Abstract
BACKGROUND
Fever is known to unmask the Brugada pattern on the electrocardiogram (ECG) and trigger ventricular arrhythmias in patients with Brugada syndrome. Genetic studies in selected cases with fever-induced Brugada pattern have identified disease-causing mutations. Thus, “fever-induced Brugada” is a recognized clinical entity. However, its prevalence has not been systematically evaluated.
OBJECTIVE
The purpose of this study was to assess the prevalence of Brugada pattern in consecutive patients with fever.
METHODS
ECGs of patients with fever admitted to the emergency department were evaluated for the presence of Brugada pattern and compared with ECGs of consecutive nonfebrile patients.
RESULTS
ECGs of 402 patients with fever and 909 without were evaluated. Type I Brugada pattern was 20 times more common in the febrile group than in the afebrile group (2% vs 0.1%, respectively, P = .0001). All patients with fever-induced type I Brugada pattern were asymptomatic and remained so during 30 months of follow-up.
CONCLUSION
Type I Brugada pattern is definitively more common among patients with fever, suggesting that asymptomatic Brugada syndrome is more prevalent than previously estimated.
Keywords: Brugada syndrome, Ventricular fibrillation, Fever
Brugada syndrome is a genetic disease presenting with a characteristic electrocardiogram (ECG) and a tendency to develop malignant polymorphic ventricular arrhythmias that may lead to syncope or cardiac arrest. The characteristic ECG, which includes a coved-type ST-segment elevation ≥2mm in the right precordial leads, is termed “type I” Brugada ECG pattern and is required to make the diagnosis of Brugada syndrome.1 Too often, however, the ECGs of patients with Brugada syndrome have lesser degrees or different contours of ST-segment elevation (“saddleback” rather than “coved”), which are termed type II or type III Brugada pattern. These ECG patterns are suggestive, but not diagnostic, of this disease. Moreover, in a given patient with Brugada syndrome there are marked day-to-day changes in ECG morphology. For example, in a large series of patients with documented Brugada syndrome who underwent repeated ECG recording over the years,2 only every third ECG was diagnostic (ie, showed the type I pattern) and every third ECG was completely normal, making the diagnosis of Brugada syndrome challenging.
In 1999, Dumaine et al3 reported the results of functional expression studies of a genetic mutation (T1620M) identified in patients with Brugada syndrome, showing that the loss of function of sodium channel current was accentuated at higher temperatures. The authors suggested the possibility that a febrile state may unmask the Brugada syndrome. Subsequent years witnessed a large number of reports documenting the ability of fever to unmask Brugada syndrome by promoting a type I Brugada ECG in susceptible individuals.4–11 Fever has been reported not only to accentuate the Brugada ECG pattern but also to actually trigger ventricular arrhythmias.4,10,12 Indeed, fever was the precipitating factor of arrhythmias in 18% of patients presenting with cardiac arrest in a large series of patients with symptomatic Brugada syndrome.13 Thus, the concept of “fever-induced Brugada syndrome” is well accepted. However, we do not really know how common (or rare) this phenomenon is because no large studies have systematically evaluated this issue. Therefore, in the present study, we prospectively analyzed ECG recordings of consecutive febrile patients (using afebrile patients as controls) and further investigated those who were positive for type I Brugada pattern.
Methods
Febrile and afebrile groups
Between January 2010 and August 2012, we prospectively collected ECG recordings of all febrile patients (defined as oral temperature >38°C [>100.4°F]) who were evaluated in our emergency medicine department. Because our hospital has a separate pediatric emergency room, only adults were studied. Also, to ensure that all patients presenting with fever in a given day were included in the study, we studied only those who presented to the emergency room during the morning shift of weekdays when specific physicians (KH, TO) were present. A control group of afebrile patients was used for comparison. This control group was randomly selected from a much larger patient population that was evaluated in the same emergency room during the same time period as the study (fever) group but were found to be afebrile (oral temperature <37.0°C [<98.6°F]). We did not specifically request ECGs with the precordial electrodes placed at higher intercostal spaces for patients with fever. All the ECGs from the fever and the control groups were evaluated separately by 2 experienced cardiologists (AA, RR) for the presence of Brugada pattern types I, II, and III. Any inconsistencies were settled by a third expert (SV). Type I, II, and III Brugada patterns were defined according to standard criteria and as endorsed by the Consensus Document,1 including recent important updates on the ECG diagnosis of Brugada syndrome14 with the only exception that an ECG with a right bundle branch block pattern and classic coved-type ST-segment elevation was considered type I even in the absence of negative T waves in leads V1–V3. This is because when a coved ST segment-elevation is obvious, (1) the presence of inverted T waves is no longer considered a requisite for defining Brugada syndrome by most experts; (2) ECGs in a patient with typical coved type I pattern may or may not have a negative T wave on different days15; and (3) the prognosis of coved-type ST-segment elevation without T-wave inversion is similar to that of the “classic” type I pattern.15
Clinical data were collected only for patients with type I Brugada pattern. These patients also underwent a standard evaluation for possible Brugada syndrome upon complete resolution of their fever and after providing informed consent. The evaluation included the following tests: (1) repeated ECG, (2) exercise test, (3) ajmaline challenge test, (4) 12-lead Holter test, and (5) echocardiogram. All of these ECG tests were performed with leads located in the standard location and once more with all 6 precordial leads located in the second and third intercostal spaces. The ajmaline test was performed according to recommended protocol (1 mg/kg over 5 minutes).1
Follow-up
All patients diagnosed with fever-induced Brugada were followed in our institution's arrhythmia clinic. All patients were evaluated for the occurrence of atrial fibrillation, syncope, or sudden death. The occurrence of these events in family members was also evaluated.
Statistical analysis
Using the observed proportion of Brugada ECG pattern, we estimated the 95% confidence interval based on the binomial distribution iterated through all possible outcomes.16 The χ2 test was used to compare the prevalence of Brugada between the febrile and afebrile groups. To compare age differences between patients with and without fever-induced Brugada ECG, we used the T-test with Welch approximation (variance not assumed equal). To compare the frequency of patients with fever-induced type I Brugada, age >60 and <60 years, we used the Fisher exact test.
Results
ECG recordings from 402 febrile and 909 afebrile patients were compared. Febrile and afebrile patients were of similar age (62 ± 22 years vs 61 ± 19 years, P = NS), but males were overrepresented in the febrile group (60% vs 49%, P <.001).
Eight of 402 patients with fever, but only 1 of 909 afebrile patients, had a type I Brugada pattern (Figure 1). Thus, a type I Brugada pattern was 20 times more prevalent among febrile patients (2% vs 0.1%, P = .0001). The estimated 95% confidence intervals for the presence of type I ECG were 1%–3.9% for patients with fever vs 0.016%–0.6% for afebrile patients. Limiting the analysis of the frequency of type I Brugada to male patients revealed similar results: 7 (3%) of males with fever but only 1 (0.2%) of afebrile males had a type I Brugada (P = .0015).
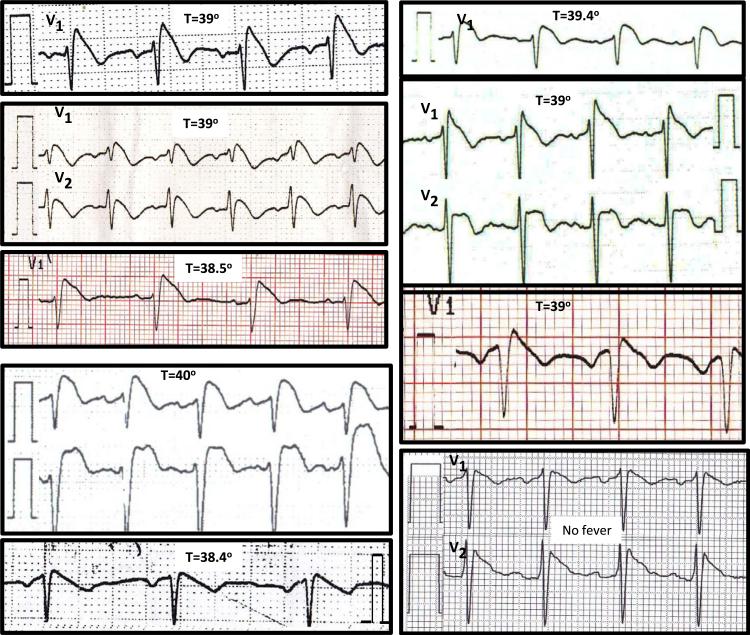
Figure 1.
Representative ECGs of the 9 patients with type I Brugada pattern, including 8 patients with fever (shown with the temperature measured when their ECG was recorded) and 1 afebrile patient.
All of the 8 patients with fever-induced type I Brugada pattern presented to the emergency room because of symptoms related to fever or the underlying infection (Table 1). Specifically, none of them reported syncope- or arrhythmia-related symptoms at the time of presentation. Thus, in all cases, the fever-induced type I Brugada pattern was an incidental finding. Mean age at the time of diagnosis of fever-induced type I Brugada was 46 years (range 31–57 years), and 7 (87%) of them were male. Interestingly, patients with fever-induced type I Brugada ECG were younger than patients with fever but no Brugada (46 ± 10 years vs 62 ± 22 years, P = .002). Mean temperature at the time of type I Brugada recording was 39°C (range 38.4°–40°C [101.1°–104°F]). A scatter plot of the heart rate and temperature of patients with fever, as recorded when the ECG was performed, failed to reveal any differences between patients with and those without type I ECG during fever (data not shown).
Table 1.
Characteristics of patients with type 1 Brugada pattern
| Patient no. | Age/gender | Temperature (°C)‡ | Fever etiology | Cardiac symptoms§ | Repeated ECG | Holter | Exercise test | Ajmaline test | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 53/F | 39 | Appendicitis | Palpitations | Type I | ND | ND | ND | |
| 2* | 47/M | 39 | Pneumonia | None | Normal | Normal | Normal | Type I | |
| 3* | 49/M | 38.5 | Bronchitis | History of vasovagal syncope | Normal | Type III | ND | Type I | |
| 4* | 56/M | 40 | Pneumonia | none | Normal | ND | ND | ND | Treated with clothiapine |
| 5* | 42/M | 39 | Viral infection | None | Normal | Normal | Normal | Type I | |
| 6* | 33/M | 38.4 | Pneumonia | Palpitations | Normal | Normal | Normal | ND | |
| 7* | 57/M | 39.4 | Gastroenteritis | None | Normal | ND | ND | ND | |
| 8* | 31/M | 39 | Pneumonia | None∥ | Normal¶ | ND | ND | ND | Treated with carbamazepine |
| 9† | 45/M | Normal | — | None | Normal | Normal | Normal | Type I | Afebrile (sauna) |
ND = not done.
Patients 1–8 had type I Brugada pattern during fever.
Patient 9 is the only patient diagnosed with type I Brugada within the afebrile group.
Celsius to Fahrenheit conversion: 38.5°C = 101.3°F, 39°C = 102.2°F, 40°C = 104°F.
None of the symptoms occurred during the index hospitalization.
Had epileptic seizures related to toxoplasma encephalitis and acquired immunodeficiency syndrome.
In the absence of fever, this patient had early repolarization in the anterolateral leads.
None of the patients had a family history suggestive of Brugada syndrome.
The only patient with a type I Brugada ECG discovered in the nonfebrile group was admitted due to smoke inhalation as a result of fire in a sauna. The finding of the Brugada pattern was incidental in this case as well.
Type II or III Brugada patterns were also found more frequently among febrile patients: 7 (1.7%) of febrile patients but only 4 (0.4%) of afebrile patients had a type II or III Brugada ECG (P = .0175). Here too, limiting the analysis to patients of male gender led to similar results: 5 (2.1%) males with fever and 2 (0.7%) without had type II or III Brugada pattern (P = .039). Mean age at the time of type II or III Brugada was 41 years (range 27–78 years), and mean temperature was 39°C (range 38.3°–39.5°C [100.9°–103.1°F]).
Further characterization of patients with fever-induced type I Brugada ECG
None of the patients with fever-induced Brugada were of southeast Asian origin or had electrolyte imbalance, significant bradycardia, or prolonged PR interval when their type I pattern was recognized. In addition, none of the 8 patients with fever-induced type I Brugada had a history suggestive of arrhythmic symptoms (Table 1). One patient had a remote history of syncope, but this was typically vagal. A second patient had a history of seizures, but those were clearly of epileptic origin and attributed to remote toxoplasma encephalitis. Also, none of the 8 patients had a family history of sudden death or Brugada syndrome. Two patients were treated with medications that probably contributed to the occurrence of type I Brugada ECG during fever (see below).
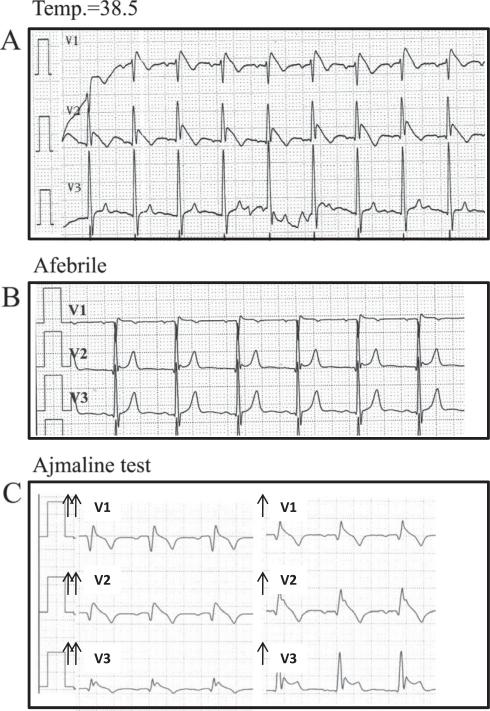
Further evaluation for possible Brugada syndrome in the absence of fever revealed the following. (1) Repeated ECG (with and without high precordial electrodes) performed in all 8 patients in the absence of fever revealed a persistent type I pattern in only one patient. (2) Exercise test (performed in 3 cases) never showed a Brugada pattern during exercise or during the recovery phase. (3) Twelve-lead Holter monitoring with high electrodes position (performed in 4 cases) were negative. (4) Ajmaline test was performed in 3 cases and was positive, revealing a type I Brugada pattern in all (Figure 2).
Figure 2.
ECG of an asymptomatic patient (no. 2) recorded during febrile illness (temperature 39°C) (A), when afebrile (B), and during ajmaline test (C). During the ajmaline test, the precordial leads are positioned on the second and third intercostal spaces. The test was stopped after administration of only 0.45 mg/kg because of the immediate ST-segment elevation.
All patients (including the one who also had persistent type I ECG in the absence of fever) were followed without antiarrhythmic therapy, and none had arrhythmic events during 30 ± 13 months (range 13–47 months) of follow-up.
Patients with fever treated with drugs possibly inducing Brugada pattern
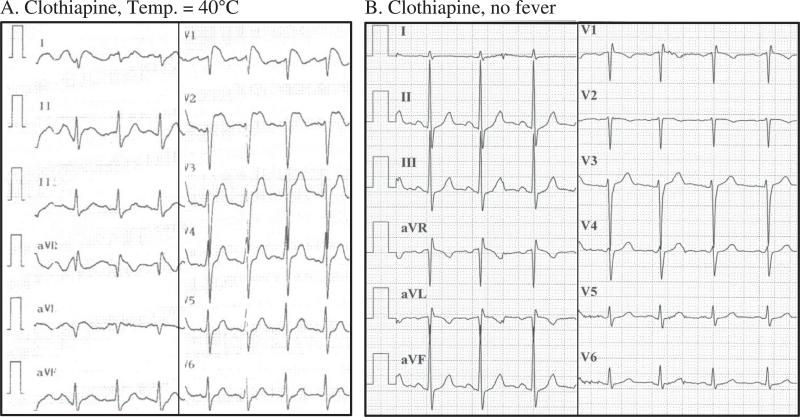
Two patients in our study are particularly interesting because they were found to have fever-induced type I Brugada pattern while being treated with drugs possibly associated with Brugada syndrome. Importantly, in both patients, the ECG normalized when the fever resolved. The first patient was treated with clothiapine and was found to have type I Brugada pattern while having high fever due to pneumonia (patient 4 in Table 1). Repeated ECG after resolution of fever was normal (Figure 3). During a follow-up of more than 2 years on the same clothiapine dosage, no cardiac events have occurred. The second patient (patient 8 in Table 1) was treated with carbamazepine. This patient had a history of epileptic seizures attributed to toxoplasma encephalitis that was a manifestation of acquired immunodeficiency syndrome. During high fever due to pneumonia, he developed type I Brugada pattern that resolved after fever subsided. After a follow-up of 2 years under the same medical regimen, he remains without cardiac events.
Figure 3.
ECG of asymptomatic patient (no. 4) treated with clothiapine recorded during febrile illness (temperature 40°C) (A) and once more after the fever resolved (B). Of note, the dosage of clothiapine was the same at the time of both recordings.
Further characterization of the single patient with type I ECG in the absence of fever
The single patient with spontaneous type I Brugada incidentally discovered among the nonfebrile control group (the male admitted after smoke inhalation in a sauna bath) had normal ECGs on repeated recordings and had negative exercise and Holter tests (all with high precordial electrodes) but had a positive ajmaline test. He remains asymptomatic without therapy.
Discussion
Fever-induced Brugada is the term used to describe the aggravation of clinical and/or ECG characteristics of this syndrome during febrile states in susceptible individuals. Although the existence of this phenomenon is well accepted, there is more than 1 proposed mechanism for its pathophysiology (see below). Moreover, little is known about the true prevalence of this phenomenon. Therefore, we studied consecutive febrile patients in order to learn how frequently fever exposes a type I Brugada ECG.
Interpretation of our main findings
The main finding of our study is that the prevalence of type I Brugada pattern is 20 times higher among patients presenting with fever than in afebrile patients (2% vs 0.1%, P = .0001). Of note, the estimated prevalence of type I Brugada among the afebrile group, with calculated 95% confidence limits of 0.016%–0.6%, is well within the estimated prevalence of asymptomatic Brugada syndrome in the general population, which is currently 0.05%.1,17 However, if one accepts the premise that fever simply unmasks the type I Brugada pattern in carriers of this disease, then one must also accept that, according to our results, the real prevalence of Brugada syndrome (at least that of asymptomatic carriers) is much higher than previously estimated. Importantly, Brugada syndrome is an autosomal dominant inherited disease that, once symptomatic, manifests with highly lethal arrhythmias. Thus, prevalence as high as 2% (95% confidence interval 1%–3.9%) is surprising because genetic traits that are lethal ultimately cause elimination of the syndrome in affected families. However, only a minority of patients with Brugada syndrome has symptoms, and those who do rarely have arrhythmias before adulthood. Accordingly, Brugada syndrome is not predicted to affect the reproduction rate of affected individuals. This situation resembles other adult-onset monogenic, autosomal, and dominant disorders that are also highly lethal. For example, about 2.7% of Ashkenazi Jews have mutations in BRCA1 or BRCA2 genes, which cause breast and/or ovarian cancer but only after the reproductive age.18
As an alternative explanation for our findings, one could argue the following:. Genetic variations present in >1% of the population are generally considered characteristic of genetic polymorphisms rather than disease-causing mutations. Thus, fever-induced type I Brugada pattern, if indeed genetically mediated, could be the phenotypic manifestation of relatively common polymorphisms. However, even if that is the case, our finding that fever-induced type I Brugada is incidentally found in 2% of febrile patients is by no means inconsequential. Of note, specific genetic polymorphisms in genes affecting the QT interval are strongly associated with drug-induced long QT syndromes.19,20 Moreover, a polymorphism in SCN5A (the gene most often involved in Brugada syndrome) that is present in 22% of Asian subjects is associated with exaggerated responses to sodium channel blockers (see below).21
The characteristics of our patients with fever-induced type I Brugada ECG, including their age (mean age 46 years, range 31–57 years) and gender (87% of male gender), are interesting because they replicate individualities reported for patients with symptomatic Brugada syndrome in larger studies.22,23 For example, in the large FINGER multicenter study, the age of patients with Brugada syndrome at the time of cardiac arrest was 43 years (range 35–54 years).22 There are too few febrile patients younger than 30 years in our series (clearly because of referral bias), but we did have 223 febrile patients older than 60 years. Based on the observed 2% incidence of type I Brugada ECG in the entire febrile cohort, one would expect to see 4 patients with type I ECG among the elderly group with fever, yet we found none (P = .001). One could speculate that the same milieu of background characteristics that “sets the stage” for the appearance of fever-induced type I Brugada in males aged 30–60 years, including their androgen hormone levels and “cardiac aging,” is the same environment that facilitates the onset of fatal ventricular arrhythmias at this particular age range.
The second finding of our study relates to the interesting interplay between fever and drug-induced Brugada syndrome. Two patients in our study were diagnosed with fever-induced Brugada while being treated with drugs that are possible inducers of the Brugada pattern. The first patient was treated with clothiapine, an antipsychotic medication. This drug is not yet listed among the drugs known to induce Brugada syndrome24 (www.brugadadrugs.org), but it belongs to the dibenzoepine family of antipsychotic drugs that also includes loxapine, a drug suspected of inducing type I Brugada and cardiac arrest.25 The second patient was treated with carbamazepine, a drug associated with Brugada pattern in an overdose case report.26 In our 2 cases, the ECG normalized once the fever resolved despite continuation of the culprit drug at the same dosage, suggesting that a “double hit” (fever plus sodium channel blockade with medications) was necessary to expose the type I Brugada pattern in these patients. This further supports our conclusion that current estimates of the prevalence of asymptomatic Brugada syndrome underestimate the true prevalence of milder forms of this disease.17
Previous studies
Only 1 previous study attempted to determine the prevalence of type I Brugada in patients with fever. This small series included 103 males with fever, none of whom had a type I Brugada pattern.27 Because of the small size of that study population, the calculated 95% confidence interval for that finding reaches 2.9%, a figure that is consistent with our results.
What do we know about fever-induced Brugada syndrome?
The prevailing view is that fever-induced Brugada syndrome is a malignant disease. For example, among 16 patients with fever-induced type I Brugada ECG described by Junttila et al,28 10 had cardiac arrest or syncope. Also, fever was the precipitating factor of arrhythmias in 18% of patients in a large series of Brugada patients presenting with cardiac arrest.13 However, in both studies, the cardiac arrest occurred mainly at the time of presentation with fever, not during long-term follow-up. This selection bias may lead to overestimation of the long-term risk for patients in whom the type I Brugada pattern is incidentally discovered during fever.
More than 1 mechanism has been proposed to explain the phenomenon of fever-induced Brugada syndrome. Expression studies performed by Dumaine et al3 with mutant cardiac sodium channels demonstrated worsening of the biophysical properties of the defective channels at higher temperatures, leading to further loss of function of sodium channel current. However, studies of other mutations tell a different story. Keller et al29 studied mutations found in patients who had fever-induced type I Brugada and fever-induced arrhythmias. These mutated channels exhibited severe to absolute loss of sodium current at physiological temperature. Thus, further loss of function during fever could not be expected and, indeed, was not found when expression studies of the same channels were performed at higher temperatures. Therefore, the authors hypothesized that in heterozygote patients, the effect of temperature on the wild-type channels is responsible for fever-induced arrhythmias. According to this model, wild-type (ie, normal) channels are less efficient at high temperatures, but this slight loss of function becomes clinically significant only in the presence of other factors that reduce the repolarization or depolarization30 reserve (eg, heterozygosity with loss-of-function mutations). This explanation is intriguing because it suggests that any patient with impaired sodium channel function, including the numerous patients treated with class IC antiarrhythmic drugs (eg, propafenone or flecainide) or patients treated with antipsychotic or antiepileptic drugs (eg, 2 of the patients in our series), potentially could be at risk for developing an “acquired Brugada syndrome” each time they develop fever. This may be true even if their ECG is near normal in the absence of fever. In a series of children with loss-of-function SCN5A (sodium channel) mutations, near-normal ECGs became frankly abnormal, sometimes leading to arrhythmia precipitation, only at the time of fever.10 Moreover, a very important population-based study recently suggested that long-term use of the antidepressant nortripty-line is associated with a 4.5-fold increased risk of sudden death.31 The proposed explanation for this observation is that multiple factors (genetic, pharmacological, and functional) conspire to reduce cardiac sodium currents and increase the risk for fatal arrhythmias. It remains to be seen if 1 of these factors is something as trivial as transient fever.
The single patient without fever found to have type I Brugada pattern in our study was admitted because of smoke inhalation as a result of fire in a sauna. A previous case report described a patient with Brugada syndrome diagnosed after an episode of syncope in a hot bath.32 Whether external heat, without fever, may serve as a trigger for uncovering Brugada is unknown, and the connection may be coincidental. Nevertheless, the higher prevalence of symptomatic Brugada syndrome reported in southeast Asia could be related, at least in part, to the higher environmental temperatures that prevail in those countries. It should be noted, however, that studies on the seasonal variation of spontaneous arrhythmias in Brugada syndrome have not conclusively demonstrated a significant peak of that phenotypic expression during warmer seasons.33–35
Study limitations
Both the febrile and afebrile control groups included in our study are not necessarily representative of the general population at large because both groups consisted of patients evaluated in an emergency department. Also, our study was conducted during morning hours only. If these limitations biased our findings, underestimation of the true prevalence of fever-induced Brugada is more likely. This is because (1) our study cohort was older (mean age >60 years) than the typical patient dying of Brugada syndrome (mean age at cardiac arrest <50 years in large studies),22,23 implying that high-risk patients were underrepresented in our series, and (2) Brugada pattern has diurnal variation with less ST-segment elevation observed during morning hours.33
The location of the recording electrodes on the chest affects the likelihood of recording a type I Brugada pattern.36 Yet, the technicians recording the ECGs in our center often fail to palpate the intercostal spaces to ensure that the V1 and V2 electrodes are placed at the correct (fourth) intercostal space. On the other hand, ECG technicians working at our center are educated to recognize ECG abnormalities that are frequently missed by physicians37 and therefore are likely to repeat the ECG with the electrodes placed at a higher intercostal space whenever an ECG raises suspicion of the Brugada syndrome. Of note, recording of a type I Brugada ECG by electrodes placed at a higher intercostal space merely reflects the correct location of right ventricular outflow in patients with Brugada syndrome.38 Accordingly, placing the V1–V2 electrodes at higher intercostal spaces only increases the sensitivity of the ECG in detecting the Brugada syndrome. Thus, any bias related to electrode position in our study (if ECGs of afebrile patients were less often recorded with higher electrodes) would be expected to lead to underdetection of the Brugada pattern in the afebrile group, without decreasing the specificity of the ECG in the febrile group. In other words, if we erred in our estimation of the prevalence of type I Brugada pattern, underestimation in the afebrile group is more likely than is overestimation in the febrile group.
Importantly, genetic results of this recently conducted study are still pending. Thus, one could argue that our patients with fever-induced type I Brugada pattern simply have an ECG that “resembles” Brugada syndrome but do not really have the disease. However, numerous case reports4,12,39,40 and series13,28 suggest that fever does not merely lead to ECG changes but may actually provoke the fatal arrhythmias that are typical of Brugada syndrome. Also, disease-causing mutations of Brugada syndrome that cause spontaneous type I Brugada in some family members but only fever-induced Brugada in others have been described.41 Thus, at the present time, patients with fever-induced type I Brugada pattern are considered to carry the disease.
Conclusion
Our study shows that the prevalence of type I Brugada ECG in patients with fever is 20 times higher than in afebrile patients, emphasizing the potency of fever in uncovering this ECG phenomenon. These findings also may imply that the number of asymptomatic Brugada patients diagnosed today is only the tip of the iceberg, as many more would have been discovered if their ECGs were recorded during febrile illnesses. This has possible implications regarding the number of patients who are potentially at risk for developing proarrhythmic complications if treated with any of the numerous medications that happen to block the cardiac sodium channel.24
Acknowledgments
We are indebted to Shelly Piterman, Nina Volensky, Ribal Kashkush, and Zinaida Reiscin, who helped collect the ECGs for the study.
ABBREVIATIONS
- AIDS
acquired immunodeficiency syndrome
- ECG
electrocardiogram
References
- 1.Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 2.Richter S, Sarkozy A, Veltmann C, et al. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J Cardiovasc Electrophysiol. 2009;20:69–75. doi: 10.1111/j.1540-8167.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 3.Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 4.Porres JM, Brugada J, Urbistondo V, Garcia F, Reviejo K, Marco P. Fever unmasking the Brugada syndrome. Pacing Clin Electrophysiol. 2002;25:1646–1648. doi: 10.1046/j.1460-9592.2002.01646.x. [DOI] [PubMed] [Google Scholar]
- 5.Kum LC, Fung JW, Sanderson JE. Brugada syndrome unmasked by febrile illness. Pacing Clin Electrophysiol. 2002;25:1660–1661. doi: 10.1046/j.1460-9592.2002.01660.x. [DOI] [PubMed] [Google Scholar]
- 6.Ortega-Carnicer J. Transient right bundle branch block unmasking anterior myocardial infarction. Resuscitation. 2003;57:313–315. doi: 10.1016/s0300-9572(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 7.Tan HL, Meregalli PG. Lethal ECG changes hidden by therapeutic hypothermia. Lancet. 2007;369:78. doi: 10.1016/S0140-6736(07)60034-8. [DOI] [PubMed] [Google Scholar]
- 8.Baranchuk A, Simpson CS. Brugada syndrome coinciding with fever and pandemic (H1N1) influenza. CMAJ. 2011;183:582. doi: 10.1503/cmaj.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki M, Shiroshita-Takeshita A, Kuruihara T, et al. Fever-induced ST-segment elevation in a syncopal patient with Brugada syndrome. Am J Emerg Med. 2012;30:263, e261–e265. doi: 10.1016/j.ajem.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Chockalingam P, Clur SA, Breur JM, et al. The diagnostic and therapeutic aspects of loss-of-function cardiac sodium channelopathies in children. Heart Rhythm. 2012;9:1986–1992. doi: 10.1016/j.hrthm.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Probst V, Denjoy I, Meregalli PG, et al. Clinical aspects and prognosis of Brugada syndrome in children. Circulation. 2007;115:2042–2048. doi: 10.1161/CIRCULATIONAHA.106.664219. [DOI] [PubMed] [Google Scholar]
- 12.Shalev A, Zeller L, Galante O, Shimony A, Gilutz H, Illia R. Symptomatic Brugada unmasked by fever. Isr Med Assoc J. 2008;10:548–549. [PubMed] [Google Scholar]
- 13.Amin AS, Meregalli PG, Bardai A, Wilde AA, Tan HL. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149:216–218. doi: 10.7326/0003-4819-149-3-200808050-00020. [DOI] [PubMed] [Google Scholar]
- 14.Bayes de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Take Y, Morita H, Wu J, et al. Spontaneous electrocardiogram alterations predict ventricular fibrillation in Brugada syndrome. Heart Rhythm. 2011;8:1014–1021. doi: 10.1016/j.hrthm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Brown LDCT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–117. [Google Scholar]
- 17.Mizusawa Y, Wilde AA. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5:606–616. doi: 10.1161/CIRCEP.111.964577. [DOI] [PubMed] [Google Scholar]
- 18.Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14:185–187. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Kanki H, Drolet B, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 20.Abbott GW, Butler MH, Bendahhou S, Dalakas MC, Ptacek LJ, Goldstein SA. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 2001;104:217–231. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 21.Bezzina CR, Shimizu W, Yang P, et al. Common sodium channel promoter haplotype in Asian subjects underlies variability in cardiac conduction. Circulation. 2006;113:338–344. doi: 10.1161/CIRCULATIONAHA.105.580811. [DOI] [PubMed] [Google Scholar]
- 22.Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 23.Kamakura S, Ohe T, Nakazawa K, et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1-V3. Circ Arrhythm Electrophysiol. 2009;2:495–503. doi: 10.1161/CIRCEP.108.816892. [DOI] [PubMed] [Google Scholar]
- 24.Postema PG, Wolpert C, Amin AS, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations. Heart Rhythm. 2009;6:1335–1341. doi: 10.1016/j.hrthm.2009.07.002. and an up-to-date website www.brugadadrugs.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouleau F, Asfar P, Boulet S, et al. Transient ST segment elevation in right precordial leads induced by psychotropic drugs: relationship to the Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:61–65. doi: 10.1046/j.1540-8167.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 26.Megarbane B, Leprince P, Deye N, et al. Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med. 2006;32:1409–1413. doi: 10.1007/s00134-006-0257-8. [DOI] [PubMed] [Google Scholar]
- 27.Erdogan O, Hunuk B. Frequency of Brugada type ECG pattern in male subjects with fever. Int J Cardiol. 2013;165:562–563. doi: 10.1016/j.ijcard.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Junttila MJ, Gonzalez M, Lizotte E, et al. Induced Brugada-type electrocardiogram, a sign for imminent malignant arrhythmias. Circulation. 2008;117:1890–1893. doi: 10.1161/CIRCULATIONAHA.107.746495. [DOI] [PubMed] [Google Scholar]
- 29.Keller DI, Rougier JS, Kucera JP, et al. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005;67:510–519. doi: 10.1016/j.cardiores.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardai A, Amin AS, Blom MT, et al. Sudden cardiac arrest associated with use of a non-cardiac drug that reduces cardiac excitability: evidence from bench, bedside, and community. Eur Heart J. 2013;34:1506–1516. doi: 10.1093/eurheartj/eht054. [DOI] [PubMed] [Google Scholar]
- 32.Smith J, Hannah A, Birnie DH. Effect of temperature on the Brugada ECG. Heart. 2003;89:272. doi: 10.1136/heart.89.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postema PG, Wilde AA. Arrhythmias in Brugada syndrome: changing throughout day and season? Heart Rhythm. 2008;5:1528–1529. doi: 10.1016/j.hrthm.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Nam GB, Baek S, et al. Circadian and seasonal variations of ventricular tachyarrhythmias in patients with early repolarization syndrome and Brugada syndrome: analysis of patients with implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2012;23:757–763. doi: 10.1111/j.1540-8167.2011.02287.x. [DOI] [PubMed] [Google Scholar]
- 35.Takigawa M, Noda T, Shimizu W, et al. Seasonal and circadian distributions of ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm. 2008;5:1523–1527. doi: 10.1016/j.hrthm.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu W, Matsuo K, Takagi M, et al. Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol. 2000;11:396–404. doi: 10.1111/j.1540-8167.2000.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 37.Viskin S, Rosovski U, Sands AJ, et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–574. doi: 10.1016/j.hrthm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Veltmann C, Papavassiliu T, Konrad T, et al. Insights into the location of type I ECG in patients with Brugada syndrome: correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm. 2012;9:414–421. doi: 10.1016/j.hrthm.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Morita H, Nagase S, Kusano K, Ohe T. Spontaneous T wave alternans and premature ventricular contractions during febrile illness in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:816–818. doi: 10.1046/j.1540-8167.2002.00816.x. [DOI] [PubMed] [Google Scholar]
- 40.Skinner JR, Chung SK, Nel CA, et al. Brugada syndrome masquerading as febrile seizures. Pediatrics. 2007;119:e1206–e1211. doi: 10.1542/peds.2006-2628. [DOI] [PubMed] [Google Scholar]
- 41.Nunez L, Barana A, Amoros I, et al. p.D1690N Nav1.5 rescues p.G1748D mutation gating defects in a compound heterozygous Brugada syndrome patient. Heart Rhythm. 2013;10:264–272. doi: 10.1016/j.hrthm.2012.10.025. [DOI] [PubMed] [Google Scholar]