Abstract
In previous studies, we observed that regulation of expression of CD200, both on cells of a transplantable breast cancer, EMT6, and of the host, as well as of the receptor, CD200R in host mice, regulated local tumor growth and metastasis in immunocompetent animals. This in turn led to an improved ability to document immunity to EMT6 in CD200R1KO mice. In the current study, we have explored the ability to cure BALB/c CD200KO or CD200R1KO mice of tumors ≤1 cm3 in size by surgical resection of localized tumor, followed by immunization with irradiated EMT6 cells along with CpG as adjuvant. While control animals treated in this fashion developed significant pulmonary and liver metastases within 30 days of surgery, significant protection was seen in both CD200KO or CD200R1KO mice, with no macroscopic lung/liver metastases observed in CD200R1KO mice on sacrifice at day 300. Following surgical resection and immunization, draining lymph nodes from control mice contained tumor cells cloned at limiting dilution in vitro even before pulmonary and hepatic metastasis was seen. In contrast, within the limits of detection of the assay used (sensitivity ~1 in 107 cells), no tumor cells were detected at limiting dilution in similarly treated CD200R1KO mice, and significant reductions were seen in CD200KO mice. Infusion of anti-CD4, but less so anti-CD8, mAb into surgically treated and immunized CD200R1KO mice attenuated protection from both macroscopic (liver/lung) and microscopic (assayed by limiting dilution of DLN) metastasis. Adoptive transfer of lymphocytes from treated CD200R1KO mice to surgically treated control mice also attenuated metastatic growth of tumor, which was abolished by pretreatment of transferred cells with anti-CD4 mAb. Our data suggest that CD200:CD200R attenuates a potentially tumor-protective CD4 host response to breast cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-013-2735-3) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Metastasis, CD200R1KO, CD200R1KO, Immunotherapy
Introduction
Increased expression of the immunoregulatory molecule CD200 has been suggested to contribute to cancer progression in human solid tumors [1, 2] and hematological tumors [3–5]. In addition, it has been suggested that human breast cancer stem cells express CD200, and that CD200+ cells, but not CD200− cells, can grow in SCID mice to form a tumor [6]. In preliminary studies (Gorczynski et al. in preparation), we have seen evidence for increased expression of human CD200 on several human breast cancer lines growing in NOD.SCIDIL−2γr−/− mice. Previous studies from our laboratory also reported that CD200 expression on cells of the transplantable EMT6 mouse breast cancer line was increased during growth in immunocompetent mice [7]. Low levels of expression persisted in NOD-SCID.IL-2γr−/− mice or mice with generalized over-expression of a CD200 transgene (CD200tg mice), despite the faster tumor growth in both of these latter strains [7].
Metastatic spread of breast cancer cells is thought to be regulated by factors intrinsic to tumor cells [8–10] as well as by host associated elements [11–13]. Local expression of TGFβ is one important factor in regulating tumor growth in vivo, and polymorphisms in TGFβR play a role in breast cancer metastasis in humans [14]. In addition, chemokine and chemokine receptor expression has been reported to regulate metastatic spread in animal models, possibly by recruiting inflammatory-type cells to the local tumor environment where they produce angiogenic factors and matrix-degrading enzymes [10, 15–17]. Factors leading to recruitment of cells which can attenuate host resistance, including Gr-1+CD11b+ myeloid-derived immune suppressor cells (MDSCs) [18, 19] and Foxp3+ Tregs [20] may also be implicated in facilitation of metastasis, e.g., a role for TGFβ in recruitment of GR-1+CD11b+ MDSCs to promote metastasis has been reported [21]. Our laboratory has also reported that in mice, CD200 expression (by the host and/or tumor cells) leads to increased seeding of tumor cells to the draining lymph node (DLN) in immunocompromised (CD200tg or NOD-SCID.IL-2γr−/−) versus immunocompetent mice, using limiting dilution cloning of tumor cells from DLN (vs contralateral lymph nodes, CLN) [7]. Neutralization of CD200 by anti-CD200mAbs decreased tumor metastasis and increased levels of cytotoxic anti-tumor immune cells in DLN [22].
Given this latter data suggesting that interference with CD200:CD200R interactions can enhance host anti-tumor resistance, we have studied the importance of CD200:CD200R expression in a model aimed at curing mice with breast cancers ≤1 cm3 in size. Our data show that surgical resection, followed by immunization with CpG as adjuvant, leads to long-term (up to 1 year) cure of tumor-bearing CD200R1KO mice, and marked prolongation of survival in CD200KO mice, but not control animals, with no tumor cells cloned from DLN of CD200R1KO at 1 year. CD4+ cells from such treated CD200R1KO mice could confer adoptive immunity to control mice.
Materials and methods (see previous publications for more details [22, 23])
Mice
Founder CD200KO and CD200R1 knockout mice are described in detail elsewhere [24]. All KO mice were derived from founder stock (on BL/6 background) and were backcrossed through ten generations with BALB/c mice obtained from Jax labs (Bar Harbour, Maine) before intercrossing to use in subsequent studies. Stock control BALB/c mice were from Jax Labs. All mice were housed 5/cage in an accredited facility at UHN. Female mice were used at 8 weeks of age.
Monoclonal antibodies
These, including a rat Mab to mouse CD200, were described previously [22, 23]. Anti-mouse CD4 mAb (GK1.5) or anti-CD8 mAb (YTS.156.7), along with rabbit complement, were purchased from Cedarlane Labs, Hornby, ON, Canada. Depletion of CD4+/CD8+ cells used mAb (1:10 dilution) for 60 min at 4 °C, followed by washing and treatment with complement (45 min at 37 °C). >95 % specific depletion was observed as assessed by subsequent FACS analysis.
CpG deoxyoligonucleotide for adjuvant use
The phosphorothioate DNA ODN with sequence 5′-TCGTCGTTTTCGGCGCGCGCCG-3′ [25] was synthesized at the Hospital for Sick Children’s Protein and Nucleic Acid Facility (HSC; University of Toronto).
EMT6 breast tumor cells, induction of tumor growth in BALB/c mice, and limiting dilution cultures to establish frequency of metastasis to DLN were as described earlier [22]: Cultures of another, more aggressive, murine breast cancer cell line, 4T1, were obtained from Dr.Nuray Erin (Antalya, Turkey).
Surgical resection and vaccination of tumor-bearing mice
Mice received 5 × 105 EMT6 tumor cells injected into the mammary fat pad in 100 μl PBS. When the tumor reached ~0.8 cm3 (generally day 15–17 post-injection) mice were anesthetized (pentobarbital) and tumors resected under sterile conditions. Mice in which local tumors re-grew within 14 days were excluded from further study as incomplete primary resection (<5 % of animals averaged over all groups in the studies described).
All mice received intraperitoneal immunization with 3 × 106 EMT6 tumor cells (irradiated with 2500Rads) mixed with 100ug CpG ODN (see above) in 100 μl PBS, emulsified with an equal volume of Incomplete Freund’s adjuvant, 2 days after surgery-in control groups (described in the text-see Fig. 3) mice received only CpG or CpG and irradiated 4T1 cells post-surgical resection. Thereafter animals were monitored × 3/week for weight loss and general health. Unless sacrificed earlier for ill-health and/or local tumor recurrence, mice were sacrificed at 2 and 4 weeks post-immunization, and at the times indicated in individual experiments, and visible tumor colonies in the lung/liver enumerated. DLN cell suspensions were prepared from individual mice and cloned under limiting dilution in 96-well flat-bottomed microtitre plates to assess tumor colony formation. CD200+ tumor colonies in these latter were enumerated after fixation using an ELISA assay described earlier [22].
Fig. 3.
Lung and liver metastases in control, CD200KO or CD200R1KO BALB/c mice receiving 5 × 105 EMT6 tumor cells subcutaneously into the mammary fat pads, followed by surgical resection 15 days later, with subsequent immunization with EMT6 with CpG as adjuvant (a), no immunization (b), or immunization with CpG alone c) or CpG with irradiated 4T1 tumor cells (d). Six mice were used per group for sacrifice at each time point (numbers surviving shown at side of each bar). Data show mean ± SD for macroscopic tumor colonies/group; ns no survivors, nd not done
Preparation of cells and cytotoxicity, proliferation, and cytokine assays: see earlier report [7].
Statistics
The frequency of cloneable tumor cells was determined as before [7]. Within experiments, comparison between groups used ANOVA, with subsequent paired Student’s t tests as indicated.
Results
Suppression of metastasis of EMT6 after surgical resection and immunization of CD200KO or CD200R1KO mice, but not control BALB/c
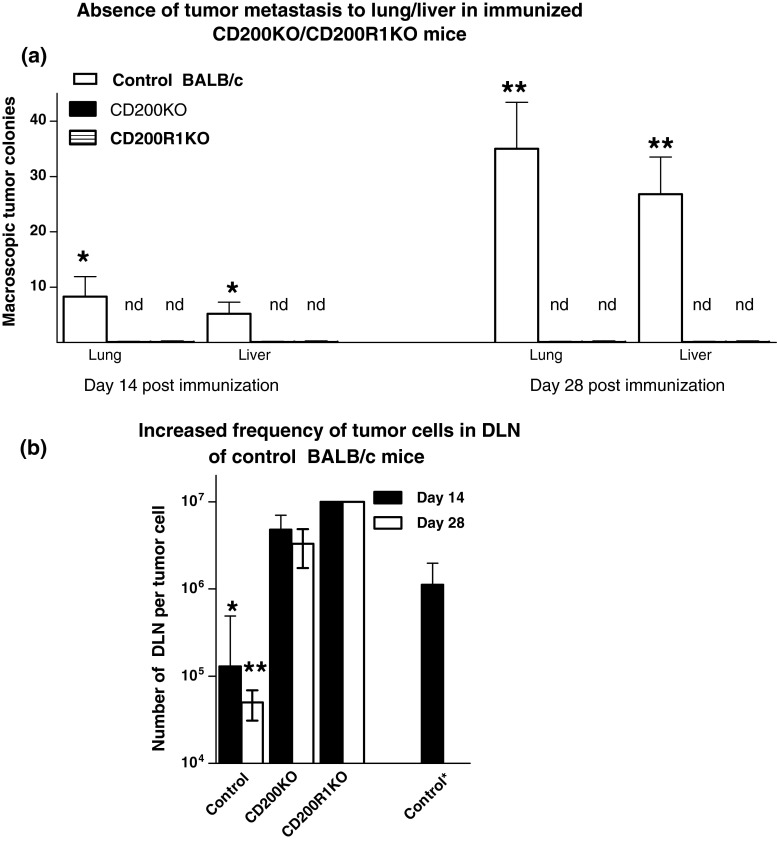
In an initial study, eight mice/group of wt BALB/c, CD200KO or CD200R1KO females received 5 × 105 tumor cells subcutaneously in the mammary fat pad. Tumors were surgically resected at day 15, and mice immunized ip with 3 × 106 irradiated EMT6 cells and CpG, emulsified in Incomplete Freund’s Adjuvant. Four mice in each group were sacrificed at 14 or 28 days post-immunization, and DLN, lung and liver harvested from individual animals. Visible (macroscopic) tumor colonies were enumerated in the liver/lung (Fig. 1a). DLN cell suspensions were cultured under limiting dilution conditions (from 103/well to 106/well) for each individual preparation, and culture plates monitored over a 21-day period for colony growth, to enumerate the frequency of tumor cells in the initial DLN samples (Fig. 1c) [22]. Note that when random colonies were tested, cells in all clones were stained (~100 % positive) with anti-BTAK (anti-tumor) antibody (data not shown). Finally, CD200+ tumor cells in the DLN were estimated by ELISA (Table 1), as described elsewhere [22].
Fig. 1.
Comparison of lung and liver metastases (a) and frequency of tumor cells cloned from DLN (b) in control, CD200KO or CD200R1KO BALB/c mice receiving 5 × 105 EMT6 tumor cells subcutaneously into the mammary fat pads, followed by surgical resection 15 days later, and immunization with EMT6 with CpG as adjuvant. Eight mice were used per group, with four of each sacrificed at 14/28 days post-surgery to measure macroscopic tumor metastases in the lung/liver (a). DLN cells harvested from individual mice were cultured under limiting dilution for 3 weeks to assess the frequency of tumor cells cloned (b). All data represent arithmetic means (±SD) for each group. Data to right in b (control *) indicates frequency of tumor cells in DLN of an independent group of mice at day 15 (the day of surgical resection). nd in a indicates no metastases detected; *,**p < 0.05 relative other groups at day 14/28, respectively
Table 1.
Frequency of CD200+/CD200−EMT6 tumor clones in DLN of control mice of Fig. 1b
| Source of DLN for tumor cloninga | EMT6 tumor clones | Mean (+SD) ODc415 | ||
|---|---|---|---|---|
| Clone frequencyb | CD200+c | CD200−c | ||
| EMT6 → wt BALB/c: at resection | 1/1.1 × 106 (77 %:102) | 7 ± 2 | 2 ± 1.6 | 0.313 ± 0.07 |
| EMT6 → wt BALB/c: day 14 post-immunization | 1/1.3 × 105 (74 %:131) | 57 + 10 | 20 + 5 | 0.322 ± 0.07 |
| EMT6 → wt BALB/c: day 28 post-immunization | 1/5.6 × 104 (74 %:143) | 1320 ± 135 | 464 ± 65 | 0.319 ± 0.08 |
Arithmetic mean (±SD) OD415 for mCD200+ clones
aTumor cells were cloned at limiting dilution as described in Fig. 1 (panel b) from DLN of mice injected with EMT6 tumor cells into the mammary fat pad, with tumors resected at 15 days, and mice receiving immunization with irradiated EMT6 2 days later. Mice were sacrificed for DLN harvest at 14 and 28 days post-immunization. Cells in limiting dilution plates were fixed at day 22 of culture and mCD200 expression assayed using rat anti-CD200 (see “Materials and methods” section). Data in row 1 shows results for DLN harvested from a separate group of EMT6-injected mice at the day of tumor resection
b,cEstimated frequency of tumor cells in DLN. % of CD200+ clones and number of tumor clones counted in parentheses. CD200+ clones had an OD415 > 3SD above the mean for control EMT6 carried in culture (CD200−); mean OD415 for control wells was 0.11 ± 0.01. Values shown are the calculated number of cloneable cells per 107 DLN cells
It is apparent from panel a of Fig. 1 that in control mice, even after surgical resection followed by immunization with irradiated EMT6 and CpG as adjuvant, significant visible metastases to both lung and liver were observed 14 and 28 days following surgery. In the absence of surgery, tumor growth was so advanced that mice in all groups became moribund before 28 days post-initial tumor inoculation, and we were unable to monitor any possible protective effect of surgery and/or immunization on metastasis in comparison with non-surgically treated animals. However, it is clear that EMT6 cells inoculated into CD200KO or CD200R1KO mice, while still able to form tumors at the site of injection (see [23]), do not produce detectable metastases to liver/lung following the treatment schedule used. Moreover, while the frequency of tumor cells cloned from DLN of control treated mice continued to increase at 14/28 days post-resection, relative to the frequency seen in DLN at the time of surgical resection (panel b, data to far left vs. far right in panel), no detectable tumor cells could be cloned from DLN of (CpG+EMT6) treated CD200R1KO mice (detection limits in assay ~1 in 1 × 107) and the numbers detected in DLN of similarly treated CD200KO were markedly reduced, and remained so following immunization. Note too that as reported in previous publications, both CD200+ and CD200− tumor cells were cloned from DLN of control mice, with no evident change in the relative percentage of these cells from the time of surgery throughout the following 28 days (Table 1).
Absence of cells attenuating ability to clone tumor from DLN of CD200KO/CD200R1KO mice
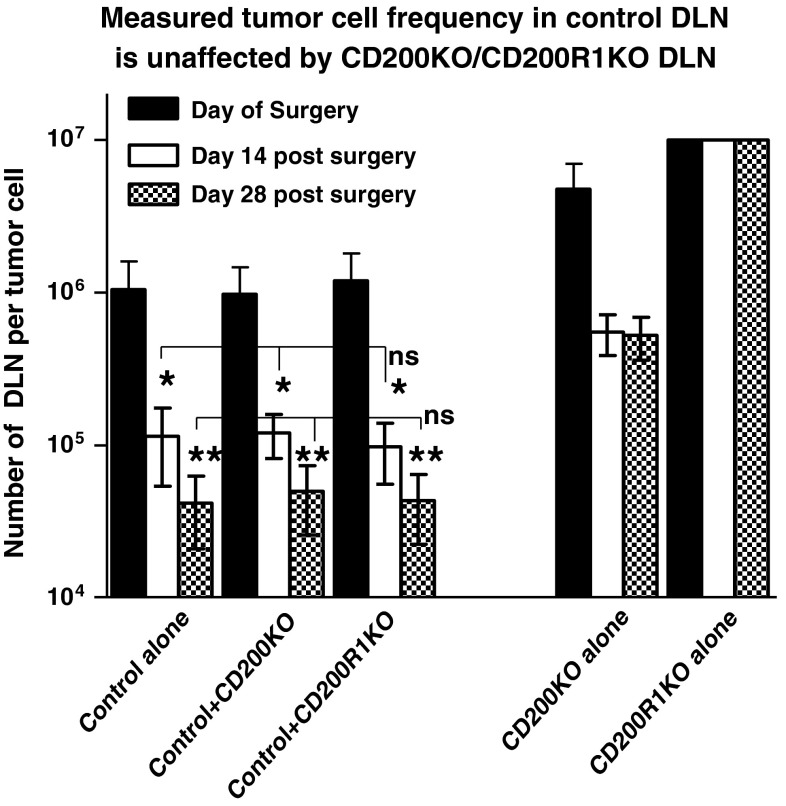
One possible explanation for the data shown in Fig. 1b was that the DLN of (CpG+EMT6) treated CD200KO/CD200R1KO mice contained populations of cells able to attenuate growth of any EMT6 tumor cells present in the population under limiting dilution conditions. While still potentially of interest, this would represent a different conclusion to the alternative, that fewer or no cloneable tumor cells existed in the DLN of these mice. In an attempt to address this issue, we injected groups of six control, CD200KO or CD200R1KO mice as in Fig. 1 with EMT6 tumor cells, followed by surgical resection and immunization with (CpG+EMT6) as before. Three mice/group were sacrificed at 14 and 28 days post-surgery. DLN cells from the control mice were cultured under limiting dilution conditions (from 2 × 103 to 1 × 105 cells/well) alone, or with a 5-fold excess of DLN cells from CD200KO or CD200R1KO mice (from 1 × 104 to 5 × 105). Cells from CD200KO or CD200R1KO mice were also cloned alone. All frequencies were subsequently calculated based on the input numbers of control cells only.
Again, as noted in Figs. 1b, and 2, the frequency of detected tumor cells in the CD200R1KO or CD200KO DLN populations alone was either below the limits of detection in this assay or less than 4-fold the frequency in control animals, respectively (see data to far right vs. far left of Figure). Importantly, addition of a 5-fold excess of cells from the DLN of CD200KO or CD200R1KO populations did not alter the measured frequency of cloneable tumor cells from DLN of control mice at any of the time points investigated (ns = not significantly different). This is consistent with the conclusion that the differences in tumor cell frequency in the DLN of these mice represents a true difference, and does not reflect the presence of a (non-tumor) population which interferes with the cloning of tumor cells in DLN of CD200KO or CD200R1KO mice.
Fig. 2.
The absence of suppression of outgrowth of tumor clones from DLN of control tumor-bearing BALB/c mice by DLN from CD200KO or CD200R1KO mice. DLN cells from control mice (3/group) were cultured under limiting dilution conditions (from 2 × 103 to 1 × 105 cells/well) alone, or with a 5-fold excess of DLN cells from CD200KO or CD200R1KO mice (from 1 × 104 to 5 × 105) at each of the time points shown (day 15 post-initial tumor cell injection represents the day of surgical resection). Cells from CD200KO or CD200R1KO mice were also cloned alone at each time point (data to far right of Figure). All frequencies of tumor cells cloned were calculated based on the input numbers of cells from DLN of control mice only. *,**p < 0.05 compared with day of surgery or day 14 post-surgery, respectively
Long-term tumor-free survival in CD200KO and CD200R1KO mice post-surgical resection
Data in Fig. 1 shows that resection of EMT6 tumor from CD200KO or CD200R1KO mice at day 15 post-inoculation of cells into the mammary fat pad, followed by immunization of mice with EMT6 and CpG as adjuvant, results in the absence of detectable lung/liver metastases for at least 28 days post-surgery, with a similar decrease in tumor cells cloned from the DLN of these mice compared with similarly treated control mice. Data in Fig. 3 and supplementary Fig. 1, pooled from similar studies, show the same findings extended over 300 days, in surgically treated animals followed by additional immunization with irradiated EMT6 cells and CpG (panel a). In addition, in panels b-d, data are shown for tumor metastases in mice receiving surgery alone (panel b), or surgery followed by CpG alone (panel c) or CpG with irradiated 4T1 tumor cells as immunogen (panel d). Numbers located beside each histogram in Fig. 3 indicate the number of animals (total 6/group) assayed per time point.
There is a number of points of interest. It is evident that additional immunization of hosts with (CpG+EMT6), beyond simply surgical tumor resection, was necessary to produce long-term tumor free survival in CD200KO and CD200R1KO mice (note difference in axis in panels a vs. b–d, and number of mice surviving to analysis/group). No significant difference in protection from metastasis was seen in surgically treated CD200KO or CD200R1KO mice following further immunization with CpG alone, or (CpG+4T1) tumor cells, relative to groups receiving no additional treatment (panels b–d). While no control animals, even with (CpG+EMT6) immunization, survived to the 150 day time point post-surgery (ns = no survivors), at least 50 % of both CD200R1KO and CD200KO mice survived to this time point after surgery and immunization with (CpG+EMT6), and 4/6 of the CD200R1KO to 300 days-CD200KO mice were not available for study to this later time point (nd in Fig. 3, panel a). Data in supplemental Fig. 1 reinforce these same conclusions, analyzing by limiting dilution the frequency of cloneable tumor cells in DLN of the different groups at the times shown. Once again, note that because of decreased survival in the absence of (CpG+EMT6) immunization the times of analysis of tumor frequency in panels b–d of supplementary Fig. 1 differ from panel a.
Decreased metastasis in CD200KO mice is not explained by a host immune response to CD200 expressed on tumor cells themselves
The data in Figs. 1, 2, and 3 show that in the absence of an intact host CD200:CD200R1 immunoregulatory axis, microscopic and macroscopic metastases from EMT6 cells implanted in the mammary fat pad are attenuated following immunization (post-surgical excision of primary tumor) with CpG and irradiated EMT6 cells. To confirm that this effect is not simply explained as a response (by the CD200KO host) to target CD00 epitopes on the EMT6 tumor itself (known to express CD2000 after growth in an immunocompetent environment [7]), as has been described for Stat6+ tumors injected into Stat6 KO mice [26], we performed the following study (note that EMT6 is CD200R1 negative by quantitative PCR-unpublished).
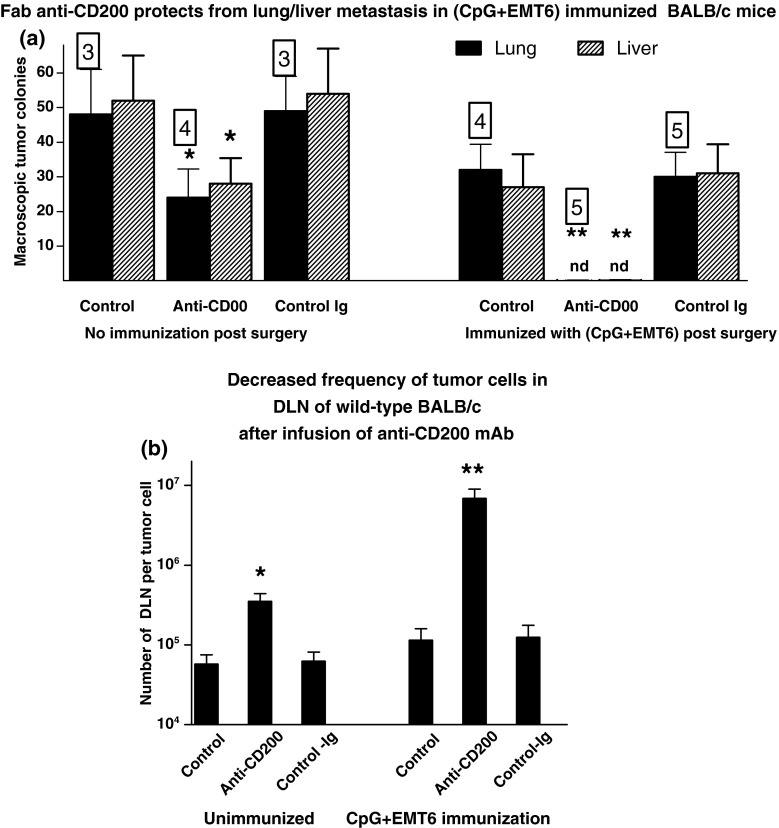
EMT6 cells were injected into the mammary fat pad of 30 wild-type control BALB/c females as before. Two groups of ten mice received iv injections (75 μg/mouse/injection) at 72 h intervals with Fab anti-CD200 Mab or isotype control Ig. Following surgical resection of tumor (day 15), five mice in each of the three groups received no further treatment, while 5 received immunization with CpG and irradiated EMT6 cells as in Figs. 1, 2, and 3. Injections with anti-CD200 or isotype control were continued for a total of 50 days, when all mice were sacrificed, and macroscopic metastases (lung/liver) and microscopic metastases (DLN limiting dilution) enumerated as before. Data are shown in Fig. 4 (panels a and b respectively). It is evident from this analysis that even when tumor CD200 expression was neutralized by Fab anti-CD200, immunization with (CpG+EMT6) protected mice from metastatic growth.
Fig. 4.
Protection from macroscopic (a) and microscopic (b) metastases in wild-type normal BALB/c females following surgical tumor resection (day 15) and immunization with (CpG + EMT6) in mice receiving ongoing infusion (at 72 h intervals) with anti-CD200 mAb. Controls received no additional treatment, or isotype control, Ig. Mice were sacrificed at 50 days post initial tumor inoculation. Data represent mean ± SD for group (numbers to side of each bar show survivors for group). *,**p < 0.05 (p < 0.01) compared with control; nd non-detectable
Role of CD4+ versus CD8+ in attenuation of metastatic tumor growth in CD200R1KO mice
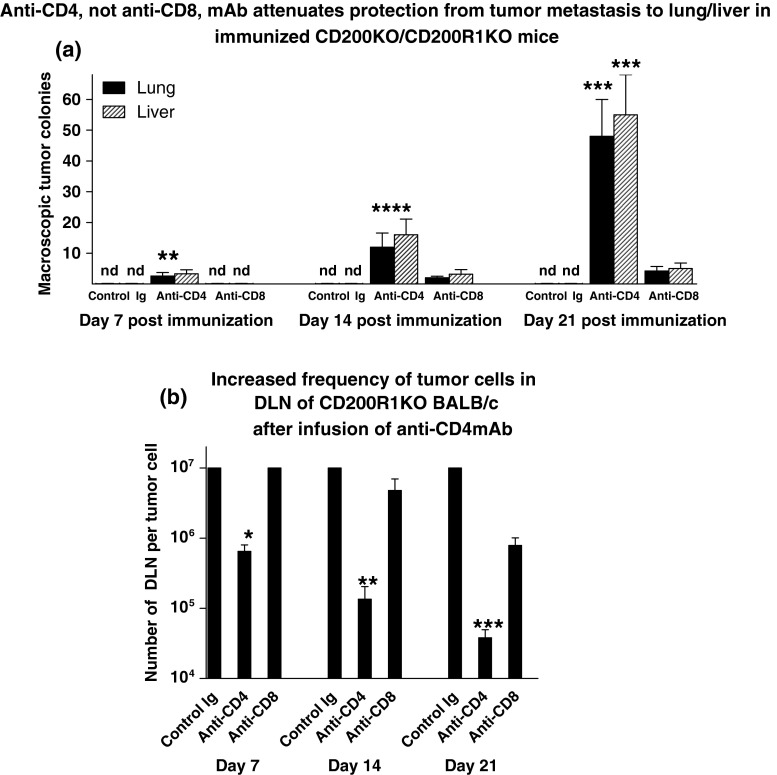
In a final series of studies, we investigated the possible mechanism whereby CD200R1KO mice were protected long-term from tumor metastasis following surgical resection and immunization with irradiated EMT6 cells with CpG. In the first of such studies, 27 CD200R1KO BALB/c mice received 5 × 105 EMT6 tumor cells subcutaneously into the mammary fat pads, followed by surgical resection 15 days later, and immunization with EMT6 with CpG as adjuvant. Mice were subsequently subdivided into groups of mice mice, each receiving either control Ig or anti-CD4/anti-CD8 mAbs (75 μg/mouse iv at 72 h intervals for four doses). Thereafter, three mice in each group were sacrificed at 14, 21, and 28 days post-surgery to measure macroscopic tumor metastases in the lung/liver (Fig. 5, panel a). In addition, DLN cells were harvested from individual sacrificed mice and cultured under limiting dilution for 3 weeks to assess the frequency of tumor cells cloned (Fig. 5, panel b).
Fig. 5.
Effect of anti-CD4/CD8 mAbs on lung and liver metastases (a) and frequency of tumor cells cloned from DLN (b) in CD200R1KO BALB/c mice receiving 5 × 105 EMT6 tumor cells subcutaneously into the mammary fat pads, followed by surgical resection 15 days later, and immunization with EMT6 with CpG as adjuvant. After immunization mice were divided into groups of nine animals, each receiving either control Ig or anti-CD4/anti-CD8 mAbs (75 μg/mouse iv at 72 h intervals for four doses). Three of each group were sacrificed at 7, 14, and 21 days post-surgery to measure macroscopic tumor metastases in the lung/liver (a). DLN cells were harvested from individual mice and cultured under limiting dilution for 3 weeks to assess the frequency of tumor cells cloned (b). All data represent arithmetic means (±SD) for each group. nd in a indicates no metastases detected; *,**,***p < 0.05 relative other groups at day 7/14/21, respectively
Interestingly, within 14 days of commencement of anti-CD4 treatment, both macroscopically visible metastases in lung/liver were seen, and the frequency of tumor cells cloned from DLN was increased ~30-fold relative to mice receiving control Ig. By 28 days post-infusion of anti-CD4, the tumor growth observed was essentially indistinguishable from that typically seen with wild-type BALB/c mice (see Fig. 1), with even greater frequencies of tumor cells cloned from DLN (~1 in 5 × 104). Importantly, while treatment with anti-CD8mAb attenuated protection (relative to control Ig: note a 20-fold increase in tumor cells cloned from DLN by d21 post-anti-CD8 treatment), the effect seen was significantly less than that observed after anti-CD4 infusion.
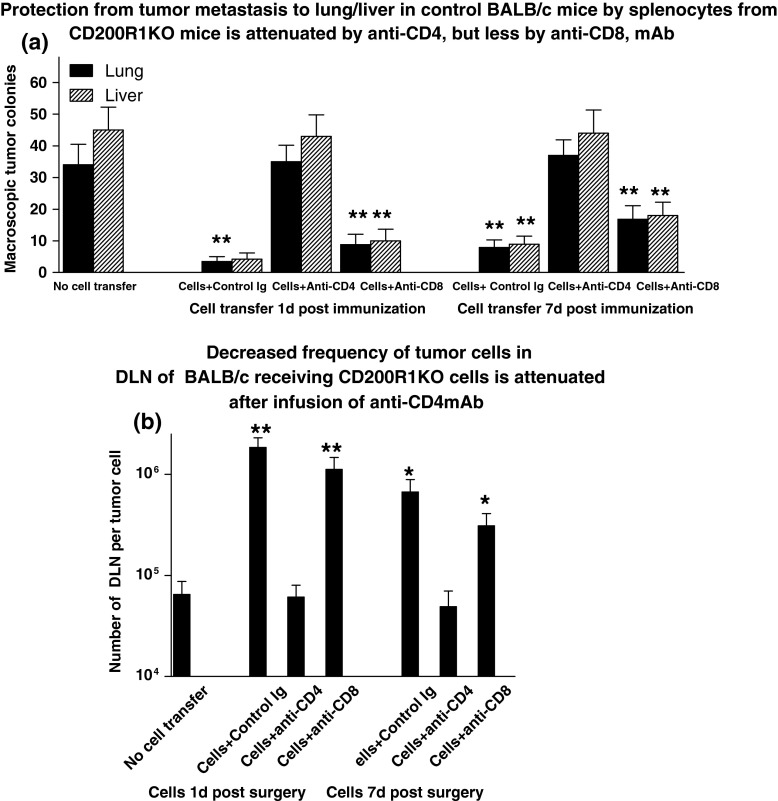
As an alternate approach to investigate the mechanism of protection from metastasis in CD200R1KO mice, we explored attenuation of lung and liver metastases (Fig. 6, panel a), and of outgrowth of tumor cells cloned from DLN (Fig. 6, panel b), in BALB/c mice treated as before by surgery/immunization after EMT6 tumor cells inoculation, and in addition receiving adoptive transfer of splenocytes from “cured” CD200R1KO mice (tumor free at 50 days post-surgery/immunization-see Fig. 1). In this study, splenocytes were independently infused into different groups of mice either 1 or 7 days after surgery/immunization. Following iv infusion of splenocytes, subsets of mice were treated with iv control Ig, anti-CD4 or anti-CD8 mAb (75 μg/mouse iv ×4 at 72 h intervals). Mice were sacrificed 28 days post initial tumor resection, and lung/liver macroscopic tumor colonies counted (panel a), along with enumeration of frequency of tumor cells in DLN using limiting dilution cultures (panel b).
Fig. 6.
Attenuation of lung and liver metastases (a), and of outgrowth of tumor cells cloned from DLN (b), in BALB/c mice receiving EMT6 tumor cells followed by surgical resection and immunization with EMT6 and CpG, along with adoptive transfer of splenocytes from “cured” CD200R1KO mice, is abolished by anti-CD4 (and less by anti-CD8) mAb. Groups of three mice received no cell transfer, or 30 × 106 splenocytes intravenously in 300 μl PBS pooled from 5 CD200R1KO mice at 50 days post tumor resection, cells being given at 1 or 7 days post-immunization of the BALB/c mice. Mice subsequently received control Ig or anti-CD4/CD8 at 72 h intervals. All animals were sacrificed 28 days post tumor resection, and macroscopic tumor colonies counted (a). DLN cells were harvested from individual mice and cultured under limiting dilution for 3 weeks to assess the frequency of tumor cells (b). All data represent arithmetic means (±SD) for each group. *,**p < 0.02, <0.05 relative to control with no cell transfer
Importantly, adoptive transfer of cells from CD200R1KO mice did indeed prevent development of macroscopic lung/liver metastases in the treated BALB/c recipients, and attenuated the increase in frequency of tumor cells cloned from DLN (Fig. 6b), even when splenocyte transfer was delayed until 7 days post-surgery/immunization. The frequency of tumor cells in DLN at 28 days (~1 in 8 × 104 in controls receiving no cell transfer-data to far left in panel b), was decreased 20-fold to ~1 in 106 in mice receiving control Ig after cell transfer, regardless of the day of cell transfer. This frequency is analogous to that seen in mice immediately after surgery (see control data to far right in Fig. 1b), implying that adoptive cell transfer from CD200R1KO mice prevents any further expansion of already metastatic DLN tumor deposits). This protection was nevertheless abolished by anti-CD4 treatment, and attenuated significantly by anti-CD8 treatment in groups receiving cell transfer 7 days post-surgery/immunization (frequency in this group ~1 in 4 × 105-far right in Fig. 6b).
Discussion
There are multiple studies to suggest that breast cancer cells are continuously monitored by host resistance mechanisms (immunosurveillance). Included in these are studies of linkage of MHC expression (Class I) with breast cancer, and of altered expression of MHC genes in cancer cells [27–29]. In addition, as with malignancies of other histological type, lymphocyte infiltration into breast tumors has been correlated with improved overall survival [30]. Peripheral blood cells of breast cancer patients have been shown to contain both T cells and antibody to antigens (MUC-1 and Her-2/neu) known to be associated with human breast cancer [31, 32]. Indeed there are preliminary experimental reports of some moderate success using Her-2/neu peptides as a cancer vaccine [33]. Buoyed by such preliminary evidence, a number of clinical trials are now underway designed to explore the efficacy of multiple antigenic moieties, many revealed through microarray analysis, which may prove of value in breast cancers of different histological types and or metastatic risk [34]. To date none of these approaches has proven to be of significant value, however, suggesting that more information on the immunobiology of host:breast cancer interactions may help shed light on this problem.
One thought as to why vaccination against cancer antigens may have had limited success has been that vaccination may augment induction of Tregs, which actually attenuate effective immunity to the tumor [35]. This concern has itself led to a newer approach in the cancer vaccine field, namely targeting T cell regulatory pathways using CTLA4 and PD-1 blockade, as immunotherapy [36]. The importance of adjuvant use in development of a clinically useful vaccine is an important issue, and independent groups have favoured the use of CpG [37], as in the studies above, or a more conventional BCG, used in combination with MUC-1 and CD80, to attenuate breast cancer growth [38]. There is concern as to what form of immunity may best reflect a suitable host-resistance strategy. While Assudani et al. [39] have stressed the value of development of CD4+ immunity in cancer vaccine trials, there is alternate evidence that CD8+ cells are linked with growth arrest of cancer cells after vaccination [40]. Nanni et al. [41] have refined their study to assess whether the vaccination strategy used was effective in reducing metastatic growth of tumor.
Using a mouse model system in which we have explored growth and metastasis of the EMT6 breast cancer cell line in female BALB/c mice, we have previously reported evidence that CD200:CD200R interactions played a significant component in regulation of host resistance. In the face of a competent hostimmune system, primary tumor cells and cells metastasizing to the DLN from tumor cells injected into the mammary fat pad were selected to over-express CD200 [7]. Anti-CD200 mAb provided protection from metastasis, while tumor cells over-expressing CD200 grew more aggressively and metastasized at higher frequency [22]. Further confirming the importance of host CD200R interactions in this immunoregulatory circuit, we showed that CD200RKO mice were more resistant both to primary and metastatic growth of tumor [23]. In additional studies in this model, data exploring tumor infiltrating cells, or cells capable of decreasing primary tumor growth on adoptive transfer, have suggested a role for myeloid-derived cells and Tregs in inhibiting host resistance, while CD8+ cells are implicated as effector cells in host immunity, with both effects modulated further by CD200:CD200R interactions.
The current studies were designed to build upon the evidence that blockade of CD200 or CD200R could enhance host tumor resistance, and ask whether additional approaches, including those used in clinical cancer care, might provide further benefit to animals with breast cancer. We opted to examine host resistance in mice following surgical resection of the primary tumor (although our previous data clearly indicates that in such animals metastatic tumor deposits in the DLN are already present, as defined by the ability of such cells to be cloned in culture under limiting dilution conditions) [22]. While a clear benefit from surgery alone was evident as assessed by both development of macroscopic metastases to lung/liver, or microscopic metastases to DLN, in both CD200KO and CD200RKO mice compared with wild-type controls, no long-term survival was seen (Fig. 3b; supplementary Fig. 1b). However, by inclusion of an immunization step following surgery, with mice receiving irradiated EMT6 tumor cells admixed with CpG in Incomplete Freunds Adjuvant 1 day later, the differences from similarly treated control mice were markedly increased (Fig. 1). Mice in the CD200KO group now showed enhanced survival out to day 150 (Fig. 3a, Supplementary Fig. 1a), while 4/6 CD200RKO mice remained alive with no detectable macroscopic or microscopic metastases at 300 days post-surgery. Protection was dependent upon EMT6 immunization, and was not recapitulated in CD200KO or CD200R1KO mice receiving either CpG alone, or CpG and irradiated 4T1 (third-party) tumor cells following surgical resection (compare panel a in Figs. 3 and supplementary Fig. 1 with panels b–d). The difference in survival of CD200KO versus CD200RKO mice is noteworthy. We have previously described that EMT6 cells growing in an immunocompetent host are selected for over-expression of CD200 on tumor cells themselves. We hypothesize that the eventual loss of control of metastatic growth in the CD200KO mice reflects suppression from such CD200+ EMT6 cells. In CD200RKO mice, regardless of the source (host/tumor) of CD200 expression, no inhibition by the CD200:CD200R axis is possible.
A control study insured that differences in tumor frequency in DLN by limiting dilution analysis was not an artifact caused by some “non-specific” inhibitory effect of CD200KO or CD200RKO DLN cells on tumor outgrowth in vitro (Fig. 2), but reflected a difference in the measured frequency of tumor cells in the DLN populations (see also [22, 23]). To eliminate the possibility that the protection afforded by immunization of CD200KO mice with CpG and EMT6 cells reflected development of an immune response to CD200-dependent epitopes, as has been described for Stat6+ adenocarcinoma cells in Stat6KO mice [26], we confirmed that protection was observed in wild-type mice receiving Fab anti-CD200 Mab (Fig. 4). Current studies (Podnos et al. in preparation) have also used EMT6 cells unable to express surface CD200 [23] in a regimen designed to immunize against metastatic growth. Finally, using infusion of anti-CD4/anti-CD8 mAbs into treated mice (Fig. 5), or by adoptive transfer of splenocytes from surgically treated and immunized CD200RKO mice to similarly treated control mice, with/without anti-CD4/CD8 mAbs (Fig. 6), we have shown that the major population implicated in resistance to both macroscopic and microscopic growth is a CD4+ population, although it is evident that CD8 depletion also diminishes the protective activity seen.
The data of Fig. 6, showing adoptive transfer of protection in vivo, are of interest in conjunction with those in Fig. 2, where a 5-fold excess of cells from CD200RKO (and CD200KO) mice did not perturb in vitro cloning of tumor cells from control mice. This discrepancy perhaps reflects a mechanism operating in vivo (to attenuate metastatic cell growth) which is not modeled in the in vitro limiting dilution cultures. Further reflective of the differences between in vitro/in vivo studies, we note that previous observations have documented CD8+ dependent-killing of tumor cells using DLN of CD200RKO mice [23], while it is evident here that a major component of the host protection revealed in the model using surgically treated and immunized mice suggests the action of a CD4+ cell population, which may act in a growth inhibitory, rather than in a cytotoxic fashion [40]. If this is indeed the case, the seemingly “cured mice” (see CD200RKO mice at 300 days in Fig. 3a, Supplementary Fig. 1a), may actually continue to harbor non-proliferating “silent” tumor cells in multiple tissues.
In sum, our current study has successfully extended previous observations in this EMT6 mammary tumor model in BALB/c mice to show that by successfully circumventing the immunosuppressive effects of the host:tumor CD200:CD200R axis, we have been able to develop a treatment regime in tumor-bearing mice, incorporating surgical resection followed by immunization with whole tumor cells +CpG, which leads to long-term cure. This “cure” is monitored by a failure to observe macroscopic metastases to lung/liver in treated mice, or to clone tumor cells by limiting dilution from lymph nodes of the same animals. Ongoing studies are exploring whether similar results can be observed in control mice treated with blocking anti-CD200/CD200R along with chemotherapy/immunomodulatory reagents (CTLA4/PD-1 blockade) which are already in clinical trial.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Supported by a Grant from the Canadian Cancer Society (CCS).
Conflict of interest
All authors confirm they have no conflicts of interest to declare.
References
- 1.Petermann KB, Rozenberg GI, Zedek D, et al. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest. 2007;117:3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreaux J, Veyrune JL, Reme T, DeVos J, Klein B. CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun. 2008;366:117–122. doi: 10.1016/j.bbrc.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 4.McWhirter JR, KretzRommel A, Saven A, et al. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Nat Acad Sci USA. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonks A. CD200 as a prognostic factor in acute myeloid leukemia. Leukemia. 2007;21:566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki BT, Mistree T, Hurt EM, Kalathur M, Farrar WL. Co-expression of the tolerogenic glycoprotein, CD200, with markers for cancer stem cells. Biochem Biophys Res Commun. 2007;364:778–782. doi: 10.1016/j.bbrc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorczynski RM, Chen Z, Diao J, et al. Breast cancer cell CD200 expression regulates immune response to EMT6 tumor cells in mice. Breast Cancer Res Treat. 2010;123:405–415. doi: 10.1007/s10549-009-0667-8. [DOI] [PubMed] [Google Scholar]
- 8.Pandit TS, Kennette W, MacKenzie L, et al. Lymphatic metastasis of breast cancer cells is associated with differential gene expression profiles that predict cancer stem cell-like properties and the ability to survive, establish and grow in a foreign environment. Int J Oncol. 2009;35:297–308. [PubMed] [Google Scholar]
- 9.Pfeffer U, Romeo F, Noonan DM, Albini A. Prediction of breast cancer metastasis by genomic profiling: where do we stand? Clin Exp Metastasis. 2009;26:547–558. doi: 10.1007/s10585-009-9254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter KW, Alsarraj J. Gene expression profiles and breast cancer metastasis: a genetic perspective. Clin Exp Metastasis. 2009;26:497–503. doi: 10.1007/s10585-009-9249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukocyte Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Kang YB. Chemokine (C-C motif) ligand 2 engages CCR2(+) stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T, Jackson CR, Link A, et al. Int7G24A variant of transforming growth factor-beta receptor type 1 is assaociated with invasive breast cancer. Clin Cancer Res. 2006;12:392–397. doi: 10.1158/1078-0432.CCR-05-1518. [DOI] [PubMed] [Google Scholar]
- 15.Liang ZX, Yoon YH, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Miyazaki H, Furihata M, et al. Chemokine CCL2/MCP-1 negatively regulates metastasis in a highly bone marrow-metastatic mouse breast cancer model. Clin Exp Metastasis. 2009;26:817–828. doi: 10.1007/s10585-009-9281-8. [DOI] [PubMed] [Google Scholar]
- 17.Ma XR, Norsworthy K, Kundu N, et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 18.Huang B, Pan PY, Li QS, et al. Gr-1(+)CD115(+) immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Debusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor GR1+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Qin FXF. Dynamic behavior and function of foxp3(+) regulatory T cells in tumor bearing host. Cell Mol Immunol. 2009;6:3–13. doi: 10.1038/cmi.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Huang JH, Ren XB, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorczynski RM, Clark DA, Erin N, Khatri I. Role of CD200 in regulation of metastasis of EMT6 tumor cells in mice. Breast Cancer Res Treat. 2011;130:49–60. doi: 10.1007/s10549-010-1259-3. [DOI] [PubMed] [Google Scholar]
- 23.Podnos A, Clark DA, Erin N, Yu K, Gorczynski RM. Further evidence for a role of tumor CD200 expression in breast cancer metastasis: decreased metastasis in CD200R1KO mice or using CD200-silenced EMT6. Breast Cancer Res Treat. 2012;136:117–127. doi: 10.1007/s10549-012-2258-3. [DOI] [PubMed] [Google Scholar]
- 24.Yu K, Chen Z, Gorczynski R. Effect of CD200 and CD200R1 expression within tissue grafts on increased graft survival in allogeneic recipients. Immunol Lett. 2013;149:1–8. doi: 10.1016/j.imlet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Landrigan A, Wong MT, Utz PJ. CpG and non-CpG oligodeoxynucleotides directly costimulate mouse and human CD4+ T cells through a TLR9- and MyD88-independent mechanism. J Immunol. 2011;187:3033–3043. doi: 10.4049/jimmunol.1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen SM, Meijer RA, Urba WJ, Hu HM, Fox BA. Regression of a mammary adenocarcinoma in STAT6−/− mice is dependent on the presence of STAT6-reactive T cells. J Immunol. 2003;170:2014–2021. doi: 10.4049/jimmunol.170.4.2014. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri S, Cariappa A, Tang M. Genetic susceptibility to breast cancer: HLA DQB*03032 and HLA DRB1*11 may represent protective alleles. Proc Natl Acad Sci USA. 2000;97:11451–11454. doi: 10.1073/pnas.97.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marincola FM, Jaffee EM, Hicklin DJ. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 29.Camploi M, Changg CC, OLdford SA. HLA antigen chnages in malignant tumors of mammary epithelial origin: molecular mechanisms and clinical implications. Breast Dis. 2004;2004:105–125. doi: 10.3233/bd-2004-20112. [DOI] [PubMed] [Google Scholar]
- 30.Menard S, Tomasic G, Casalini P. Lymphoid infiltration as a prognostic variable for early onset breast carcinomas. Clin Cancer Res. 1997;3:817–819. [PubMed] [Google Scholar]
- 31.Disis ML, Calenoff E, McLaughlin G. Existent T cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16–20. [PubMed] [Google Scholar]
- 32.Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993;151:1654–1662. [PubMed] [Google Scholar]
- 33.Baxevanis CN, Sotiriadou NN, Gritzapis AD, et al. Immunogenic HER-2/neu peptides as tumor vaccines. Cancer Immunol Immunother. 2006;55:85–95. doi: 10.1007/s00262-005-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson KS. Tumor vaccines for breast cancer. Cancer Invest. 2009;27:361–368. doi: 10.1080/07357900802574421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novakovic S, Stegel V, Kopitar A, Han A, Novakovic BJ. Preventive and therapeutic antitumor effect of tumor vaccine composed of CpG ODN class C and irradiated tumor cells is triggered through the APCs and activation of CTLs. Vaccine. 2007;25:8241–8256. doi: 10.1016/j.vaccine.2007.09.067. [DOI] [PubMed] [Google Scholar]
- 38.Yuan SF, Shi CH, Lv YG, Wang T, Wang H, Han W. A novel bacillus calmette-guerin-based breast cancer vaccine that coexpresses multiple tandem repeats of MUC1 and CD80 breaks the immune tolerance and inhibits MUC1-positive breast cancer growth. Cancer Biother Radiopharm. 2009;24:607–613. doi: 10.1089/cbr.2009.0622. [DOI] [PubMed] [Google Scholar]
- 39.Assudani DP, Horton RBV, Mathieu MG, McArdle SEB, Rees RC. The role of CD4(+) T cell help in cancer immunity and the formulation of novel cancer vaccines. Cancer Immunol Immunother. 2007;56:70–80. doi: 10.1007/s00262-006-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyer M, Karbach J, Mallmann MR, et al. Cancer vaccine enhanced, non-tumor-reactive CD8(+) T cells exhibit a distinct molecular program associated with ‘‘division arrest anergy’’. Cancer Res. 2009;69:4346–4354. doi: 10.1158/0008-5472.CAN-08-3796. [DOI] [PubMed] [Google Scholar]
- 41.Nanni P, Nicoletti G, Palladini A, et al. Antimetastatic activity of a preventive cancer vaccine. Cancer Res. 2007;67:11037–11044. doi: 10.1158/0008-5472.CAN-07-2499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.