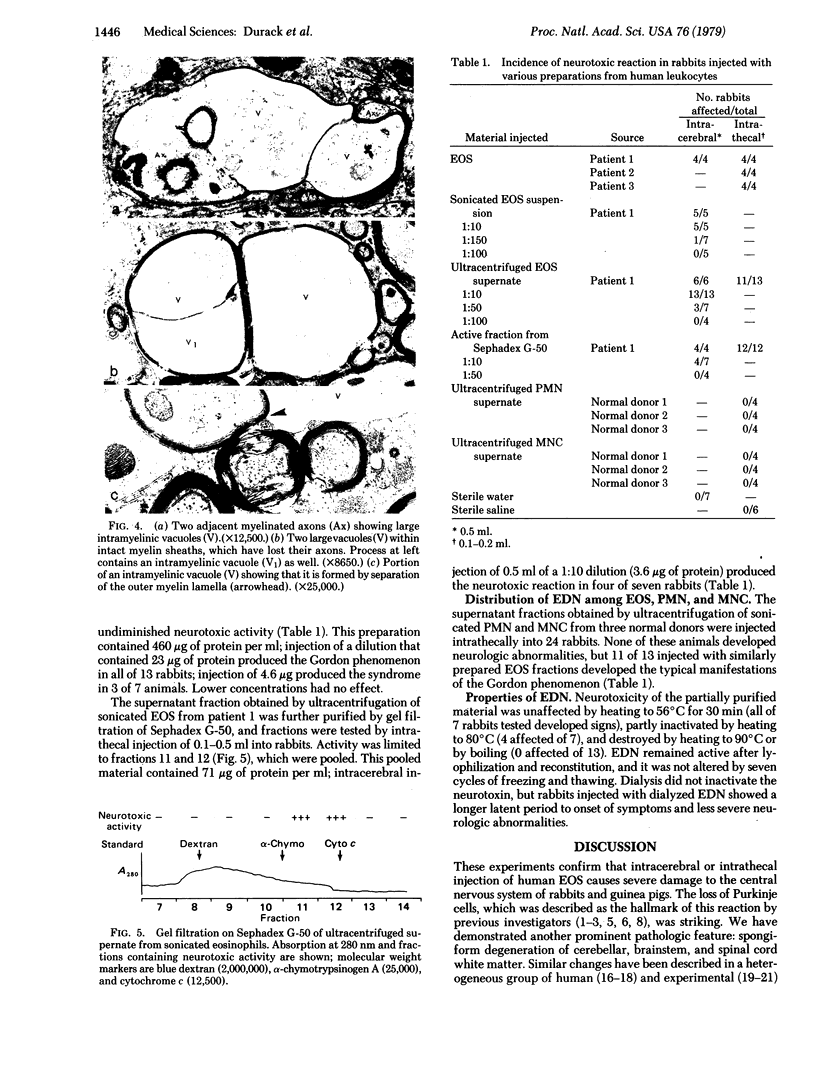

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Schneck L., Cara J., Volk B. W. Spongy degeneration of the central nervous system (van Bogaert and Bertrand type; Canavan's disease). A review. Hum Pathol. 1973 Sep;4(3):331–347. doi: 10.1016/s0046-8177(73)80098-x. [DOI] [PubMed] [Google Scholar]
- Aleu F. P., Katzman R., Terry R. D. Fine structure and electrolyte analyses of cerebral edema induced by alkyl tin intoxication. J Neuropathol Exp Neurol. 1963 Jul;22(3):403–413. doi: 10.1097/00005072-196307000-00003. [DOI] [PubMed] [Google Scholar]
- Benvenisti D. S., Ultmann J. E. Eosinophilic leukemia. Report of five cases and review of literature. Ann Intern Med. 1969 Oct;71(4):731–745. doi: 10.7326/0003-4819-71-4-731. [DOI] [PubMed] [Google Scholar]
- Carlton W. W., Kreutzberg G. Isonicotinic acid hydrazide-induced spongy degeneration of the white matter in the brains of Pekin ducks. Am J Pathol. 1966 Jan;48(1):91–105. [PMC free article] [PubMed] [Google Scholar]
- Chusid M. J., Dale D. C., West B. C., Wolff S. M. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975 Jan;54(1):1–27. [PubMed] [Google Scholar]
- Kelser R. A., King L. S. Studies of a Paralysis Syndrome Produced in Rabbits and Guinea Pigs by Extracts of Normal Primate Bone Marrow. Am J Pathol. 1936 May;12(3):317–332.3. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Schochet S. S. Electron microscopic observations on experimental spongy degeneration of the cerebellar white matter. J Neuropathol Exp Neurol. 1968 Apr;27(2):210–220. doi: 10.1097/00005072-196804000-00003. [DOI] [PubMed] [Google Scholar]
- Lampert P., O'Brien J., Garrett R. Hexachlorophene encephalopathy. Acta Neuropathol. 1973;23(4):326–333. doi: 10.1007/BF00687462. [DOI] [PubMed] [Google Scholar]
- MEYER J. S., FOLEY J. M. The encephalopathy produced by extracts of eosinophils and bone marrow. J Neuropathol Exp Neurol. 1953 Oct;12(4):349–362. doi: 10.1097/00005072-195312040-00002. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell H., Swarner O., Gluck L., Lampert P. Hexachlorophene myelinopathy in premature infants. J Pediatr. 1973 Jun;82(6):976–981. doi: 10.1016/s0022-3476(73)80428-7. [DOI] [PubMed] [Google Scholar]
- Schwartz A. M., Lapham L. W., van den Noort S. Cytologic and cytochemical studies of neuroglia. IV. Experimentally induced protoplasmic astrocytosis in the Bergmann glia of cerebellum. Neurology. 1966 Nov;16(11):1118–1126. doi: 10.1212/wnl.16.11.1118. [DOI] [PubMed] [Google Scholar]
- Seiler G., Westerman R. A., Wilson J. A. The role of specific eosinophil granules in eosinophil-induced experimental encephalitis. Neurology. 1969 May;19(5):478–488. doi: 10.1212/wnl.19.5.478. [DOI] [PubMed] [Google Scholar]
- Snead O. C., 3rd, Kalavsky S. M. Cerebrospinal fluid eosinophilia. A manifestation of a disorder resembling multiple sclerosis in childhood. J Pediatr. 1976 Jul;89(1):83–84. doi: 10.1016/s0022-3476(76)80935-3. [DOI] [PubMed] [Google Scholar]
- TERPLAN K., KRAUS R., BARNES S. Eosinophilic meningo-encephalitis, with predominantly cerebellar changes caused by Trichinella infection. J Mt Sinai Hosp N Y. 1957 Nov-Dec;24(6):1293–1309. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yii C. Y. Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg. 1976 Mar;25(2):233–249. doi: 10.4269/ajtmh.1976.25.233. [DOI] [PubMed] [Google Scholar]