Abstract
OBJECTIVE
To describe the methods for assigning the cause of death for stillbirths enrolled in the Stillbirth Collaborative Research Network (SCRN).
METHODS
A complete evaluation, including postmortem examination, placental pathology, medical record abstraction, and maternal interview was available on 512 stillbirths among 500 women. These 512 stillbirths were evaluated for cause of death using the definitions outlined in this report. Using the best available evidence, SCRN investigators developed a new methodology to assign the cause of death of stillbirths using clinical, postmortem, and placental pathology data. This new tool, designated the Initial Causes of Fetal Death, incorporates known causes of death and assigns them as possible or probable based on strict diagnostic criteria, derived from published references and pathophysiologic sequences that lead to stillbirth.
RESULTS
Six broad categories of causes of death are accounted for, including maternal medical conditions; obstetric complications; maternal or fetal hematologic conditions; fetal genetic, structural, and karyotypic abnormalities; placental infection, fetal infection, or both; and placental pathologic findings. Isolated histologic chorioamnionitis and small for gestational age were not considered causes of death.
CONCLUSION
A new system, Initial Causes of Fetal Death, to assign cause of death in stillbirths was developed by the SCRN investigators for use in this study but has broader applicability. Initial Causes of Fetal Death is a standardized method to assign probable and possible causes of death of stillbirths based on information routinely collected during prenatal care and the clinical evaluation of fetal death.
The Stillbirth Collaborative Research Network (SCRN) is a consortium of five academic health centers, one data coordinating and analysis center, and the Pregnancy and Perinatology Branch at the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD). The Stillbirth Collaborative Research Network has conducted a population-based study of stillbirth with the specific aims being 1) to obtain a geographic population-based determination of the incidence of stillbirth defined as fetal death at 20 or more weeks of gestation; 2) to determine the causes of stillbirth using a standard stillbirth postmortem protocol to include review of clinical history, protocols for postmortem and pathologic examinations of the fetus and placenta, and other postmortem testing; and 3) to elucidate risk factors for stillbirth. Between March 2006 and August 2008, the SCRN investigators recruited and enrolled women who had stillbirth (defined as fetal death at 20 or more weeks of gestation) while residing in five geographically defined regions. Women were recruited in 59 hospitals, including academic health centers and community hospitals. These geographically defined areas included the counties of Bexar, Brazoria, and Galveston in Texas; Salt Lake County in Utah; DeKalb County in Georgia; Bristol County in Massachusetts; and the entire state of Rhode Island.
A major aim of SCRN is to determine the cause of fetal death using the best available evidence including all available clinical and pathologic data. For the more than 500 stillborn neonates enrolled in this study on whom a complete postmortem examination was performed, we sought clinical, pathologic, and pathophysiologic findings that might be considered the cause of death. To classify these findings, the SCRN investigators developed the following tool to assign a cause of death for stillbirth cases ascertained and enrolled during the course of this study.
MATERIALS AND METHODS
The SCRN Initial Causes of Fetal Death has been devised to provide a structured system so that the definitions used to ascertain the most likely cause of fetal death are uniform and that those reviewing the potential cause of fetal death can communicate using a common language. An important goal of this system is to use the best available evidence and rigorous definitions determined before case review when assigning a cause of death.
Among the 663 women with stillbirths enrolled in the study, 620 delivered a single stillborn neonate, 42 delivered twins (13 sets with 2 stillborn neonates and 29 sets with 1 stillborn and 1 liveborn neonate), and 1 delivered triplets (1 stillborn and 2 liveborn neonates), for a total of 676 stillborn neonates (707 total neonates). A complete evaluation, including postmortem examination, placental pathology, medical record abstraction, and maternal interview was available on 512 stillbirths among 500 women. These 512 stillbirths were evaluated for cause of death using the definitions outlined in this report. One novel aspect of SCRN is that we also recruited and enrolled 1932 women with live births to serve as contemporaneous women in a control group. These women underwent the same study protocol (except the postmortem examination) as women who had stillbirth. Therefore, an important aspect of this system is the ability to consider potential newly discovered causes or risk factors for fetal death. As a result, one of our major goals will be to compare the initial clinical cause of death data with our final cause of death once all data from the study are available.
During the course of the study, causes of death were reported to individual participants as they became evident. Only the finding of carcinoma of the placenta was reported back to control participants. This study was reviewed and approved by the institutional review boards at each of the academic centers, the data coordinating center (RTI International), and all of the participating hospitals.
The SCRN investigators recognize the difficulty of assigning a cause of fetal death with a significant degree of certainty, especially before the availability of all laboratory evaluations. The cause of death can be assigned with certainty in a relatively small proportion of cases (eg, hydrops fetalis due to Rh isoimmunization, direct catastrophic fetal trauma). However, in most cases, defining the precise cause of death is a complex and iterative process. Given the degree of uncertainty in the majority of cases regarding the event or condition that led directly to fetal death, we adopted a hierarchical system wherein a condition that was a potential cause of fetal death was graded as being a present condition, a possible cause of death, or a probable cause of death.
To better define the approach to ascertaining the cause of death, we created subcommittees in specific areas: Surveillance and Epidemiology, Maternal Disease Mechanisms, Genetics, Immunology and Infectious Diseases, and Placenta/Postmortem Evaluation. These subcommittees were charged with reviewing available evidence to develop a working classification system for case reviews of each stillbirth enrolled in SCRN. The goal of Initial Causes of Fetal Death is to provide common definitions of conditions that may be potential causes of death so that the investigators in the study could use a common language in determining the most likely cause of death in each specific case.
As more definitive data come to light during the analytic phase of the SCRN study, we anticipate that Initial Causes of Fetal Death likely will need to be revised, refined, and updated. This current version of Initial Causes of Fetal Death is based on clinically available data, not on the extensive genetic, microbiological, and biochemical data that will be analyzed over the course of the study. Over time, as new tests become available or new questions raised, the SCRN cases will be evaluated for potentially novel causes of stillbirth and Initial Causes of Fetal Death will be modified to reflect these new insights. The original system, as well as any modifications, will be available on the publicly accessible area of the SCRN web site (https://scrn.rti.org).
In the development of this system, each subcommittee reviewed the literature and used individual and group expertise in identifying conditions of interest. A condition of interest is a maternal, fetal, or placental condition that might be a potential cause of fetal death and therefore must be evaluated in relation to a probable or possible cause of death. If, from evidence collected by medical record review, maternal interview, or pathologic evaluation, the condition of interest is present, then the following criteria are used to further classify a condition as a probable or possible cause of death:
-
Probable Cause of Stillbirth.
The identified condition is, with high likelihood, the cause of the fetal death. For example, maternal diabetes would be the probable cause of fetal death for a case in which the mother has type 1 diabetes mellitus with a stillbirth that occurred during an episode of diabetic ketoacidosis.
-
Possible Cause of Stillbirth.
The identified condition cannot with high likelihood be considered the cause of death, but there is reasonable certainty that this condition may be involved in a pathophysiologic sequence that led to the fetal death. For example, a fetal death occurs in a woman with poorly controlled type 1 diabetes mellitus and an elevated hemoglobin A1C with normal fetal growth. A fetal death in this circumstance cannot be definitely considered caused by the diabetes, as there are many other potential conditions that may affect this outcome.
Notably, not all conditions of interest can be further classified as a probable or possible cause of death based on the best available evidence or even expert opinion. In this circumstance, the condition would be considered present. For example, a fetal death case in which the mother had well-controlled gestational diabetes would be identified as “gestational diabetes present,” as there is no evidence to suggest that well-controlled gestational diabetes is a cause of fetal death. In the course of data analysis for SCRN, future studies may indicate that such a condition might be considered a cause of death. In this circumstance, a specific case may be re-coded in the future as having a possible or probable cause of death. For the most part, “present” conditions are potential risk factors for, rather than causes of, stillbirth.
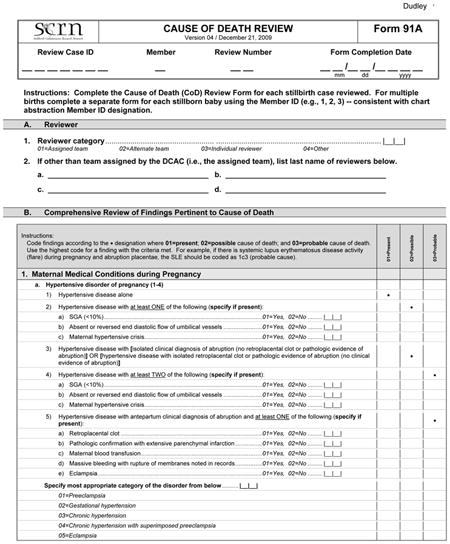
After the SCRN subcommittees created the first version of the Initial Causes of Fetal Death instrument, each case of stillbirth that underwent postmortem examination was reviewed by one of five teams of physicians who have been involved in the study. Each team consisted of two physicians who would independently review the abstracted medical records and autopsy records, along with a narrative of each case including pertinent hospital records and pathology reports. After independent review, the two investigators in each team reviewed the cases together and then completed the Initial Causes of Fetal Death instrument.
After the initial cause of death review, the first version of Initial Causes of Fetal Death required modification to account for new findings and nuances that were not readily evident before performing the initial review. Through this iterative process, all investigators involved in SCRN provided input into Initial Causes of Fetal Death based on the best available scientific evidence. The stillbirth cases were then reviewed a second time in a similar fashion as described, and causes of death assigned as appropriate for each case using the version of Initial Causes of Fetal Death presented in this report. The data from these reviews will be presented in other forthcoming reports.
RESULTS
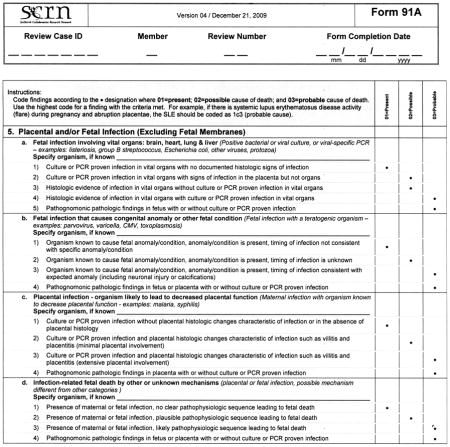
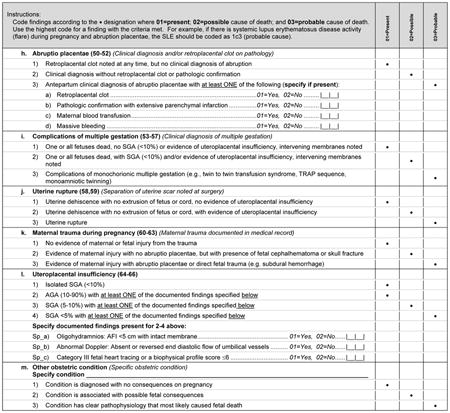
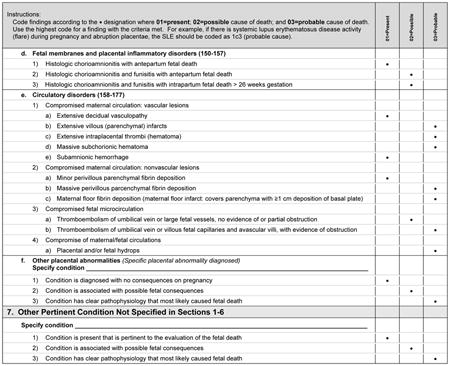
Initial Causes of Fetal Death is divided into six sections, correlating with six general categories identified by the SCRN subcommittees that describe a constellation of findings that could be a potential cause of fetal death. Appendix 1 is an example of the system that we used to assign cause of death in the cases collected during SCRN, in this instance the criteria for assigning fetal death due to maternal or fetal infection (section 5 in Appendix 2). A fully referenced version of Initial Causes of Fetal Death is available in Appendix 2 online at http://links.lww.com/AOG/A186. As can be noted in Appendix 1, we end the section allowing for “other” as a cause of death to so that investigators may expand this list as new conditions are described, characterized, and defined.
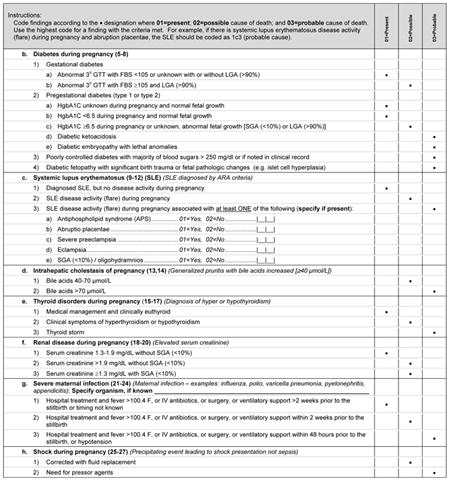
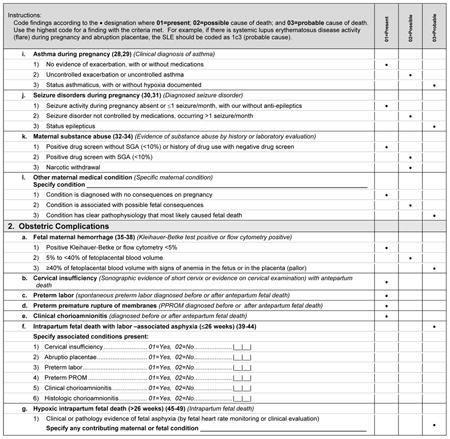
The medical conditions included in Appendix 2 are the most common medical complications during pregnancy. A medical condition described as present requires that the condition be diagnosed using standard criteria (eg, definitions by professional organizations, common definitions established in the medical literature) before the stillbirth or that the condition be diagnosed during the evaluation of the stillbirth. For a condition to be considered a possible cause of stillbirth, literature showing an association of the condition with stillbirth must be present and referenced. For a condition to be considered a probable cause of stillbirth, medical literature showing pathophysiology leading to fetal death must be referenced.
Many noninfectious obstetric conditions can be a direct cause of fetal death. These conditions are not associated with other medical conditions in the mother or fetus and have no other specific known placental or fetal cause.
When considering fetal-maternal hemorrhage (FMH) as a cause of death, we made the following assumptions:
Fetoplacental blood volume=125 mL/kg fetal weight
FMH red cell volume=maternal blood volume×maternal hematocrit×% fetal red blood cells in maternal sample
FMH whole blood volume=(FMH red cell volume)/(fetal hematocrit)
Conditional on these assumptions, we chose to define a FMH of greater than 40% of fetal blood volume before delivery as sufficient to cause fetal death.1
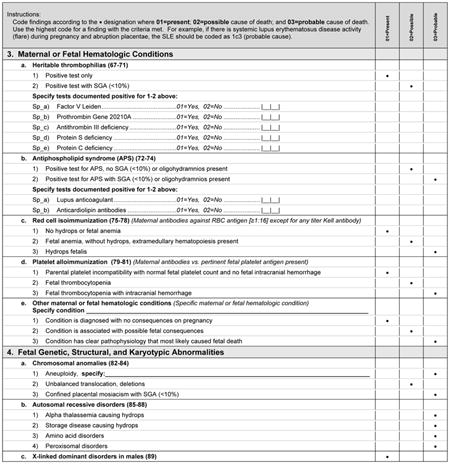
We list specific hematologic conditions separate from the broader category of maternal medical conditions, because they are of contemporary interest as potential causes of fetal death. In addition, many of these conditions represent unique interactions between the mother and fetus via the maternal immune system.
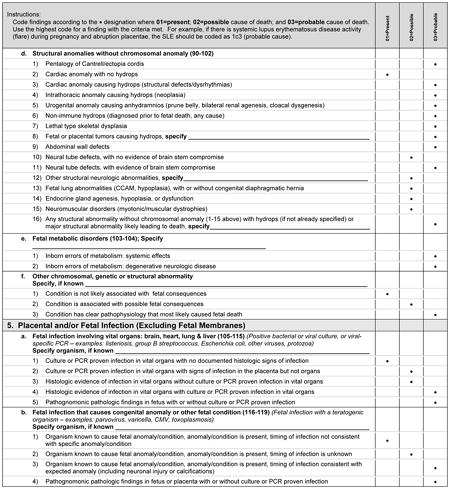
A variety of fetal conditions have been implicated as causes of fetal death. These conditions are intentionally general, as specific abnormalities may be part of a syndrome or sequence such that detailed listing of all associated anomalies would likely be incomplete.
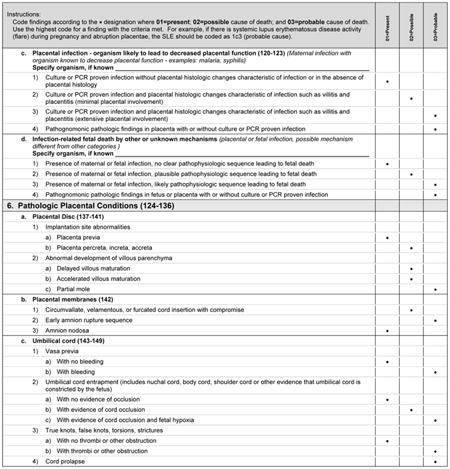
To better clarify the relationship between various infections and stillbirth, we defined three potential mechanisms by which infections are thought to cause stillbirth and provide criteria by which each of these mechanisms may be considered a present condition, or rise to be considered a possible or probable cause of stillbirth. We are aware that not all cases in which an infection causes stillbirth will fit exactly into one of these mechanistic categories. If an infection is considered a possible or probable cause of a specific stillbirth, yet the mechanism is not clear, the infection is classified as an infectious cause of death of other or unknown mechanisms.
Although placental pathologic findings are characteristically found in many of the conditions in Initial Causes of Fetal Death (eg, fibrosis or infarcts in association with antiphospholipid syndrome), placental conditions were included as an independent condition for those cases in which placental findings predominate and without a previously diagnosed maternal or fetal condition. When the placental findings occur in association with a maternal or fetal condition, then the condition itself is considered to be a possible or probable cause of death, rather than the placental anatomic abnormality.
Histologic chorioamnionitis with funisitis is a special condition that requires more thorough consideration, as funisitis indicates a fetal inflammatory response to an infectious process. Defined by extensive polymorphonuclear cell invasion of the membranes and umbilical cord, respectively, these conditions are believed by some authors in some cases to be causal for stillbirth.2 In the case of funisitis with extensive polymorphonuclear cell invasion, we agree with this opinion. However, we have chosen to consider isolated histologic chorioamnionitis as a present condition, as there is insufficient evidence to include this pathologic diagnosis as a probable or possible cause of death.
Small for gestational age will not be treated as a cause of death but rather as a confirmatory finding that the maternal or placental condition adversely influenced fetal well-being. Although SGA is commonly associated with fetal death, it can only be considered as a consequence of other intrinsic conditions that are possible or probable causes of fetal death and cannot be considered to be a proximate cause of death. In this regard, SGA is a common modifier of other underlying maternal and fetal conditions, but not a specific condition itself. For the purpose of this algorithm SGA will be defined as a birth weight below the 10th percentile for gestational age based on population norms.3 Gestational age at delivery is based on last menstrual period and ultrasound criteria in an algorithm used by the Maternal-Fetal Medicine Units Network,4 and the gestational age of the stillbirth at death is adjusted for the difference between the time when the fetus was last alive and time of diagnosis of death, using pathologic criteria to most accurately determine the time of death. We feel this is an improvement over arbitrarily using the time of delivery to reflect the time of death, as a significant proportion of fetuses (usually preterm) die days or weeks before the actual delivery. If unknown, the time of fetal death will be based on postmortem examination. In the future, we plan to assess the influence of using individualized fetal growth curves to identify SGA fetuses.5–7
DISCUSSION
The SCRN Initial Causes of Fetal Death has been developed to provide a common set of definitions that can be applied to each case of fetal death enrolled in the SCRN study. By using strict diagnostic criteria, we assigned cause of death based on pathophysiologic evidence acquired from the postmortem examination, placental evaluation, and maternal evaluation. In many cases, a probable cause of death was not assigned because we lacked conclusive evidence of a specific pathologic sequence that meets these strict criteria.
Many classification schemes for assigning cause of fetal death are currently used throughout the world.1,8–14 These classification schemes have recently been thoroughly reviewed by Gordijn et al.14 They listed the 35 different classification systems that have been reported in the medical literature since 1954 and noted that each system was created with a specific purpose by the investigators. These systems have the common theme of attempting to assign a cause of death but also to reveal trends in perinatal mortality and to guide prevention strategies and audits of perinatal care. One area of weakness of these systems identified by Gordijn et al14 is the problem of incomplete registration or incomplete ascertainment of all women who suffered stillbirth in the population of study. In SCRN, we addressed this problem by developing a surveillance program designed to identify more than 90% of potentially eligible women over the time of enrollment on a population basis.
Another weakness noted by Gordijn et al14 is the lack of clear and uniform definitions and guidelines in at least half of the currently available classification systems. They advocated developing a multilayered classification system to encompass all perinatal mortality including stillbirth and neonatal death. Because neonatal death was beyond the scope of SCRN, we developed our system to address only stillbirths at 20 or more weeks gestation. When developing our system, we incorporated the three key aspects of an evaluation of fetal death recommended by these authors. Our system includes consideration of three important features: 1) “when,” with an analysis of the clinical condition of the pregnancies at the time of stillbirth; 2) “what,” with a detailed and systematic pathologic protocol for postmortem examination and placental evaluation; and 3) “why,” or the reason for the fetal death taking into account all clinical and pathologic characteristics of the stillbirth. These features were then evaluated using Initial Causes of Fetal Death to determine the cause of death in as many cases as possible using rigorous and standardized definitions.
We developed our system after a workshop convened by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development12 where commonly used classification systems were discussed and evaluated. Numerous issues came to light in the course of the workshop. Perhaps most evident was that determination of the precise cause of death, from a pathophysiologic perspective, can be very complex and often there is no unequivocal cause of death. Second, the classification systems that are currently in use were designed for a variety of purposes including serving as public health tools that are intended to catalog as many cases of stillbirth as possible. Third, none of the classification systems currently in use or previously reported were deemed sufficiently complete to account for the quantity and depth of data being collected during the course of the SCRN study. The SCRN investigators desired an instrument in which we could assign causes of death based on the best available evidence. In exchange for potentially greater certainty regarding causes of death, Initial Causes of Fetal Death may leave a substantial proportion of cases as unexplained or unclassified, while providing a more accurate depiction of the multifactorial nature of many cases of stillbirth. Moreover, many stillbirths may not have only a single cause of death. Instead, there may be a cumulative effect of several risk factors leading to multiple potential causes of death. Rigorously defining causes of death should facilitate both the clinical and experimental investigation of unexplained cases.
In addition to facilitating our research, providing rigorous definitions and more accurately attributing the cause of fetal death have useful clinical implications beyond the scope of SCRN. For example, the opportunity to find the actual cause of death may be missed by assigning causation to any associated conditions that are also present in many live births (eg, maternal thrombophilias). Accurately assigning a probable cause of fetal death is critically important for counseling grieving families.
The SCRN Initial Causes of Fetal Death is not designed to be static, rather it is intended to be flexible so that new conditions can be added as they become evident. In addition to the 512 cases of stillbirth with complete evaluations, we enrolled approximately 1,932 women with live births as a contemporaneous control group. Assays of biospecimens and detailed statistical analyses of the case–control data are ongoing. Should evolving results from the case–control study provide information regarding new possible and probable causes of fetal death, we may need to modify the coding criteria and revise some of the causes of fetal death that were initially assigned to specific cases. Conditions may be changed from each general assignment as present, possible cause of death, or probable cause of death as new information comes to light. For example, if we find that a specific genetic polymorphism may be present, but in the case–control study is strongly associated with fetal death, then the assignment for that polymorphism would change our level of certainty. Any modifications to the SCRN Initial Causes of Fetal Death will be available online on the public portion of the SCRN web site (https://scrn.rti.org).
Even with modifications, Initial Causes of Fetal Death may remain useful in stillbirth cases where extensive evaluation is not available. Most cases of stillbirth do not undergo all the tests that will be analyzed and used in future iterations of Initial Causes of Fetal Death. In these cases, this initial version of Initial Causes of Fetal Death may be used as it relies on clinically available tests and the postmortem examination.
In conclusion, we have devised a structured system to assign the causes of fetal death based on the best available evidence using a detailed protocol encompassing clinical, pathologic, and pathophysiologic data obtained through a rigorous study process. We anticipate that Initial Causes of Fetal Death will be useful for the researcher and clinician when faced with determining and explaining the cause of a fetal death.
Acknowledgments
Supported in part by grant funding from the Stillbirth Collaborative Research Network sites: U10-HD045953 (Brown University, Rhode Island); U10-HD045925 (Emory University, Georgia); U10-HD045952 (University of Texas Medical Branch at Galveston, Texas); U10-HD045955 (University of Texas Health Science Center at San Antonio, Texas); U10-HD045944 (University of Utah Health Sciences Center, Utah); and U01-HD-45954 (RTI International, North Carolina); and by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Appendix 1. Cause of Death Review
Appendix 2


Footnotes
For a list of criteria used to assign causes of fetal death in the SCRN cases, see Appendix 1 on page 260. For a fully referenced version of Initial Causes of Fetal Death, see Appendix 2 online at http://links.lww.com/AOG/A186.
Financial Disclosure
Robert L. Goldenberg is a consultant to United Healthcare, Research Triangle Institute; is employed by Drexel University College of Medicine; and has received grants or has grants pending from the Global Network funded by the NICHD. The other authors did not report any potential conflicts of interest.
References
- 1.Varli IH, Petersson K, Bottinga R, Bremme K, Hofsjö A, Holm M, et al. The Stockholm classification of stillbirth. Acta Obstet Gynecol Scand. 2008;87:1202–12. doi: 10.1080/00016340802460271. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375:1482–90. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogen M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 4.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 5.Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population-based percentiles. Am J Obstet Gynecol. 2009;201:28.e1–8. doi: 10.1016/j.ajog.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Gardosi J, France A. A customized standard to assess fetal growth in a US population. Am J Obstet Gynecol. 2009;201:25.e1–7. doi: 10.1016/j.ajog.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Kady SM, Gardosi J. Perinatal mortality and fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2004;18:397–410. doi: 10.1016/j.bpobgyn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Frøen JF, Pinar H, Flenady V, Bahrin S, Charles A, Chauke L, et al. Causes of death and associated conditions (Codac): a utilitarian approach to the classification of perinatal deaths. BMC Pregnancy Childbirth. 2009;9:22. doi: 10.1186/1471-2393-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flenady V, Frøen JF, Pinar H, Torabi R, Saastad E, Guyon G, et al. An evaluation of classification systems for stillbirth. BMC Pregnancy Childbirth. 2009;9:24. doi: 10.1186/1471-2393-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–35. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg RL, Kirby R, Culhane JF. Stillbirth: a review. J Matern Fetal Neonatal Med. 2004;16:79–94. doi: 10.1080/14767050400003801. [DOI] [PubMed] [Google Scholar]
- 12.Reddy UM, Goldenberg R, Silver R, Smith GC, Pauli RM, Wapner RJ, et al. Stillbirth classification—developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;114:901–14. doi: 10.1097/AOG.0b013e3181b8f6e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57:1–19. [PubMed] [Google Scholar]
- 14.Gordijn SJ, Korteweg FJ, Erwich JJ, Holm JP, van Diem MT, Bergman KA, et al. A multilayered approach for the analysis of perinatal mortality using different classification systems. Eur J Obstet Gynecol Reprod Biol. 2009;144:99–104. doi: 10.1016/j.ejogrb.2009.01.012. [DOI] [PubMed] [Google Scholar]


