Abstract
Several outbreaks of avian influenza (AI) caused by H9N2 subtype, have been reported in the poultry industry during 1990 around the globe. Currently, H9N2 are endemic in the large area of Middle and Far East, including Pakistan. Since H9N2 AI viruses are sporadically reported from humans, extensive incidence of H9N2 in poultry imposes a great risk for human health. In this context, continuous monitoring of the poultry and determining the genetic nature of these viruses are fundamental to predict any future threat. Thus gene sequences of one isolate of H9N2, isolated from commercial poultry flocks, were analyzed. The results of this investigation, based on hemagglutinin (HA), neuraminidase (NA) and non-structural genes, showed that Pakistani H9N2 isolates are closely related to each other and to other H9N2 isolates from the Middle East. However, several unusual substitutions with unknown functional consequences were observed in HA and NA proteins and thus warrant further investigations for their possible role in viral biology. In conclusion, these findings provide information regarding the genetic nature of H9N2 avian influenza viruses in Pakistani poultry and necessitate the sequencing of more H9N2 viruses from both naturally infected and vaccinated flocks.
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-013-0144-1) contains supplementary material, which is available to authorized users.
Keywords: Influenza, H9N2, DNA sequencing, Phylogenetic analysis, Pakistan
Introduction
Wild aquatic birds are considered natural reservoir for avian influenza A viruses. These viruses usually infect the lower intestinal tract of different avian species and transmit via the fecal-oral route [32]. Intriguingly, influenza viruses have been detected in at least 105 avian species [29]. Among 144 (16 × 9) theoretically possible combination of hemagglutinin (HA) and neuraminidase (NA) influenza subtypes, at least 116 have been reported in birds [10]. Avian influenza A viruses (AIV) belonging to only H5, H7 and H9 subtypes gained adaptations to cause severe disease in domestic poultry. However, among them, only H9 subtype exists as low pathogenic AIV (LPAIV) [32].
The H9N2 subtype has become established in poultry population of many Asian and Middle East countries since late 1990s, and H9N2 AIVs have been responsible for serious disease outbreaks in commercial chickens in Pakistan and in many neighboring countries such as Iran [2, 6, 10]. Currently, the H9N2 AIV has become endemic among poultry in large areas of the Middle East [2]. The first H9N2 outbreak in Pakistani poultry was reported in 1998. However, since 1995, there have been five severe influenza outbreaks in Pakistan caused either by H5, H7 or H9 subtypes of AIVs [17, 27]. During these epidemics, primary focus was on the diagnosis of these infections and to control by enforcing biosecurity measures and strategic vaccinations. The knowledge behind genetic and biological characteristics of the H9N2 viruses largely remained unexplored.
Beside substantial economical losses for the commercial poultry, H9N2 also poses a serious threat to public health. After infection of H9N2 in Chinese children in 1999, these viruses were reported sporadically among humans; however, they were unable to establish widespread infections [7]. Provided that H9N2 viruses have potential to infect humans, monitoring of such cases would be of importance to ascertain their genetic variability and their ability to switch hosts [11].
The primary objective of this study was to evaluate the genetic relationships of H9N2 viruses currently circulating in the poultry industry in Pakistan. Additionally, efforts were made to determine the molecular features between sequences of the HA, NA and non-structural gene (NS) genes of Pakistani H9N2 strains and gene sequences from different influenza A viruses available in GenBank.
Materials and Methods
Sampling history and screening
A total of eight blood samples were collected from 18 days old healthy chickens (n = 184) kept in a commercial broiler poultry flock during April 2010 in a region close to Lahore, Pakistan. These birds were maintained in a semi-controlled shed with relatively poor sanitary and biosecurity conditions. As recommended by the local authorities, birds were vaccinated for Newcastle disease and infectious bronchitis viruses, due to high prevalence of these diseases in the region. Blood was stored on QIAcard FTA Indicator Four Spots (Qiagen, Hilden, Germany), and shipped at ambient temperature from Pakistan to the Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden, for processing. These QIAcards have the ability to inactivate the samples and preserve nucleic acids, which is proven to be an efficient and economical means of sample transportation [24–26]. In parallel, tissue samples were also collected and transported to Veterinary Research Institute (VRI), Lahore, Pakistan.
The RNA was extracted from QIAcard FTA Indicator (Qiagen) impregnated with clinical samples as recommended by the manufacturer (preparation of isolated RNA from FTA Cards, Rev 1 10/17/07; Whatman, Hilden, Germany) with the modifications, as described earlier [24–26]. The extracted RNA was screened for the presence of influenza A viruses by real-time RT-PCR for the matrix protein gene [27] according to the recommendations from the Community Reference Laboratory (CRL; AHVLA Addlestone) [19].
Virus isolation and pathogenicity assessment
Only one sample was detected positive in real-time PCR, which was processed for virus isolation in 10-day old embryonated hens’ eggs at the Veterinary Research Institute (VRI), Lahore, Pakistan. Initial virus typing was performed using standard hemagglutination-inhibition and NA inhibition assays according to the recommended procedures [4]. Allantoic fluids from the samples showing high HA titre were assessed for intravenous pathogenicity index (IVPI) in 6-week old chickens to determine the pathogenicity, as previously described [3]. For genome detection and characterization, the allantoic fluid was again stored on QIAcard FTA Indicator Four Spots (Qiagen, Germany), and shipped to SLU Uppsala, Sweden, for further processing.
Reverse transcription (RT)-PCR and nucleotide sequencing
The RNA was eluted from Qiacard FTA Indicator (Qiagen), and was used for viral gene amplification and sequencing, as described previously [17, 24, 25]. One positive sample was selected for sequence analysis considering that all the samples were collected from same flock and therefore may predict the same genetic nature. The amplified PCR products were gel extracted and directly processed for sequencing using ABI PRISM BigDye Terminator version 3.1 (Applied Biosystems), according to the manufacturer’s instructions. Sequences were analyzed with an automated nucleic acid analyzer (ABI PRISM 3100; Applied Biosystems). Each DNA fragment was sequenced at least twice in both directions.
Phylogenetic analysis
Sequence assembly and editing were performed using the SEQMAN program from DNASTAR Lasergene suite 8 (version 8.0.2 13; DNASTAR, Inc., Madison, WI, USA). The sequence was phylogenetically analyzed and compared with virus sequence data available in GenBank. The genetic pattern was determined and phylogenetic trees were constructed using the neighbour-joining method (Kimura 2 parameter) with 2000 bootstrap replicates using the Molecular Evolutionary Genetics Analysis (MEGA, version 5) software package (CEMI, Tempe, AZ, USA) [31]. To confirm the genetic pattern demonstrated by the neighbour-joining method, trees were constructed using Bayesian Inference with the program MrBayes version 3.1.2 [30]. Two independent Monte Carlo Markov (MCM) chains were executed and sampled every 1,000 generations using the default parameters of the priors. Trees saved in this last step were used to construct a majority rule consensus tree. The NetNGlyc 1.0 Server, freely available at http://www.cbs.dtu.dk/services/NetNGlyc/, was used to predict the potential glycosylation sites in HA and NA proteins of H9N2 isolate.
Results
Phylogenetic analysis
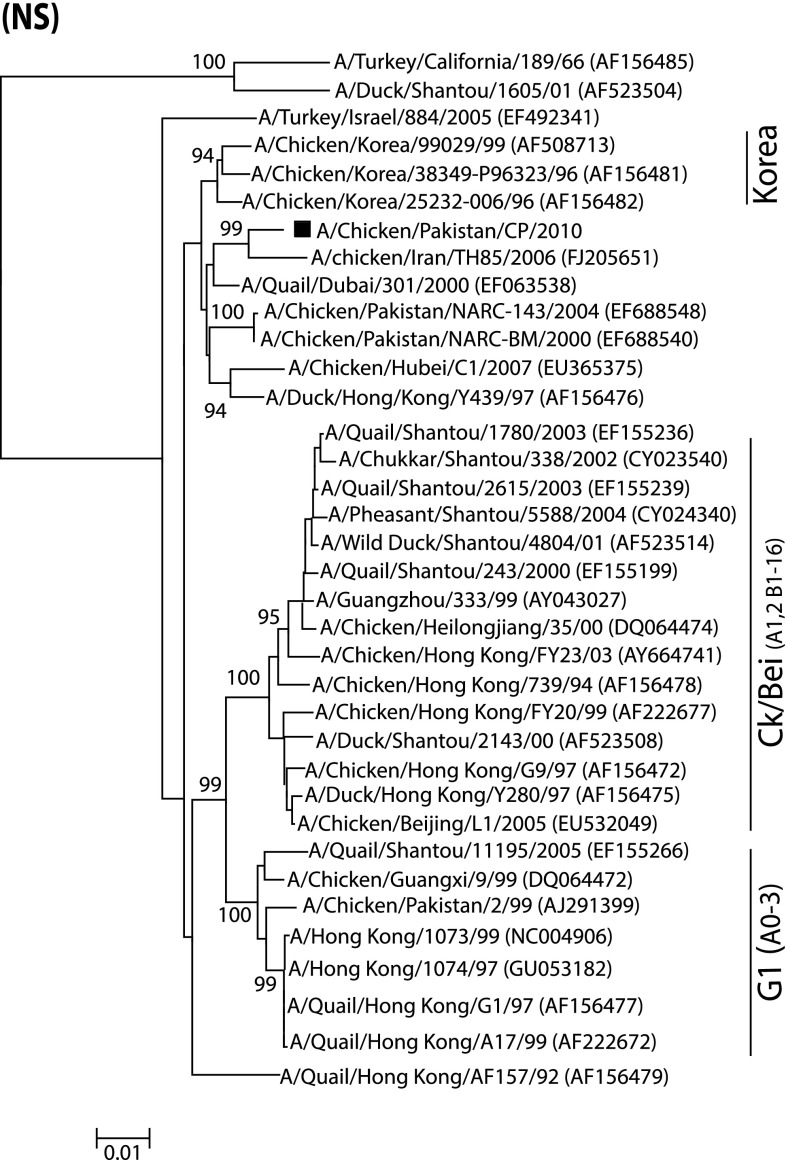
In order to genetically characterize H9N2 virus isolated from a commercial broiler poultry flock, complete gene segments of two surface glycoproteins (HA and NA) of one isolate were sequenced. The isolate was phylogenetically analyzed based on these genes, and compared with the representative strains of avian origin H9N2 viruses from Eurasian lineages [17, 21, 33]. Earlier reports demonstrated that H9N2 viruses causing infection in poultry are mainly divided into three distinct lineages. These include Ch/BJ/1/94, Qa/HK/G1/97 and Y439 or Korean-like, which are represented by A/Chicken/Beijing/1/94, A/Quail/Hong Kong/G1/97 and A/Duck/Hong Kong/Y439/97, respectively [17]. In the present study, the phylogenetic tree based on the HA gene revealed that the isolate A/Chicken/Pakistan/CP/2010 (accession number: JQ905259) clustered with the G1 lineage and formed a distinct group with closely related isolates (95 % nucleotide identity) (Fig. 1a). Additionally, viruses isolated from China and the Middle East also clustered in the same group (G1) as that of A/Chicken/Pakistan/CP/2010 [8, 9]. This isolate showed the highest nucleotide similarity with a previously characterized isolate (A/Chicken/Pakistan/UDL-2/08) from Pakistan. A corresponding tree topology was observed based on the NA gene tree, in which A/Chicken/Pakistan/CP/2010 (accession number: JQ905260) clustered with previously reported Pakistani isolates and the isolates from the Middle East and China (Fig. 1b). Consistent with the branching pattern of A/Chicken/Pakistan/CP/2010 in HA and NA based phylogenetic trees, analysis of the NS gene (accession number: KC711044) indicated that this isolate clustered to Iranian influenza isolates obtained in 2006 (Fig. 2). A Bayesian tree for each gene also reflected the same clustering pattern as NJ method (Fig. S1).
Fig. 1.
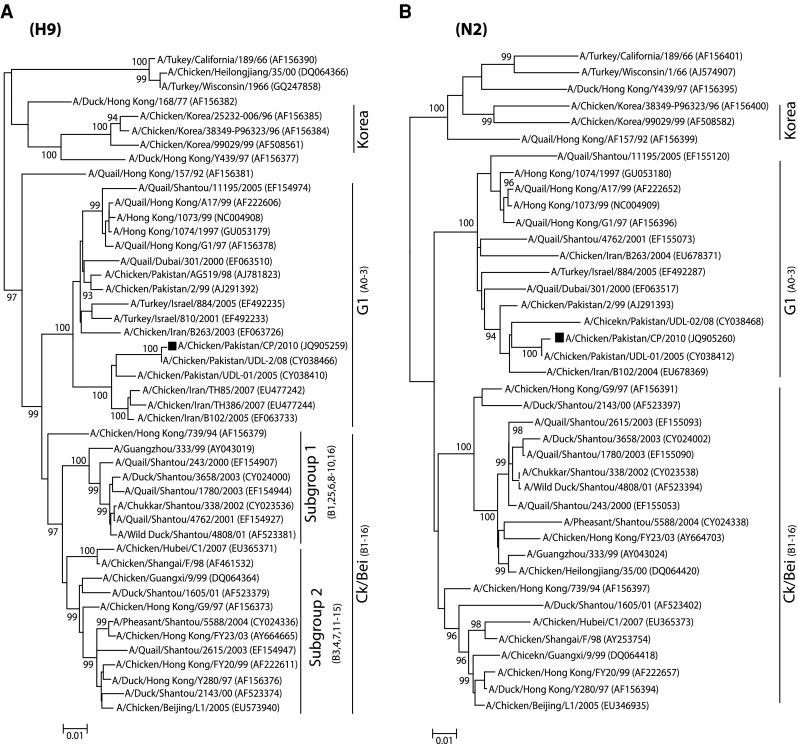
Phylogenetic analysis of HA and NA genes of representative influenza A viruses isolated in Asia. Trees (unrooted) were generated by the neighbor-joining method with the MEGA programme (Bayesian analysis revealed similar relationships). Numbers above the branches indicate neighbor-joining bootstrap values. Only bootstrap values above 80 % are shown. The analysis was based on nucleotides 129–1,042 of the HA gene, and 231–1,297 of the NA gene. The isolate characterized in this study is labeled with a black square. A bar represents 0.01 substitutions per site whereas codes in the trees represents the cross-immunity based genotypes
Fig. 2.
Phylogenetic analysis of NS genes of representative influenza A viruses and comparison with the NS gene of H9N2 isolated and reported in this study. Tree (unrooted) was constructed as described in Fig. 1
Sequence analysis
To investigate the significant substitutions and the molecular markers that may correlate with the possible pathogenic properties of A/Chicken/Pakistan/CP/2010, deduced amino acid sequences of the two surface glycoproteins, HA and NA, were compared with the representative strains of Eurasian lineages of H9N2 viruses.
Hemagglutinin (HA)
It is noteworthy that A/Chicken/Pakistan/CP/2010, the isolate under study, has an Arginine (R) to Lysine (K) substitution at the −4 position of the cleavage site (Table 1), and this substitution has been reported occasionally in H9N2 viruses [21]. The association of this mutation to the viral pathogenicity is not fully elucidated yet but it is speculated that it may alter the host adaptation of these viruses [21]. Despite this substitution, this isolate remained LPAIV since IVPI was calculated to be 0.01.
Table 1.
Sequence features of H9N2 (A/Chicken/Pakistan/CP/2010) characterized in this study
| Influenza proteins | Properties of HA and NA proteins | ||
|---|---|---|---|
| Hemagglutinin (HA) | HA receptor binding sites | Glycosylation sites | Cleavage site |
| P110, W161, T163, H191, A198, L234, I235, G238 | 29NSTD33 (++a, 9/9b), 82NPSC86 (+++, 9/9), 105NGTC109 (+++, 9/9), 141NVTH145 (++, 9/9), 298NSTL302 (++, 9/9), 305NISK309 (++, 9/9), 492NGTY496 (+, 7/9), 551NGSC554 (−, 3/9) | KSSR | |
| Neuraminidase (NA) | Presence of NA stalk deletion | Glycosylation sites | Haemadsorbing sites |
| Nil at 38–39, 46–50, 62–64 | 44NSSK48 (+a, 8/9b), 61NITK65 (+++, 9/9), 69NGTI73 (++, 9/9), 86NWSK90 (++, 9/9), 146NGTT150 (++, 9/9), 200NATA204 (−, 8/9), 234NGTC238 (+++, 9/9), 402NRSG406 (−−, 9/9) | 366IKKDSRAG373, 400S, 402N, 403R, 432Q | |
aProbability and strength for glycosylation at this site if crossing the threshold of 0.5 (+), 0.75 (++) or 0.75 as well as jury agreement (+++) or non-glycosylated sites with threshold of less than 0.5 (−)
bJury agreement, which indicates how many of the nine networks support the prediction
The receptor binding site motif of HA is critical for cellular receptor specificity and determining virus host range [12]. Residues at positions 110, 161, 163, 191, 198, 234, 235 and 238 are major components of the receptor binding site of the HA molecule (Table 1). The A/Chicken/Pakistan/CP/2010 isolate showed following residues at P110, W161, T163, H191, A198 and I235 in the receptor-binding pocket. The left edge of the binding pocket motif at amino acid position 232–239 was NGLIGRIN whereas at the right edge (at position 146–150), the sequence was observed to be GTSKS. Eight potential glycosylation sites were predicted using the NetNGlyc 1.0 Server: 29NSTD33, 82NPSC86, 105NGTC109, 141NVTH145, 298NSTL302, 305NISK309, 492NGTY496, and 551NGSC554. Two glycosylation sites at position 206 and 218, which have been observed in some of the influenza strains from the Middle East and in representative isolates of the G1 lineage, respectively, were missing in this Pakistani isolate [1, 17, 21]. These alterations might be associated with switching of influenza viruses to poultry [16, 17]. It is tempting to suggest the lack of glycosylation may facilitate the selective adaptation of H9N2 viruses in poultry [17].
Neuraminidase (NA)
Three functional motifs have been described in the NA proteins that play essential roles in the pathogenicity of influenza viruses: the stalk length, sialic acid binding site and glycosylation sites. The two amino acid (at position 38–39 aa) and three amino acid deletions (at position 63–65 aa) in the NA stalk region as present in G1-like viruses (A/Quail/Hong Kong/G1/97) and Y280-like viruses (A/Duck/Hong Kong/Y280/97) [14] were absent in A/Chicken/Pakistan/CP/2010. Further, the substitution (46–50 aa) associated with oseltamivir and zanamivir resistance was also not detected. In the sialic acid binding site (also called hemadsorbing site), amino acids from 366 to 373, 400S, 402N, 403R, 432Q are conserved in G1-like and Y280-like viruses and in H9N2 viruses isolated from wild birds and adapted to poultry [20]. All of these substitutions were found to be present in A/Chicken/Pakistan/CP/2010.
Non-structural gene (NS)
The H9N2 influenza viruses, previously characterized from Pakistan during 2005–2008, have shown reassortment in which NS gene belong to Z-genotypes of highly pathogenic H5N1 and carry a five amino acid deletion in the middle of NS1 protein sequence [17]. However, no deletion or insertion was observed in the putative sequence of NS1 protein of the isolate reported here. The last four residues of the NS1 protein at the C-terminus form the PDZ ligand domain. The large-scale sequence analysis of avian influenza viruses revealed that two residues are rare in this PDZ ligand: a C-terminal isoleucine (I) residue and lysine (K) at the -4 position of the PDZ ligand [28]. Interestingly, there is a distinct PDZ ligand in the isolate A/Chicken/Pakistan/CP/2010, which carried KSEI. It has been shown that this ligand determines the influenza virus pathogenicity for mice [18]; however, no such report is available that describes the role of PDZ ligand of H9N2 viruses in poultry.
Discussion
It has been shown that the HA and NA gene sequences have drifted over the years [9, 33]. Based on the topology of the trees, it is evident that the genetic nature of the H9N2 viruses remains conserved at least between isolates obtained in 2008 and 2010 (16 and this study) in different parts of the country. Moreover, the H9N2 lineage-defining residues were observed to be similar in the under study isolate, A/Chicken/Pakistan/CP/2010. These include eight amino acid residues in HA (N17, Y96, N179, T209, P262, C290, H304 in HA1 and V91 in HA2) and seven amino acids (L10, T43, S77, S153, T212, V307, G346) residues in NA protein. Taken together, the pattern of nucleotide similarity indicates that the A/Chicken/Pakistan/CP/2010 isolate retained surface glycoproteins similar to the previously characterized Pakistani isolates belong to H9N2 G1-lineage. However, it is necessary to sequence a large collection of H9N2 isolates from naturally infected and vaccinated flocks, over a period of several years to ascertain the nature of the viruses that are both genetically and epidemiologically representative of this subtype of influenza virus.
It has been demonstrated that amino acid sequences connecting HA1/HA2 at the cleavage site and the glycosylation sites not only play a central role in the switch of low pathogenic to high pathogenic phenotype but also facilitate interspecies transmission [5, 23]. The majority of the described H9N2 influenza viruses carry RSSR motif at the cleavage site with no poly-basic residues, which reflects the low pathogenic phenotype of H9N2. This motif has also been noted in the H9N2 viruses adapted to chickens in recent years in Asia and the Middle East [1, 13, 22, 33].
It has been observed that glycosylation sites play a significant role in increased virulence of influenza viruses, either due to alteration in sialic acid activity or change in antigenicity [16]. Comparison of glycosylation sites between G1-like H9N2 viruses and A/Chicken/Pakistan/CP/2010 demonstrated the presence of eight conserved potential glycosylation sites at 44NSSK48, 61NITK65, 69NGTI73, 86NWSK90, 146NGTT150, 200NATA204, 234NGTC238, and 402NRSG406. Among them, the 44NSSK48 glycosylation site has also been reported in A/Chicken/Hong Kong/G9/97 and A/Chicken/India/2048/03, with unknown significance [21]. However, addition or loss of potential glycosylation may contribute to increased virulence due to altered antigenicity or sialidase activity [16].
The great wealth of reports demonstrating the continuous circulation of H9N2 viruses in Asian poultry highlights the potential of these viruses to evolve rapidly, and attain increased virulence [7, 8, 17, 27]. A recent report of increased replication of poultry-origin H9N2 viruses in a mouse model provides further evidence of adaptation of these viruses to mammals [15]. This situation is complicated by backyard poultry practices, where apparent absence of clinical symptoms may increase the chance of human exposure and reassortment. These observations are supported by isolation of H9N2 viruses from humans and animals, predominantly from Southeast Asia [17]. Genetic analysis of the Hong Kong H9N2 isolates that caused disease in human has shown high genetic similarity to H9N2 Pakistani isolate, A/Chicken/Pakistan/2/99. This indicates that H9N2 strains similar to those that caused human infections in China also circulated in Pakistan during the second half of the 1990s [9]. These H9N2 have not become established in human population and were unable to cause widespread infections. All these developments have resulted in increasing concern about the human health implications of H9N2 subtype viruses. In summary, we have reported the genetic analysis of a H9N2 avian influenza isolate collected from broiler chicken in Pakistan and found that the virus belonged to G1-like sublineage. These findings provide additional information regarding the genetic homogeneity of H9N2 avian influenza viruses circulating in Pakistani poultry over the years. With the possible threat of H9N2 virus, continuous surveillance and monitoring of the gene constellation would help to improve the understanding of the evolution and the emergence of influenza strains in the region.
Electronic supplementary material
Bayesian inference trees with the program MrBayes version 3.1.2 for HA, NA and NS1 protein genes of H9N2 isolate
References
- 1.Aamir UB, Wernery U, Ilyushina N, Webster RG. Characterization of avian H9N2 influenza viruses from United Arab Emirates 2000 to 2003. Virology. 2007;361:45–55. doi: 10.1016/j.virol.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander DJ. Report on avian influenza in the Eastern Hemisphere during 1997–2002. Avian Dis. 2003;47:792–797. doi: 10.1637/0005-2086-47.s3.792. [DOI] [PubMed] [Google Scholar]
- 3.Alexander DJ. Highly pathogenic avian influenza. Manual of diagnostic tests and vaccines for terrestrial animals. Paris: Office International des Epizooties; 2004. pp. 258–269. [Google Scholar]
- 4.Alexander DJ, Spackman D. Characterisation of influenza A viruses isolated from turkeys in England during March–May 1979. Avian Pathol. 1981;10:281–293. doi: 10.1080/03079458108418477. [DOI] [PubMed] [Google Scholar]
- 5.Baigent SJ, McCauley JW. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays. 2003;25:657–671. doi: 10.1002/bies.10303. [DOI] [PubMed] [Google Scholar]
- 6.Banks J, Speidel EC, Harris PA, Alexander DJ. Phylogenetic analysis of influenza A viruses of H9 haemagglutinin subtype. Avian Pathol. 2000;29:353–359. doi: 10.1080/03079450050118485. [DOI] [PubMed] [Google Scholar]
- 7.Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, Xu KM, Lim W, Webster RG, Yuen KY, Peiris JS, Guan Y. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt AM, Siddique S, Tahir S, Nasrullah I, Hussain M, Idrees M, Lu J, Tong Y. Comparative sequence, antigenic and phylogenetic analysis of avian influenza (H9N2) surface proteins isolated in Pakistan between 1999 and 2008. J Infection Dev Ctries. 2011;5:413–424. doi: 10.3855/jidc.1372. [DOI] [PubMed] [Google Scholar]
- 9.Cameron KR, Gregory V, Banks J, Brown IH, Alexander DJ, Hay AJ, Lin YP. H9N2 subtype influenza A viruses in poultry in Pakistan are closely related to the H9N2 viruses responsible for human infection in Hong Kong. Virology. 2000;278:36–41. doi: 10.1006/viro.2000.0585. [DOI] [PubMed] [Google Scholar]
- 10.Capua I, Alexander DJ. Avian influenza: recent developments. Avian Pathol. 2004;33:393–404. doi: 10.1080/03079450410001724085. [DOI] [PubMed] [Google Scholar]
- 11.Capua I, Alexander DJ. Ecology, epidemiology and human health implications of avian influenza viruses: why do we need to share genetic data? Zoonoses Public Health. 2008;55:2–15. doi: 10.1111/j.1863-2378.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 12.Gambaryan A, Webster R, Matrosovich M. Differences between influenza virus receptors on target cells of duck and chicken. Arch Virol. 2002;147:1197–1208. doi: 10.1007/s00705-002-0796-4. [DOI] [PubMed] [Google Scholar]
- 13.Golender N, Panshin A, Banet-Noach C, Nagar S, Pokamunski S, Pirak M, Tendler Y, Davidson I, Garcia M, Perk S. Genetic characterization of avian influenza viruses isolated in Israel during 2000–2006. Virus Genes. 2008;37:289–297. doi: 10.1007/s11262-008-0272-7. [DOI] [PubMed] [Google Scholar]
- 14.Guo YJ, Krauss S, Senne DA, Mo IP, Lo KS, Xiong XP, Norwood M, Shortridge KF, Webster RG, Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 15.Hossain MJ, Hickman D, Perez DR. Evidence of expanded host range and mammalian-associated genetic changes in a duck H9N2 influenza virus following adaptation in quail and chickens. PLoS One. 2008;3:e3170. doi: 10.1371/journal.pone.0003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulse DJ, Webster RG, Russell RJ, Perez DR. Molecular determinants within the surface proteins involved in the pathogenicity of H5N1 influenza viruses in chickens. J Virol. 2004;78:9954–9964. doi: 10.1128/JVI.78.18.9954-9964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal M, Yaqub T, Reddy K, McCauley JW. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS One. 2009;4:e5788. doi: 10.1371/journal.pone.0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson D, Hossain MJ, Hickman D, Perez DR, Lamb RA. A new influenza virus virulence determinant: the NS1 protein four C-terminal residues modulate pathogenicity. Proc Natl Acad Sci USA. 2008;105:4381–4386. doi: 10.1073/pnas.0800482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson D, Ronquist F. Skeletal morphology of Opius dissitus and Biosteres carbonarius (Hymenoptera: Braconidae), with a discussion of terminology. PLoS One. 2012;7:e32573. doi: 10.1371/journal.pone.0032573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobasa D, Rodgers ME, Wells K, Kawaoka Y. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J Virol. 1997;71:6706–6713. doi: 10.1128/jvi.71.9.6706-6713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Liu X, Cheng J, Peng D, Jia L, Huang Y. Phylogenetic analysis of the hemagglutinin genes of twenty-six avian influenza viruses of subtype H9N2 isolated from chickens in China during 1996–2001. Avian Dis. 2003;47:116–127. doi: 10.1637/0005-2086(2003)047[0116:PAOTHG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Mase M, Imada T, Sanada Y, Etoh M, Sanada N, Tsukamoto K, Kawaoka Y, Yamaguchi S. Imported parakeets harbor H9N2 influenza A viruses that are genetically closely related to those transmitted to humans in Hong Kong. J Virol. 2001;75:3490–3494. doi: 10.1128/JVI.75.7.3490-3494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munir M, Abbas M, Khan MT, Zohari S, Berg M. Genomic and biological characterization of a velogenic Newcastle disease virus isolated from a healthy backyard poultry flock in 2010. Virol J. 2012;9:46. doi: 10.1186/1743-422X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munir M, Cortey M, Abbas M, Qureshi ZU, Afzal F, Shabbir MZ, Khan MT, Ahmed S, Ahmad S, Baule C, Stahl K, Zohari S, Berg M. Biological characterization and phylogenetic analysis of a novel genetic group of Newcastle disease virus isolated from outbreaks in commercial poultry and from backyard poultry flocks in Pakistan. Infect Genet Evol. 2012;12:1010–1019. doi: 10.1016/j.meegid.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Munir M, Zohari S, Saeed A, Khan QM, Abubakar M, LeBlanc N, Berg M. Detection and phylogenetic analysis of peste des petits ruminants virus isolated from outbreaks in Punjab, Pakistan. Transbound Emerg Dis. 2012;59:85–93. doi: 10.1111/j.1865-1682.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 27.Naeem K, Siddique N, Ayaz M, Jalalee MA. Avian influenza in Pakistan: outbreaks of low- and high-pathogenicity avian influenza in Pakistan during 2003–2006. Avian Dis. 2007;51:189–193. doi: 10.1637/7617-042506R.1. [DOI] [PubMed] [Google Scholar]
- 28.Obenauer JC, Denson J, Mehta PK, Su X, Mukatira S, Finkelstein DB, Xu X, Wang J, Ma J, Fan Y, Rakestraw KM, Webster RG, Hoffmann E, Krauss S, Zheng J, Zhang Z, Naeve CW. Large-scale sequence analysis of avian influenza isolates. Science. 2006;311:1576–1580. doi: 10.1126/science.1121586. [DOI] [PubMed] [Google Scholar]
- 29.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 30.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu KM, Smith GJ, Bahl J, Duan L, Tai H, Vijaykrishna D, Wang J, Zhang JX, Li KS, Fan XH, Webster RG, Chen H, Peiris JS, Guan Y. The genesis and evolution of H9N2 influenza viruses in poultry from southern China, 2000 to 2005. J Virol. 2007;81:10389–10401. doi: 10.1128/JVI.00979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bayesian inference trees with the program MrBayes version 3.1.2 for HA, NA and NS1 protein genes of H9N2 isolate