ABSTRACT
BACKGROUND
Arthritis affects 20 % of the adult US population and is associated with comorbid depression. Depression screening guidelines have been endorsed for high-risk groups, including persons with arthritis, in the hopes that screening will increase recognition and use of appropriate interventions.
OBJECTIVE
To examine national rates of depression and depression screening for patients with arthritis between 2006 and 2010.
PARTICIPANTS AND DESIGN
We used nationally representative cross-sections of ambulatory visits in the United States from the National Ambulatory Medical Care Survey from 2006 to 2010, which included 18,507 visits with a diagnosis of arthritis. When weighted to the US population, this total represents approximately 644 million visits.
MEASUREMENTS
Visits where arthritis was listed among diagnoses. Outcomes were survey-weighted estimates of depression and prevalence of depression screening among patients with arthritis across patient and physician characteristics.
KEY RESULTS
Of the 644,419,374 visits with arthritis listed, 83,574,127 (13 %) were associated with a comorbid diagnosis of depression. The odds ratio for comorbid depression with arthritis was 1.42 (95 % CI 1.3, 1.5). Depression screening occurred at 3,835,000 (1 %) visits associated with arthritis. When examining the rates of depression screening between ambulatory visits with and without arthritis listed, there was no difference in depression screening rates; both were approximately 1 %. There was no difference in screening rates by provider type. Compared to visits with other common, chronic conditions, the prevalence of depression at arthritis visits was high (13 per 100 visits), although the prevalence of depression screening at arthritis visits was low (0.68 per 100 visits).
CONCLUSIONS
Despite the high prevalence of depression with arthritis, screening for depression was performed at few arthritis visits, representing missed opportunities to detect a common, serious comorbidity. Improved depression screening by providers would identify affected patients, and may lead to appropriate interventions such as mental health referrals and/or treatment with anti-depressants.
KEY WORDS: arthritis, depression, screening
Arthritis affects one in five adults,1 and is one of the most frequent reasons for ambulatory visits to the primary care physician.2 Arthritis impacts patients both physically and psychologically,3 and often leads to depressed mood4–6 with subsequent worse health outcomes, including increased mortality.7,8 Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease,9,10 myocardial infarction,11 and suicide.12,13 Patients with arthritis and associated depression have increased health service utilization14 and are less likely to be adherent with their medications.15,16 In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.17,18
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the UK National Institute of Clinical Excellence.19 The U.S. Preventive Service Task Force (USPSTF) and Canadian Task Force for Preventive Health Care recommend depression screening in all adults.20,21 Before screening for depression in specific patients groups can be recommended, however, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening.22 Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent, with rates ranging from 18 % to 42 %.23,24 Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis (OA), but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.25,26 Implicit in the use of depression screening is the assumption that the patients so recognized would benefit from treatment,27 and, in fact, a recent Canadian study showed that patients with hip and/or knee OA screened for depression were more likely to receive mental health services compared to patients with OA who were not screened.28 While small studies have identified patients with different types of arthritis as high-risk for developing depression,29 there is no data about screening practices for depression in patients with all forms of arthritis in ambulatory clinics nationwide.
Our objectives were to describe national rates of current comorbid depression and depression screening for patients with arthritis at outpatient clinics. Our findings will be useful to determine if current depression screening practices need to be improved at ambulatory visits for patients with arthritis.
MATERIALS AND METHODS
Data Source
We examined ambulatory visits coded for arthritis using nationally representative data from the National Ambulatory Medical Care Survey (NAMCS),30–32 an annual visit-based cross-sectional survey conducted in physicians’ offices. NAMCS data collection is carried out by the United States Census Bureau. The NAMCS is an annual probability survey, and is designed to generate nationally representative estimates of nonfederal, office-based physicians providing direct patient care in the 50 states and District of Columbia, excluding radiologists, anesthesiologists, and pathologists. Physicians and their office staff were trained to complete the survey for all visits in a randomly sampled week. NAMCS uses a multistage cluster strategy, selecting physicians by geographic location and provider specialty. Details on the sampling and estimation process for the survey are available at http://www.cdc.gov/nchs/. The purposeful sampling strategy and weights allows one to generalize to the ≈ 650 million office visits made annually to physicians in the US. Participating physicians and their patients vary from year to year, so there is no longitudinal follow-up. Since NAMCS data is publicly available and de-identified, it is considered not to be human subject data by our Institutional Review Board (IRB), and is thus exempt from the need for IRB approval.
Case Definition for Arthritis, Depression, and Depression Screening
Ambulatory care visits were selected if the provider affirmed, “regardless of the primary diagnoses related to this visit, does the patient now have arthritis?” Even if the arthritis was judged to be not clinically significant at the current visit, providers still identified it. “Arthritis” included any rheumatic disease in which there was inflammation involving joints (e.g., osteoarthritis, rheumatoid arthritis, acute arthritis, juvenile chronic arthritis, hypertrophic arthritis, Lyme arthritis, or psoriatic arthritis). Current depression was defined as present if the provider established “regardless of the primary diagnoses related to this visit, does the patient now have depression?” Depression included affective disorders and major depressive disorders. Depression screening was a simple yes/no question in the diagnostic/screening services section of the NAMCS survey, and did not require specific screening procedures.
Potential Correlates of Depression and Depression Screening
The NAMCS visit data included variables possibly related to depression and depression screening. These included sociodemographic variables concerning the presenting patient, such as age, sex, race (white, African American, and other), ethnicity (Hispanic and non-Hispanic), and region of residence (Northeast, South, Midwest, West). Type of provider who performed the visit is defined as primary care, medical specialty, or surgical specialty where primary care includes family practice, general practice, internal medicine, obstetrics and gynecology, adolescent medicine, pediatrics, and sports medicine doctors. The “medical specialty” category includes sub-specialties of internal medicine such as rheumatology. We also evaluated the odds of depression being comorbid with arthritis compared to other chronic diseases (chronic obstructive pulmonary disease, congestive heart failure, hypertension, diabetes, ischemic heart disease, and chronic renal failure).
Statistical Analyses
In order to obtain national estimates from the sample, each record is assigned an inflation factor called the “patient visit weight”. Using the survey data, we created sub-populations of patient visits associated with arthritis. The unit of analysis was “visit coded for arthritis”, not persons with arthritis. Different providers were sampled each year and arthritis cases were not followed longitudinally.
Differences in baseline characteristics between depressed and non-depressed patients were assessed using a two-sided t-test or the Pearson’s chi-square test. Odds ratios were estimated for the prevalence of depression in a number of common chronic conditions, including arthritis, using multivariate logistic regression. Based on previous studies showing that outcomes in arthritis often differ at the level of the individual,33–36 we adjusted for the effects of age, gender, race (white, black, other), ethnicity, geographical region (Northeast, Midwest, South, West), and provider type on depression for patients with arthritis. Ethnicity was not a significant predictor of comorbid depression amongst visits coded for arthritis in the univariate model, and was omitted from multivariate analyses.
To obtain national estimates for depression screening, we examined only visits without a diagnosis of depression, since physicians were unlikely to screen for depression in patients who already carried the diagnosis. All statistical analyses were performed using STATA 12 software (StataCorp) and accounted for the components of the complex survey design. All statistical tests were two-tailed, with p < 0.05 considered significant.
RESULTS
We identified 18,507 surveyed ambulatory visits with a diagnosis of arthritis over the 5-year study period. When weighted to the US population, this total represents 644,419,374 (13 %) ambulatory visits that included a diagnosis of arthritis from 2006 to 2010. Characteristics of these arthritis visits are described by depression status in Table 1. The majority of patients were older than 50 years, and 64 % were women. Most patients were white and non-Hispanic.
Table 1.
Characteristics of Arthritis Visits by Depression Status
| Characteristics | Weighted to all ambulatory US visits* | |||
|---|---|---|---|---|
| All visits n = 644,419,374 | Not depressed n = 560,845,247 (87 %) | Depressed n = 83,574,127 (13 %) | p value | |
| Age | < 0.0001 | |||
| 0–30 | 25,841,220 (4) | 23,585,750 (4) | 2,255,468 (.4) | |
| 31–50 | 116,446,600 (18) | 96,534,020 (15) | 19,977,000 (3) | |
| 51–70 | 278,969,100 (43) | 240,046,200 (37) | 38,922,930 (6) | |
| ≥ 70 | 223,162,400 (35) | 200,736,600 (31) | 22,425,790 (3) | |
| Sex | < 0.0001 | |||
| Female | 413,781,700 (64) | 350,693,000 (54) | 63,088,660 (10) | |
| Male | 230,637,700 (36) | 210,145,200 (33) | 20,492,540 (3) | |
| Race | < 0.0001 | |||
| White | 555,876,200 (86) | 478,996,900 (74) | 76,943,670 (12) | |
| African American | 63,733,080 (10) | 59,093,260 (9) | 4,704,261 (1) | |
| Other | 24,745,700 (4) | 22,812,450 (4) | 1,997,700 (1) | |
| Ethnicity† | 0.25 | |||
| Hispanic | 56,837,790 (9) | 50,329,150 (8) | 6,508,636 (1) | |
| Non-Hispanic | 587,581,600 (91) | 510,057,900 (79) | 77,459,210 (12) | |
| Geographic region | 0.05 | |||
| Northeast | 121,730,800 (19) | 104,331,500 (16) | 17,399,320 (3) | |
| Midwest | 158,462,700 (25) | 135,392,500 (21) | 23,134,660 (4) | |
| South | 238,370,700 (37) | 211,498,400 (33) | 26,872,290 (4) | |
| West | 125,855,100 (20) | 109,680,200 (17) | 16,174,930 (3) | |
| Physician specialty | < 0.0001 | |||
| Primary care | 296,239,600 (46) | 250,421,400 (39) | 45,818,220 (7) | |
| Surgical specialty | 211,047,300 (33) | 195,839,000 (30) | 15,272,740 (2) | |
| Medical specialty | 137,132,400 (21) | 114,577,800 (18) | 22,554,680 (4) | |
*Values are numbers (percentages). Some column totals may not equal 100 % due to estimation and rounding
†Ethnicity with 28 % of missing data imputed
Of the more than 644 million visits, 83,574,127 (13 %) were associated with a comorbid diagnosis of current depression. Those with visits in which both arthritis and comorbid depression were identified were more likely to be > 50 years old, white, non-Hispanic women and seen by a primary care physician. The odds ratio for current comorbid depression with arthritis was 1.42 (95 % CI 1.3, 1.5), adjusted for age, gender, race, geographic region, and other common chronic conditions. When compared to other common, chronic diseases, arthritis had the second highest prevalence of comorbid depression (Table 2).
Table 2.
Prevalence and Adjusted Odds Ratios of Depression and Depression Screening in Patients with Arthritis and Other Common Chronic Conditions
| Condition | Prevalence* of depression | OR for depression† (95 % CI) | p value | Prevalence‡ of depression screening |
|---|---|---|---|---|
| COPD | 14 | 1.64 (1.5, 1.8) | < 0.0001 | 0.76 |
| Arthritis | 13 | 1.42 (1.3, 1.5) | < 0.0001 | 0.68 |
| CHF | 13 | 1.34 (1.1, 1.6) | < 0.0001 | 1.1 |
| Hypertension | 11 | 1.30 (1.2, 1.4) | < 0.0001 | 0.87 |
| Diabetes | 11 | 1.12 (1.03, 1.2) | 0.008 | 0.90 |
| Ischemic Heart Disease | 10 | 0.95 (.84, 1.07) | 0.36 | 0.84 |
| Chronic Renal Failure | 8 | 0.83 (.60, 1.1) | 0.24 | 0.68 |
Adjusted odds ratio for current comorbid depression and prevalence of depression screening with arthritis are in bold
*Prevalence is per 100 visits
†Adjusted for age, gender, race (white, black, other), geographic region (Northeast, Midwest, South, West), and other chronic conditions (Chronic Obstructive Pulmonary Disease [COPD], Congestive Heart Failure [CHF], Arthritis, Diabetes, Hypertension, Ischemic Heart Disease). Reference group is general population without the chronic condition
‡Prevalence is per 100 visits among patients without depression
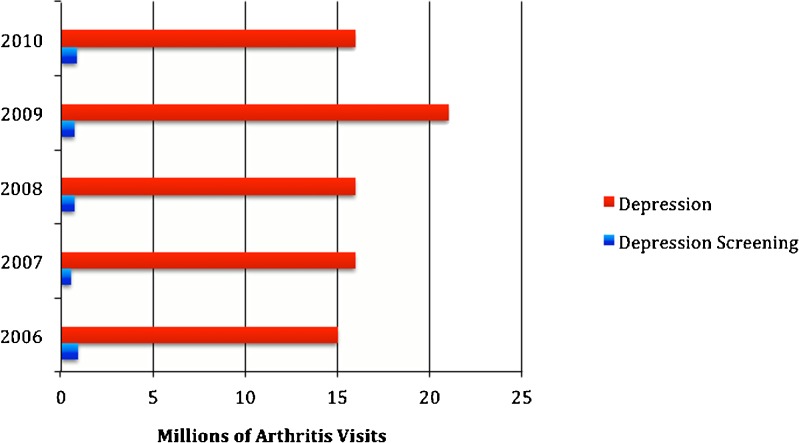
We identified 3,835,000 (1 %) visits in which depression screening occurred out of an estimated 560 million total visits in which depression was not already identified. When comparing the rates of depression screening between ambulatory visits with and without arthritis, there was no difference in depression screening rates; both were approximately 1 % (p = 0.17). Although the prevalence of depression at arthritis visits (13 per 100 visits) was among the highest for visits with other common, chronic conditions, the prevalence of depression screening at arthritis visits was the lowest (0.68 per 100 visits) compared to visits with other common, chronic conditions (Table 2). The yearly prevalence for depression far outweighed depression screening practices (Fig. 1). In fact, depression screening with arthritis visits decreased over the 5-year study. There was no difference in screening rates by general practitioners, internists, rheumatologists, or orthopedic surgeons at patient visits coded for arthritis. Rheumatologists and orthopedic surgeons had no arthritis visits at which depression screening was coded.
Figure 1.
National estimates of depression and depression screening at arthritis visits in the United States by year (2006–2010).
DISCUSSION
When correctly diagnosed, depression is a treatable condition. In order to manage depression in patients with arthritis, better identification of the problem is required. We examined data from a US national survey conducted over a 5-year time period to determine, among ambulatory visits of physician diagnosed arthritis, the prevalence of depression and depression screening, and whether age, gender, race, ethnicity, geographic region, and/or physician specialty were associated with differences in depression screening practices at ambulatory visits for patients with arthritis.
Our results concur with previous studies about the prevalence of current comorbid depression and arthritis;37 13 % of arthritis visits were coded for comorbid depression. Similar to previous reports, arthritis visits by women and those of older age were associated with comorbid depression. While our investigation identified 13 % of persons at visits coded for arthritis as currently depressed, a prior study showed that only 4.5 % of ambulatory visits where the primary diagnosis was arthritis had a drug mention where an antidepressant was ordered, supplied, administered, or continued.38 This demonstrates a potential gap between recognition of comorbid depression with arthritis and treatment, recognizing that providers may choose to treat with non-pharmacologic strategies.
Since arthritis can serve as a “red-flag” to raise suspicion for depression, it was important to evaluate screening practices in the US. Screening for depression was done at few arthritis visits, representing missed opportunities to identify a serious comorbidity. Even with increasing evidence that depression is common among patients with arthritis, depression screening among these patients remained constant over time. This may be due to the fact that depression screening is not necessarily a priority among preventive services. The relative importance of depression screening compared to other preventive services was evaluated in a systematic review of all 25 services recommended by the USPSTF.39 Depression screening ranked poorly for clinically preventable burden and cost-effectiveness and was outscored by colorectal screening, hypertension screening, vision screening, and several other preventive services. However, the analysis included depression screening for all adults and did not focus on patients at high risk for depression, such as those with arthritis. Given that depression is more prevalent in patients with a chronic physical health problem and/or physical impairment, depression screening in this selected population is likely to be more cost-effective.
Regardless of the type of provider, this study shows that all physicians need to do better with regard to identifying depression. While it is true that some patients identified by screening techniques may have transient symptoms and not true depressive disorders, our results show that patients in the US with arthritis are at high risk for comorbid depression, and we recommend depression screening by health-care providers in these high-risk patients. Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 min to a visit.40,41 Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments.41 Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes.42 Thus, while it is our hope that patients with arthritis will be screened for depression, provision of or referral to treatment is a necessary follow-up to screening. To this point, the U.S Preventive Service Task Force recommends screening adults seen in a primary care setting only when collaborative care programs are in place.43 These “collaborative care” programs typically involve nonmedical specialists such as case managers, who work with primary care physicians and mental health specialists to provide management and follow-up.44 However, it is important to remember that the USPSTF’s recommendations pertain to all adults in primary care and are not targeted to patients at high-risk for depression. We believe that depression screening for highly susceptible patients, such as those with arthritis, should be universal, which is reflected in the UK NICE guidelines.
This study has limitations. Our estimates for prevalence of depression and depression screening among patients with arthritis may be conservative for three reasons. First, we only included visits where the provider recorded arthritis, and in some cases, physicians may have omitted the diagnosis. Additionally, the majority of surveys are filled out by office staff and NAMCS representatives based on clinic notes, rather than by the physicians themselves. Thus, there may be additional visits occurring among persons with undiagnosed arthritis.45 Second, depression and depression screening may be underestimated for similar reasons.46 Third, estimates from this survey exclude potential ambulatory care settings, such as federally employed physicians, federal military and Veteran’s Administration clinics, community health centers, and tertiary care academic centers. The potential implications of underestimation are important, because it may be that depressed patients are not being identified to receive therapy for a treatable condition, which can have significant effects on health. However, a positive depression screen is not the same as a diagnosis of depression, and it is unclear what proportion of patients who receive a positive screen should progress to more complete evaluation or initiation of depression-related treatment.
In conclusion, we found no difference in depression screening rates in patients with arthritis compared to the general population, despite patients with arthritis being considered “high-risk”. Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase (1) the recognition of depression in high-risk groups, and (2) the use of appropriate interventions such as mental health referrals and/or treatment with anti-depressants.
Acknowledgements
Support
Dr. Margaretten is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2TR000143. This papers’ contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr. Margaretten also received support from The Rosalind Russell Medical Research Center for Arthritis Research. The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, or preparation of final approval of the manuscript prior to publication.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 2.Wright EA, Katz JN, Cisternas MG, Kessler CL, Wagenseller A, Losina E. Impact of knee osteoarthritis on health care resource utilization in a US population-based national sample. Med Care. 2010;48(9):785–791. doi: 10.1097/MLR.0b013e3181e419b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih M, Hootman JM, Strine TW, Chapman DP, Brady TJ. Serious psychological distress in U.S. adults with arthritis. J Gen Intern Med. 2006;21(11):1160–1166. doi: 10.1111/j.1525-1497.2006.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawker GA, Gignac MA, Badley E, et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res. 2011;63(10):1382–1390. doi: 10.1002/acr.20298. [DOI] [PubMed] [Google Scholar]
- 5.Sale JE, Gignac M, Hawker G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J Rheumatol. 2008;35(2):335–342. [PubMed] [Google Scholar]
- 6.Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol. 2005;32(6):1013–1019. [PubMed] [Google Scholar]
- 8.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51(5):636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 9.Treharne GJ, Hale ED, Lyons AC, et al. Cardiovascular disease and psychological morbidity among rheumatoid arthritis patients. Rheumatology (Oxford) 2005;44(2):241–246. doi: 10.1093/rheumatology/keh441. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Mortality in osteoarthritis. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S120–S124. [PubMed] [Google Scholar]
- 11.Scherrer JF, Virgo KS, Zeringue A, et al. Depression increases risk of incident myocardial infarction among Veterans Administration patients with rheumatoid arthritis. Gen Hosp Psychiatry. 2009;31(4):353–359. doi: 10.1016/j.genhosppsych.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Fuller-Thomson E, Shaked Y. Factors associated with depression and suicidal ideation among individuals with arthritis or rheumatism: findings from a representative community survey. Arthritis Rheum. 2009;61(7):944–950. doi: 10.1002/art.24615. [DOI] [PubMed] [Google Scholar]
- 13.Timonen M, Viilo K, Hakko H, et al. Suicides in persons suffering from rheumatoid arthritis. Rheumatology (Oxford) 2003;42(2):287–291. doi: 10.1093/rheumatology/keg082. [DOI] [PubMed] [Google Scholar]
- 14.Dunlop DD, Manheim LM, Song J, Chang RW. Health care utilization among older adults with arthritis. Arthritis Rheum. 2003;49(2):164–171. doi: 10.1002/art.11003. [DOI] [PubMed] [Google Scholar]
- 15.Mattey DL, Dawes PT, Hassell AB, Brownfield A, Packham JC. Effect of psychological distress on continuation of anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. J Rheumatol. 2010;37(10):2021–2024. doi: 10.3899/jrheum.100050. [DOI] [PubMed] [Google Scholar]
- 16.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 17.Rosemann T, Gensichen J, Sauer N, Laux G, Szecsenyi J. The impact of concomitant depression on quality of life and health service utilisation in patients with osteoarthritis. Rheumatol Int. 2007;27(9):859–863. doi: 10.1007/s00296-007-0309-6. [DOI] [PubMed] [Google Scholar]
- 18.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56(5):1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Collaborating Centre for Mental Health (UK). Depression in adults with a chronic physical health problem: treatment and management. Leicester (UK): British Psychological Society, 2010. (NICE Clinical Guidelines, No. 91.) Available from: http://www.ncbi.nlm.nih.gov/books/NBK82916/ [PubMed]
- 20.MacMillan HL, Patterson CJ, Wathen CN, et al. Screening for depression in primary care: recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2005;172(1):33–35. doi: 10.1503/cmaj.1030823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pignone MP, Gaynes BN, Rushton JL, et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776. doi: 10.7326/0003-4819-136-10-200205210-00013. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam Pan Am Sanit Bur. 1968;65(4):281–393. [PubMed] [Google Scholar]
- 23.Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumatol. 2011;6(6):617–623. doi: 10.2217/ijr.11.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy LB, Sacks JJ, Brady TJ, Hootman JM, Chapman DP. Anxiety and depression among US adults with arthritis: prevalence and correlates. Arthritis Care Res. 2012;64(7):968–976. doi: 10.1002/acr.21685. [DOI] [PubMed] [Google Scholar]
- 25.Sleath B, Chewning B, de Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum. 2008;59(2):186–191. doi: 10.1002/art.23347. [DOI] [PubMed] [Google Scholar]
- 26.Newman S, Mulligan K. The psychology of rheumatic diseases. Baillieres Best Pract Res Clin Rheumatol. 2000;14(4):773–786. doi: 10.1053/berh.2000.0112. [DOI] [PubMed] [Google Scholar]
- 27.Luchins DJ. Depression screening as a quality indicator. Ment Health Fam Med. 2010;7(2):107–113. [PMC free article] [PubMed] [Google Scholar]
- 28.Gleicher Y, Croxford R, Hochman J, Hawker G. A prospective study of mental health care for comorbid depressed mood in older adults with painful osteoarthritis. BMC Psychiatry. 2011;11:147. doi: 10.1186/1471-244X-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med. 2005;165(11):1260–1266. doi: 10.1001/archinte.165.11.1260. [DOI] [PubMed] [Google Scholar]
- 30.Middleton K, Hing E, Xu J. National hospital ambulatory medical care survey: 2005 outpatient department summary. Adv Data. Jun 29 2007;(389):1–34. [PubMed]
- 31.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National ambulatory medical care survey: 2006 summary. Natl Health Stat Rep. Aug 6 2008;(3):1–39. [PubMed]
- 32.Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National ambulatory medical care survey: 2007 summary. Natl Health Stat Rep. Nov 3 2010;(27):1–32. [PubMed]
- 33.Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and severity of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64. [PMC free article] [PubMed] [Google Scholar]
- 34.Allen KD, Oddone EZ, Coffman CJ, Keefe FJ, Lindquist JH, Bosworth HB. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthr Cartil / OARS, Osteoarthr Res Soc. 2010;18(2):160–167. doi: 10.1016/j.joca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Bruce B, Fries JF, Murtagh KN. Health status disparities in ethnic minority patients with rheumatoid arthritis: a cross-sectional study. J Rheumatol. 2007;34(7):1475–1479. [PubMed] [Google Scholar]
- 36.Barton JL, Trupin L, Schillinger D, et al. Racial and ethnic disparities in disease activity and function among persons with rheumatoid arthritis from university-affiliated clinics. Arthritis Care Res. 2011;63(9):1238–1246. doi: 10.1002/acr.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosemann T, Backenstrass M, Joest K, Rosemann A, Szecsenyi J, Laux G. Predictors of depression in a sample of 1,021 primary care patients with osteoarthritis. Arthritis Rheum. 2007;57(3):415–422. doi: 10.1002/art.22624. [DOI] [PubMed] [Google Scholar]
- 38.Hootman JM, Helmick CG, Schappert SM. Magnitude and characteristics of arthritis and other rheumatic conditions on ambulatory medical care visits, United States, 1997. Arthritis Rheum. 2002;47(6):571–581. doi: 10.1002/art.10791. [DOI] [PubMed] [Google Scholar]
- 39.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31(1):52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA: J Am Med Assoc. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 41.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12(7):439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissman MM, Neria Y, Gameroff MJ, et al. Positive screens for psychiatric disorders in primary care: a long-term follow-up of patients who were not in treatment. Psychiatr Serv. 2010;61(2):151–159. doi: 10.1176/appi.ps.61.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Screening for depression in adults: U.S. preventive services task force recommendation statement. Ann Intern Med. Dec 1 2009;151(11):784–92. [DOI] [PubMed]
- 44.Katon WJ, Seelig M. Population-based care of depression: team care approaches to improving outcomes. J Occup Environ Med / Am Coll Occup Environ Med. 2008;50(4):459–467. doi: 10.1097/JOM.0b013e318168efb7. [DOI] [PubMed] [Google Scholar]
- 45.Rao JK, Callahan LF, Helmick CG., 3rd Characteristics of persons with self-reported arthritis and other rheumatic conditions who do not see a doctor. J Rheumatol. 1997;24(1):169–173. [PubMed] [Google Scholar]
- 46.Gilchrist VJ, Stange KC, Flocke SA, McCord G, Bourguet CC. A comparison of the National Ambulatory Medical Care Survey (NAMCS) measurement approach with direct observation of outpatient visits. Med Care. 2004;42(3):276–280. doi: 10.1097/01.mlr.0000114916.95639.af. [DOI] [PubMed] [Google Scholar]